Abstract
Serotonin (5-hydroxytryptamine; 5-HT) is abundantly present throughout the gastrointestinal tract and stored mostly in enterochromaffin (EC) cells, which are located on the mucosal surface. 5-HT released from EC cells stimulate both intrinsic and extrinsic nerves, which results in various physiological and pathophysiological responses, such as gastrointestinal contractions. EC cells are believed to have the ability to respond to the chemical composition of the luminal contents of the gut; however, the underlying molecular and cellular mechanisms have not been identified. Here, we demonstrate that the transient receptor potential (TRP) cation channel TRPA1, which is activated by pungent compounds or cold temperature, is highly expressed in EC cells. We also found that TRPA1 agonists, including allyl isothiocyanate and cinnamaldehyde, stimulate EC cell functions, such as increasing intracellular Ca2+ levels and 5-HT release, by using highly concentrated EC cell fractions and a model of EC cell function, the RIN14B cell line. Furthermore, we showed that allyl isothiocyanate promotes the contraction of isolated guinea pig ileum via the 5-HT3 receptor. Taken together, our results indicate that TRPA1 acts as a sensor molecule for EC cells and may regulate gastrointestinal function.
Keywords: gastrointestinal tract, RIN14B
The gastrointestinal tract has many functions, such as secretion, motility, and absorption. These functions are affected by various signals from the luminal contents, including nutrient and non-nutrient chemicals, mechanical factors, and microorganisms (1). The endocrine cells of the gut (hereafter enteroendocrine cells) are thought to be highly specialized mucosal cell subpopulations that receive luminal signals. There are more than 10 different types of enteroendocrine cells, and each type produces distinct transmitters/hormones (2). Serotonin (5-HT)-containing enterochromaffin (EC) cells, which are located throughout the gut, are considered to be the most prevalent enteroendocrine cells (3, 4). The 5-HT released from EC cells activate the submucosal sensory branch of the enteric nervous system and also control gastrointestinal motility and chloride secretion via interneurons and motor neurons (5, 6). Hence, EC cells are considered to be a major component of both the physiology and pathophysiology of gastrointestinal function (7, 8). It has been suggested that EC cells respond to the contents of the lumen through the activation of receptor-operated or voltage-dependent Ca2+ channels (9), however, the details of the cellular and molecular mechanisms have not yet been clarified.
Many ion channels, like the transient receptor potential (TRP) channels expressed in sensory neurons, respond to natural compounds, especially spices and herbal medicines. For example, the vanilloid receptor (TRPV1) responds to the plant component capsaicin (the pungent ingredient in chili peppers), which produces the psychophysical sensation of heat or burning, whereas TRPM8 responds to menthol (found in peppermint), which produces a cooling sensation (10, 11). Recently, the TRP channel TRPA1 (formerly ANKTM1) was cloned and characterized (12, 13). Like the other TRPs, TRPA1 is an excitatory ion channel, and is thought to function in diverse sensory processes, including cold nociception, hearing, and inflammatory pain (14–16). TRPA1 is also activated by several natural plant-derived products including mustard, cinnamon, and garlic, which produce a pungent or sharp sensation (17–20). Although TRPA1 has been found in sensory neurons and the sensory terminals of target organs (21, 22), little is known about the functional role of TRPA1 in the gastrointestinal tract, especially in non-neuronal cells such as enteroendocrine cells.
Here, we report that TRPA1 is highly expressed in human and rat EC cells, and that TRPA1 agonists cause Ca2+ influx and 5-HT release in EC cells. In addition, TRPA1 agonists are shown to promote the contraction of isolated strips of intestine via the 5-HT3 receptor. These results suggest that TRPA1 acts as a sensor molecule in EC cells for the regulation of gastrointestinal functions.
Results
TRPA1 in EC Cells.
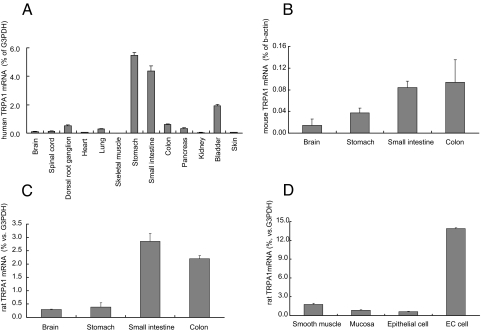
Analyses of the tissue distribution of TRPA1 mRNA using real time RT-PCR showed abundant expression of TRPA1 in gastrointestinal tissues in humans (Fig. 1A), mice (Fig. 1B), and rats (Fig. 1C). In addition, we determined the amount of TRPA1 mRNA using cDNAs from 4 distinct samples of rat small intestine: the mucosal layer (scraped off of the intact intestine), the smooth muscle layer (the layer remaining after scraping), the epithelial cell fraction (chemically isolated cells from the full thickness of the intestine), and the enriched EC cell fraction (EC cells further concentrated from the epithelial cell fraction). The degree of EC cell enrichment in the preparation was estimated by immunostaining the 5-HT. Over 400 cells were rondomly counted in the enriched EC cell fraction, 5-HT was positively immunostained in 81% of the cells, in contrast to much less in the epithelial cell fraction (Fig. S1). Real time RT-PCR analysis revealed that the expression of TRPA1 was higher in the enriched EC cell fraction than in the other preparations (Fig. 1D). The level of TRPA1 mRNA in the enriched EC cell fraction was approximately 16 times greater than that in the mucosal layer. TPH1, chromogranin A, VMAT1, and synaptophysin, all of which have been reported to be expressed abundantly in EC cells (4, 8), were also extensively expressed in the enriched EC cell fraction (Table 1).
Fig. 1.
Distribution of transient receptor potential cation channel A1(TRPA1) mRNA in human (A), mouse (B), and rat (C) tissues, and localization in rat small intestine (D). Data represent the ratio of TRPA1 to glyceraldehyde-3-phosphate dehydrogenase (G3PDH) or β-actin mRNA. Smooth muscle, smooth muscle layer; mucosa, mucosal layer; epithelial cell, isolated epithelial cell fraction; EC cell, enriched EC cell fraction. Data represent the mean ± S.D. (n = 3).
Table 1.
mRNA levels of EC cell markers and TRPA1 in mucosal layer (Mucosa) and enriched EC cell fraction (EC cell) from rat small intestine
| Marker | Ratio, % |
||||
|---|---|---|---|---|---|
| TPH1 | Chromogranin A | VMAT1 | Synaptophysin | TRPA1 | |
| Mucosa | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 |
| EC cell | 7,310.5 | 7,837.2 | 3,777.6 | 2,288.4 | 1,626.7 |
Data represent the ratio of the G3PDH-normalized mRNA levels in EC cells to that in the mucosa.
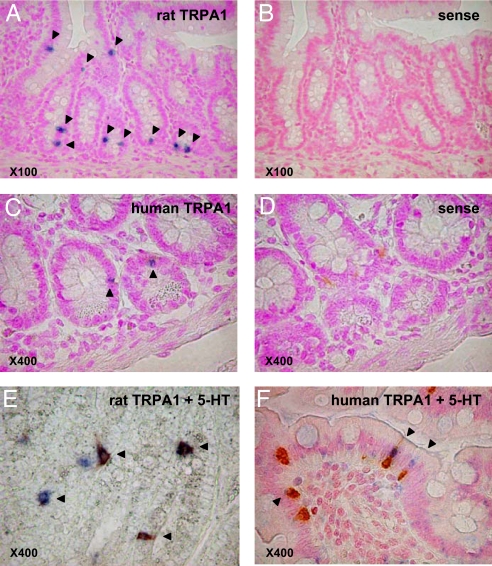
To further confirm the high expression of TRPA1 in EC cells, we performed double staining experiments coupled with in situ hybridization (ISH) for TRPA1 mRNA and immunohistochemistry (IHC) for 5-HT in rat or human duodenum. Dense staining of TRPA1-specific ISH was observed in the epithelial layer, but not in the smooth muscle layer or lamina propria in either rats or humans (Fig. 2 A and C). Examination using the TRPA1 sense probe detected no specific staining (Fig. 2 B and D). Double staining for TRPA1 and 5-HT showed that TRPA1 mRNA was expressed in the 5-HT-positive intraepithelial enteroendocrine cells in both species (Fig. 2 E and F). The numbers of TRPA1-stained cells in the rat duodenum and 5HT-expressing epithelial cells facing the intestinal lumen were approximately the same (around 70%).
Fig. 2.
In situ hybridization (ISH) of TRPA1 mRNA in rats (A and B) and humans (C and D), as well as ISH combined with 5-HT antibody immunohistochemistry (IHC) in rats (E) and humans (F) using duodenal tissues. TRPA1 staining was restricted to epithelial cells facing the intestinal lumen in both rats and humans (arrows in A and C). No sense probes were stained (B and D). Many TRPA1-expressing cells (blue) were double-stained with the 5-HT antibody (brown) (E and F; arrow heads).
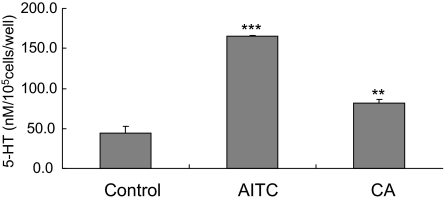
To investigate the functional significance of TRPA1 in EC cells, we used enriched EC cell fractions to examine the effects of TRPA1 agonists on 5-HT release. Allyl isothiocyanate (AITC) and cinnamaldehyde (CA) increased 5-HT release from the enriched EC cell fraction (Fig. 3).
Fig. 3.
The release of 5-HT from rat enriched small intestine EC cell fractions induced by TRPA1 agonists. AITC (500 μM) and CA (500 μM) stimulated the release of 5-HT. Data represent the mean ± S.D. (n = 3). **, P < 0.01; ***, P < 0.001 vs. control group (Dunnett's t test).
Characterization of the Rat Endocrine Cell Line RIN14B.
We isolated an EC cell lineage for the detailed study of EC cell functions because a completely pure EC cell culture could not be achieved. Slight amounts of somatostatin and GIP mRNA were detected in the EC cell fraction, which suggested that the contaminating cells may be an enteroendocrine cell subset, such as D-cells or K-cells (Fig. S2).
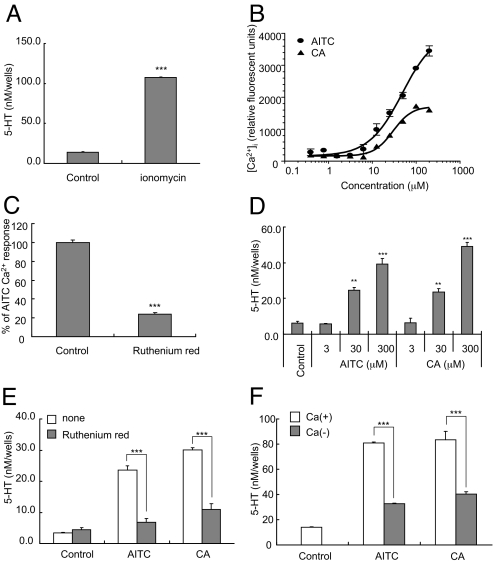
Several dozen cell lines which were derived from gastrointestinal or endocrine tissue were examined by means of gene expression of EC cell markers such as TPH1, chromogranin A, VMAT1, and synaptophysin. This yielded the rat pancreatic endocrine cell line RIN14B, which highly expresses EC cell marker genes (Table 2). As in the rat enriched EC cell fraction, TRPA1 mRNA was also found to be highly expressed in RIN14B cells, but not in other intestinal cells, including NCI-H716, CaCo-2, and IEC-6 (data not shown). The expression levels of the EC cell markers and TRPA1 genes in RIN14B were comparable to those in the enriched EC cell fraction (Table 2). It is generally accepted that the elevation of intracellular Ca2+ ([Ca2+]i) triggers 5-HT release by EC cells (9). Therefore, the potential of RIN14B cells to release 5-HT by means of stimulation with Ca2+ ionophore was subsequently tested. As shown in Fig. 4A, ionomycin at 10 μM stimulated 5-HT release from RIN14B cells. These results indicated that RIN14B cells share functional similarities with EC cells, and can be a good model for studying the EC cell function.
Table 2.
mRNA levels of EC cell markers and TRPA1 in RIN 14B and enriched EC cells
| Marker | Ratio, % |
||||
|---|---|---|---|---|---|
| TPH1 | chromogranin A | VMAT1 | synaptophysin | TRPA1 | |
| EC cell | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 |
| RIN14B | 917.4 | 7,2646.8 | 3,442.6 | 98.8 | 159.7 |
Data represent the ratio of G3PDH-normalized mRNA levels in RIN14B cells to that in EC cells.
Fig. 4.
Stimulated 5-HT release and intracellular Ca2+ in RIN14B cells. Ionomycin (10 μM) induced the release of 5-HT from RIN14B cells (A). The dose-response curves of TRPA1 agonist-induced increase in [Ca2+]i in RIN14B cells (B). Blockade of the AITC (30 μM)-evoked increase in [Ca2+]i induced by ruthenium red (3 μM) in RIN14B cells (C). TRPA1 agonist-induced release of 5-HT from RIN14B cells (D). Effects of the TRPA1 inhibitor ruthenium red (30 μM) on the AITC (300 μM)- or CA (300 μM)-induced 5-HT release from RIN14B cells (E). Role of Ca2+ in the TRPA1 agonist-induced release of 5-HT from RIN14B cells (F). The cells were stimulated by AITC (300 μM) or CA (300 μM) in HBSS (open column) and Ca2+-free HBSS (solid column). All data represent the mean ± S.D. (n = 3). **, P < 0.01; ***, P < 0.001 vs. control group (Dunnett's test for D and Student's t test for A, C, E, and F).
Effects of TRPA1 Agonists on the [Ca2+]i Increase and 5-HT Release Induced in RIN14B Cells.
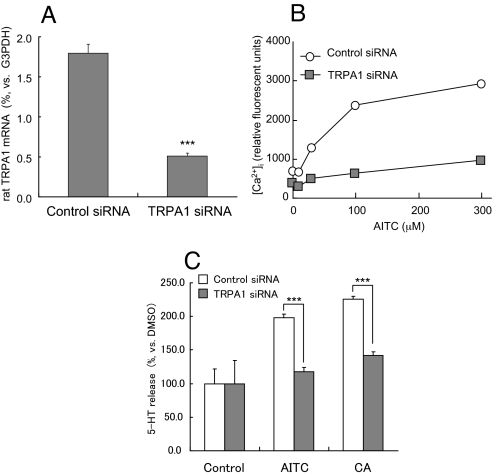
To investigate the mechanisms behind TRPA1-mediated 5-HT release, we examined the effects of TRPA1 agonists on the concentration of [Ca2+]i in RIN14B cells using a fluorometric imaging plate reader (FLIPR) system. AITC and CA were found to evoke a dose-dependent rise in [Ca2+]i (Fig. 4B). This TRPA1 agonist-induced [Ca2+]i increase was blocked by ruthenium red, a TRPA1 inhibitor (Fig. 4C). Furthermore, these TRPA1 agonists had a stimulatory effect on 5-HT release from RIN14B (Fig. 4D). Both treatment with ruthenium red and the use of a Ca2+-free medium inhibited the release of 5-HT (Fig. 4 E and F). A small interfering RNA (siRNA) was used to further confirm that the 5-HT release induced by these compounds were TRPA1-mediated. Real time RT-PCR of RIN14B cells showed that the transfection of rat TRPA1-specific siRNA reduced TRPA1 mRNA expression by approximately 70% (Fig. 5A). The TRPA1 agonist-induced increase in [Ca2+]i seen in RIN14B cells was also inhibited by siRNA transfection (Fig. 5B). Moreover, the transfection of siRNA reduced the TRPA1 agonist-induced release of 5-HT from RIN14B cells (Fig. 5C). These results indicate that the [Ca2+]i increase and 5-HT release induced by AITC and CA in the EC cell model, RIN14B, were mediated by TRPA1.
Fig. 5.
Analyses for TRPA1 agonist-induced 5-HT release mechanisms using TRPA1-specific siRNA. Effects of siRNA on either TRPA1 mRNA expression (A) or TRPA1 agonist-induced [Ca2+]i (B) in RIN14B cells. (C) Effects of siRNA on TRPA1 agonist-induced 5-HT released from RIN14B cells. Three to 5 independent experiments were performed and typical results are shown, respectively. Data represent the mean (B) or the mean ± S.D. (n = 3) (A and C), respectively. ***, P < 0.001 vs. control group (Student's t test).
Contractile Response to TRPA1 Agonists.
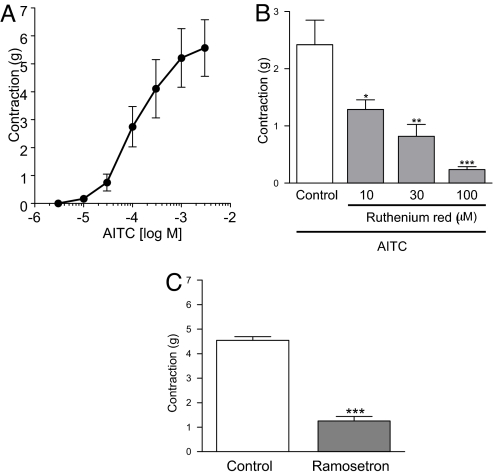
The 5-HT released from EC cells is thought to stimulate gastrointestinal contractions primarily through 5-HT3 receptors located on intrinsic neurons (3, 23). Organ bath experiments were performed to determine whether TRPA1 agonists could induce gastrointestinal contractions through this mechanism. In these experiments, the application of AITC caused concentration-dependent contractions in isolated guinea pig ileum (Fig. 6A). Ruthenium red inhibited these AITC-induced contractions in a concentration-dependent manner (Fig. 6B). Ruthenium red's ability to inhibit is not the result of non-specific effects, such as inhibition of Ca2+ channels, as previously reported (24). In fact, the contractile responses to AITC were significantly reduced by ramosetron, which is known to be highly specific 5-HT3 antagonist (25) (Fig. 6C). The fact that it has an inhibitory effect indicates that AITC contracts ileum through a 5-HT3 receptor-mediated pathway. In addition, both ruthenium red and ramosetron inhibited the contraction of guinea pig ileum induced by CA in preliminary experiments. These results indicated that TRPA1 plays a role in the regulation of gastrointestinal motility through serotonergic mechanisms.
Fig. 6.
Contractile effect of AITC in guinea pig-isolated ileum. AITC dose-dependently induced contractions (A). Effect of ruthenium red (10–100 μM) (B) and ramosetron (0.3 μM) (C) on the contractile responses to AITC (300 μM). Data represent the means ± S.E. of 4 experiments. *, P < 0.05; **, P < 0.01; ***, P < 0.001 vs. control group (Dunnett's test for B and Student's t test for C).
Discussion
Gastrointestinal functions such as motility and secretion are regulated not only by neurons, but also endocrine cells; these accumulate and release neurotransmitters and hormones. More than 10 different types of endocrine cells are present in the gastrointestinal tract, each with a distinct distribution (2). In the small and large intestine, major populations of the endocrine cells are 5-HT-releasing EC cells. These cells are found on the mucosal surface and release 5-HT into the gut wall in response to mechanical stimuli or nutrients such as glucose and fatty acids (26–28). This released 5-HT can stimulate vagal afferents and enteric nerves, which results in various gastrointestinal reactions, such as vomiting and peristaltic reflux (3, 29). These observations led to the idea that EC cells act as sensors in the gastrointestinal mucosa. However, the cellular and molecular mechanisms behind these sensory functions have not yet been clarified. In this study, new insights into the sensory mechanism of EC cells were gained using purified EC cells and RIN14B cells.
It has been reported that TRPA1 is expressed primarily in small diameter, nociceptive neurons, where its activation likely contributes to a variety of sensory processes, including thermal nociception, mechanosensation, and inflammatory hyperalgesia (14–16). TRPA1 is an excitatory ion channel targeted by irritant compounds derived from plants, such as mustard, cinnamon, and garlic (17–20). These compounds can depolarize the nociceptors in nerve endings, which convey harmful information to the brain (17–19). These studies resulted in the proposal that the TRPA1 expressed in neuronal cells acts as a sensor for reactive chemicals in the body (30). However, little is known about the function of TRPA1 in non-neuronal cells, including enteroendocrine cells, although recent evidence suggests that TRPA1 expression is more widespread than originally thought (22). In the present study, TRPA1 was found to be highly expressed in the gastrointestinal tract, from the stomach to the colon, as well as in the dorsal root ganglia. Moreover, our studies showed that TRPA1 is highly expressed in EC cells, but not in submucosal or smooth muscle layers that involve enteric nerves, and can act functionally. Interestingly, some TRPA1 stimulants, including methyl salicylate, eugenol, hypertonic solution, and cold temperatures, increase the release of 5-HT from EC cells (31–34). These observations support our data, which indicates that TRPA1 is expressed in EC cells and contributes to the release of 5-HT. Previous tracing studies showed that intestinal nerves do not enter the epithelium, which excludes the possibility that they sense anything from the intestinal contents directly (35). Given this, it is possible that the EC cells dispersed along the gastrointestinal mucosa could be sensors for detecting the chemical makeup of the luminal contents. Further, the TRPA1 in the EC cells could serve as a luminal sensory molecule.
The 5-HT released from EC cells via stimulation by TRPA1 can activate intrinsic neurons located in the submucosal and myenteric plexuses. In fact, we demonstrated that TRPA1 agonist evokes intestinal contractions, and that the contractile response was largely inhibited by the 5-HT3 receptor antagonist. It is well known that 5-HT3 receptors contribute to the initiation and/or transmission of gastrointestinal motility and secretory reflexes (36–39). However, in the guinea pig, 5-HT4 and/or 5-HT1P receptors in the enteric neuron are also involved in the modulation of peristaltic activity by 5-HT (40). The 5-HT3 antagonist-resistant contractions in our experiments could be mediated by these receptors.
In addition to the intrinsic nerves, the extrinsic nerve endings in the gastrointestinal mucosa can also be activated by the 5-HT released from EC cells (3). In fact, cancer chemotherapeutic agent-induced nausea and vomiting are mediated by extrinsic afferent nerves activated by the 5-HT released from EC cells (3, 41). Recent reports showed that acrolein, a metabolite of chemotherapeutic agents (42), is a TRPA1 agonist (14), and we have observed that acrolein increased the release of 5-HT from purified EC cells and RIN14B cells (data not shown). These results suggest that the TRPA1 in EC cells could be involved in chemotherapeutic agent-induced nausea and vomiting.
Like EC cells, the rat somatostatin-producing pancreatic delta cell line RIN14B was found to be capable of releasing 5-HT in response to TRPA1 agonists. There are several endocrine cells known to co-express more than one hormone, such as the rat insulinoma cell line RINm5F (43, 44). This cell line was isolated using the same cloning method as that for RIN14B, and expresses multiple hormones, including insulin and 5-HT (44, 45). Moreover, endocrine cells are descendent from multipotent progenitor cells, and both somatostatin- and 5-HT-expressing cells can be derived from the same progenitors (46); therefore, it is not surprising that RIN14B can release both somatostatin and 5-HT. In addition to its 5-HT-releasing ability, RIN14B also expressed both endocrine (chromogranin A, synaptophysin, and VMAT1) and EC cell (TPH1) markers. These results indicate that the RIN14B cell line could be a useful model for studying the physiological and pharmacological nature of EC cells. However, it should be noted that some functional differences can exist between these cells, since the mRNA levels of some markers vary. Further investigation is needed to elucidate the function of EC cell markers in RIN14B cells.
Previous studies have shown that EC cells respond to nutrients, such as glucose and fatty acids, and the mechanical stimulation generated by a shaker (26–28). As shown in Figs. 3 and 4, both the EC cell fraction and RIN14B cells released 5-HT, even under control conditions. These results suggest that the glucose in the assay buffer and/or mechanical stimulation generated by movement of the assay buffer might influence the basal release of 5-HT. There is also a possibility that some contaminating cells could also stimulate basal release in the EC cell fraction because the level of 5-HT was higher than that in RIN14B cells.
To summarize, we showed that TRPA1 is highly expressed in EC cells, and that stimulation of TRPA1 by agonists evokes the release of 5-HT from EC cells. This can then produce contractions via the 5-HT3 receptor. Furthermore, the RIN14B cell line was found to be a useful model for the investigation of EC cell function. These findings will provide insights into the physiology and pathophysiology of EC cell function.
Materials and Methods
Animals.
Male Sprague-Dawley rats (160–200 g) and male ddY mice (30–35 g) were used for the gene expression study, and male Hartley guinea pigs (350–500 g) were used for the organ bath study. All animals were obtained from Japan SLC, Inc., and housed in a temperature-controlled environment (22 ± 2 °C) under a 12-h light/dark cycle with food and water provided ad libitum until use. All animal experimental procedures were approved by the Committee for Animal Experiments of Astellas Pharma Inc.
Chemicals.
In this study, AITC, CA, ruthenium red, ionomycin calcium (Wako Pure Chemical Industries), acetylcholine chloride (Sigma-Aldrich Japan), and ramosetron hydrochloride (Astellas Pharma, Inc.) were used. AITC and CA were diluted in media containing a final dimethyl sulfoxide (DMSO) concentration of 0.3%, and the other drugs were dissolved in deionized water.
Cell Culture.
RIN14B cells were obtained from ATCC (ATCC No. CCL 89), and cultured in RPMI1640 medium (Invitrogen-Japan) supplemented with 10% FCS and antibiotics (100 U/mL penicillin and 100 μg/mL streptomycin) at 37 °C in a 5% CO2 humidified atmosphere.
Preparations of Tissues and Enriched EC Cell Fraction.
For each tissue and cell isolation experiment, mice and rats were anesthetized with ether and killed by exsanguination; the brain, stomach, small intestine, and colon of each were then removed. For the assessment of the contribution of EC cells to TRPA1 expression and function, EC cells from rat small intestine were concentrated as follows. The small intestinal segments were everted, end-ligated, and preincubated in 1 mM DTT containing HBSS (Hanks' balanced salt solution, Invitrogen-Japan) to remove mucus. The sacs were then incubated for 20 min, without stirring, at 37 °C in chelating digestive buffer (70 mM NaCl, 5 mM KCl, 20 mM NaHCO3, 0.5 mM NaH2PO4, 1 mM Na2HPO4, 50 mM Hepes, 11 mM Glucose, 1 mM EDTA, 0.5% BSA, and 0.05 mM DTT) infused with 95% O2/5% CO2 gas. The buffer was then changed, and incubation continued for another 20 min. After the second incubation, the sacs were transferred to HBSS containing 0.5% BSA and gently stirred for 10 min twice. The suspended cells (epithelial cell fraction) were collected by pouring the suspension through a nylon mesh filter. The epithelial cell fraction was further separated by counterflow elutriation. The cell fraction was suspended in elutriation buffer (140 mM NaCl, 1.2 mM MgSO4, 1 mM CaCl2, 10 mM Hepes, 11 mM glucose, 0.5% BSA, and 0.05 mM DTT) and loaded into a JE6B rotor in a J2 small elutriation chamber (Beckman Coulter). After centrifugation at 2,000 rpm, the cells were collected at the rate of 21 mL/min. The cells were further concentrated by performing step density gradient centrifugation (47). The bottom and the intermediate layers were adjusted to a density of 1.100 and 1.070 g/mL, respectively. After centrifugation at 1,100 rpm for 8 min with slow deceleration, enriched EC cells were collected at the 1.070 interface. The cells collected were washed in HBSS to remove cellular debris and obtain the enriched EC cell fraction. The fractions were used immediately afterward for 5-HT release and RT-PCR experiments. The mucosal layer was scraped off the intact small intestine using the edge of a glass slide. The remaining smooth muscle containing intrinsic nerves was also used as reference.
Real-Time RT-PCR.
All experimental procedures using human mRNA and tissues were approved by the Astellas Research Ethics Committee. The mRNAs from human tissues, containing whole stomach, whole small intestine, and whole colon, were purchased from Clontech Laboratories, Inc. for the analysis of gene expression. Total RNA was isolated from the animal tissues, rat cell fractions, and RIN14B cells using an RNeasy Kit (Qiagen-Japan) and reverse transcribed into cDNA using the SuperScript-II enzyme (Invitrogen-Japan) according to the manufacturer's instructions. The coding sequences for the corresponding receptors published in GenBank were used to generate specific oligonucleotide primers for the selected gene sequence (Table S1). Quantitative PCR was performed using an ABI PRISM 7900HT sequence detection system (Applied Biosystems) with Power SYBR Green PCR master mix. Negative control experiments were performed using RNA isolated from tissue without subsequent RT-PCR, but they did not yield specific products.
5-HT Release Experiments.
RIN14B cells were seeded in 24-well plates at the rate of 2 × 105 cells/0.5 mL RPMI1640 containing 10% FCS/well and cultured for 72 h. The cells of the enriched EC cell fraction were also seeded under the same conditions, and cultured for 2–4 h. The medium was removed before washing the cells with HBSS containing 0.1% BSA and 2 μM fluoxetine (Tocris). The HBSS was again removed and replaced with 0.25 ml HBSS containing different stimulants at the indicated concentrations, after which the solution was incubated further for 20 min at 37 °C. The assay buffer was collected and centrifuged for 5 min to remove any detached cells. The supernatants were collected and stored at −80 °C until 5-HT measurement using an enzyme immunoassay (EIA) kit (Beckman Coulter). DMSO was used as the control in 5-HT release experiments.
[Ca2+]i Analysis.
RIN14B cells were plated at 2 × 104 cells per well in black 96-well clear-bottom plates (Biocoat, Becton Dickinson) and incubated for 48 h at 37 °C, 5% CO2 to achieve 80–100% confluence. The cell culture medium was removed, and the cells of each well were washed once with 100 μL of assay buffer (140 mM NaCl, 0.15 mM CaCl2, 3.3 mM KH2PO4, 0.8 mM K2HPO4, 1.2 mM MgCl2, 10.0 mM glucose, and 20.0 mM Hepes, pH 7.4). The cells were incubated with assay buffer containing 2 μM Fluo-4 a.m. for 120 min at 37 °C, 5% CO2 before each experiment to allow for the equilibration of Fluo-4 a.m. across the cell membranes. Fluorescence was recorded using a fluorometric imaging plate reader (FLIPR) at an excitation wavelength of 488 nm and an emission wavelength of 540–590 nm. Assays were carried out at room temperature.
siRNA Experiments.
Four siRNAs were designed from the rat TRPA1 sequence using the siDirect designing program (RNAi Co.). These 21-nucleotide siRNAs were obtained from Sigma-Aldrich Japan. In the experiments to suppress TRPA1 expression in RIN14B cells, the most effective siRNA among these was used (sense primer; 5′-CUGGCAGACUACCUAAUUUCA-3′, and antisense primer; 5′- AAAUUAGGUAGUCUGCCAGGU-3′). Negative control siRNA was used as a control (Ambion). The siRNAs were transfected using Lipofectamin RNAimax (Invitrogen-Japan) according to the manufacturer's instructions. Two days after the transfection, the cells were used for gene expression and 5-HT release experiments.
In Situ Hybridization and Immunohistochemistry.
For detecting rat and human TRPA1 mRNA, digoxigenin-labeled probes were synthesized by means of a digoxigenin RNA Labeling Kit (Roche Diagnostics). The probe for human TRPA1 contained 491 bp (covering amino acid residues 957–1,120) and the probe for rat TRPA1 contained 447 bp (covering amino acid residues 821–970). Both probes were cloned into the pBluescript II SK (+) vector. The sense and antisense probes for both constructs were transcribed with T7 and T3 primers, respectively. Six-micrometer paraffin-embedded sections of rat and human duodenum were obtained from Genostaff for in situ hybridization (ISH). The sections were hybridized with the probe, and the signal was detected using NBT/BCIP solution (Roche Diagnostics). After the ISH, the probes underwent a second staining for immunohistochemistry testing. The same sections were then incubated with anti-5-HT rabbit antibody (Sigma-Aldrich Japan), and 5-HT immunoreactivity was visualized using DAB solution.
Organ Bath Experiments.
Guinea pigs were exsanguinated under ether anesthesia. The terminal ileum was isolated and divided into longitudinal segments approximately 20 mm in length. Each segment was opened along the mesenteric attachment, and vertically suspended in an organ bath containing 10 mL of Krebs-Henseleit solution (118 mM NaCl, 4.7 mM KCl, 1.2 mM KH2PO4, 20 mM NaHCO3, 1.2 mM MgSO4, 2.5 mM CaCl2, and 11 mM glucose) gassed with a mixture of 95% O2 and 5% CO2 at 37 °C under a resting tension of 1 g. The contractions were monitored isometrically using a force-displacement transducer (TB-611T, Nihon Kohden) connected to a pen recorder (LR 4210, Yokogawa) through an amplifier (AP-610J, Nihon Kohden). After 1 h of equilibration, 10 μM acetylcholine was applied at 10-min intervals until constant contractions were achieved. A non-cumulative concentration-response curve for AITC was obtained by testing only one concentration of AITC for each preparation. For the characterization of the contractile response of the agonist, ruthenium red (10–100 μM) or ramosetron (5-HT3 antagonist, 0.3 μM) was added to the bath and preequilibrated for 15 min before the application of a submaximal concentration of AITC (300 μM). The concentrations of these antagonists were determined on the basis of previous data (38, 48).
Statistical Analyses.
In the 5-HT release experiments of TRPA1 receptor agonists in EC cells and RIN14B cells, all values were expressed as the mean ± S.D. For the experiments designed to study the contractile effect of TRPA1 receptor agonists in isolated guinea-pig ileum, all values were expressed as the mean ± S.E. or as the mean with 95% confidence limits. The EC50 values (concentration of agonist eliciting half-maximal contraction) were estimated from the concentration-response data via nonlinear regression analysis using version 8.2 of the Statistical Analysis System (SAS Institute Japan). The statistical significance of differences between 2 groups were assessed using the Student's t test. Multiple comparisons were made using 1-way ANOVA followed by Dunnett's test. P values less than 0.05 (P < 0.05) were considered statistically significant.
Supplementary Material
Acknowledgments.
We thank K. Takano, M. Yamano, Y. Takinami, R. Takezawa, Y. Takemoto, T. Goto, H. Tanaka, and H. Kamada for their excellent technical assistance and discussions.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0805323106/DCSupplemental.
References
- 1.Dockray GJ. Luminal sensing in the gut: an overview. J Physiol Pharmacol. 2003;54(Suppl 4):9–17. [PubMed] [Google Scholar]
- 2.Sundler F, Bottcher G, Ekblad E, Hakanson R. The neuroendocrine system of the gastrointestinal tract. Nord Med. 1988;103:8–11. [PubMed] [Google Scholar]
- 3.Gershon MD, Tack J. The serotonin signaling system: from basic understanding to drug development for functional GI disorders. Gastroenterology. 2007;132:397–414. doi: 10.1053/j.gastro.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 4.Rindi G, Leiter AB, Kopin AS, Bordi C, Solcia E. The “normal” endocrine cell of the gut: changing concepts and new evidences. Ann N Y Acad Sci. 2004;1014:1–12. doi: 10.1196/annals.1294.001. [DOI] [PubMed] [Google Scholar]
- 5.Gershon MD. Plasticity in serotonin control mechanisms in the gut. Curr Opin Pharmacol. 2003;3:600–607. doi: 10.1016/j.coph.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 6.Kellum JM, Albuquerque FC, Stoner MC, Harris RP. Stroking human jejunal mucosa induces 5-HT release and Cl- secretion via afferent neurons and 5-HT4 receptors. Am J Physiol. 1999;277:G515–520. doi: 10.1152/ajpgi.1999.277.3.G515. [DOI] [PubMed] [Google Scholar]
- 7.Thomson AB, et al. Small bowel review: Normal physiology part 1. Dig Dis Sci. 2001;46:2567–2587. doi: 10.1023/a:1012794505897. [DOI] [PubMed] [Google Scholar]
- 8.Thomson AB, et al. Small bowel review: Normal physiology part 2. Dig Dis Sci. 2001;46:2588–2607. doi: 10.1023/a:1012746622735. [DOI] [PubMed] [Google Scholar]
- 9.Racke K, Reimann A, Schworer H, Kilbinger H. Regulation of 5-HT release from enterochromaffin cells. Behav Brain Res. 1996;73:83–87. doi: 10.1016/0166-4328(96)00075-7. [DOI] [PubMed] [Google Scholar]
- 10.Caterina MJ, et al. The capsaicin receptor: A heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 11.Peier AM, et al. A TRP channel that senses cold stimuli and menthol. Cell. 2002;108:705–715. doi: 10.1016/s0092-8674(02)00652-9. [DOI] [PubMed] [Google Scholar]
- 12.Jaquemar D, Schenker T, Trueb B. An ankyrin-like protein with transmembrane domains is specifically lost after oncogenic transformation of human fibroblasts. J Biol Chem. 1999;274:7325–7333. doi: 10.1074/jbc.274.11.7325. [DOI] [PubMed] [Google Scholar]
- 13.Story GM, et al. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell. 2003;112:819–829. doi: 10.1016/s0092-8674(03)00158-2. [DOI] [PubMed] [Google Scholar]
- 14.Bautista DM, et al. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell. 2006;124:1269–1282. doi: 10.1016/j.cell.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 15.Kwan KY, et al. TRPA1 contributes to cold, mechanical, and chemical nociception but is not essential for hair-cell transduction. Neuron. 2006;50:277–289. doi: 10.1016/j.neuron.2006.03.042. [DOI] [PubMed] [Google Scholar]
- 16.Nagata K, Duggan A, Kumar G, Garcia-Anoveros J. Nociceptor and hair cell transducer properties of TRPA1, a channel for pain and hearing. J Neurosci. 2005;25:4052–4061. doi: 10.1523/JNEUROSCI.0013-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bandell M, et al. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron. 2004;41:849–857. doi: 10.1016/s0896-6273(04)00150-3. [DOI] [PubMed] [Google Scholar]
- 18.Bautista DM, et al. Pungent products from garlic activate the sensory ion channel TRPA1. Proc Natl Acad Sci USA. 2005;102:12248–12252. doi: 10.1073/pnas.0505356102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jordt SE, et al. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature. 2004;427:260–265. doi: 10.1038/nature02282. [DOI] [PubMed] [Google Scholar]
- 20.Macpherson LJ, et al. The pungency of garlic: activation of TRPA1 and TRPV1 in response to allicin. Curr Biol. 2005;15:929–934. doi: 10.1016/j.cub.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 21.Penuelas A, et al. Contractile effect of TRPA1 receptor agonists in the isolated mouse intestine. Eur J Pharmacol. 2007;576:143–150. doi: 10.1016/j.ejphar.2007.08.015. [DOI] [PubMed] [Google Scholar]
- 22.Stokes A, et al. TRPA1 is a substrate for de-ubiquitination by the tumor suppressor CYLD. Cell Signal. 2006;18:1584–1594. doi: 10.1016/j.cellsig.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 23.Tuladhar BR, Kaisar M, Naylor RJ. Evidence for a 5-HT3 receptor involvement in the facilitation of peristalsis on mucosal application of 5-HT in the guinea pig isolated ileum. British J Pharmacol. 1997;122:1174–1178. doi: 10.1038/sj.bjp.0701503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cibulsky SM, Sather WA. Block by ruthenium red of cloned neuronal voltage-gated calcium channels. J Pharmacol Exp Ther. 1999;289:1447–1453. [PubMed] [Google Scholar]
- 25.Ito H, et al. Characterization of YM060, a potent and selective 5-hydroxytryptamine3 receptor antagonist, in rabbit nodose ganglion and N1E-115 neuroblastoma cells. J Pharmacol Exp Ther. 1992;263:1127–1132. [PubMed] [Google Scholar]
- 26.Fukumoto S, et al. Short-chain fatty acids stimulate colonic transit via intraluminal 5-HT release in rats. Am J Physiol Regul Integr Comp Physiol. 2003;284:R1269–1276. doi: 10.1152/ajpregu.00442.2002. [DOI] [PubMed] [Google Scholar]
- 27.Kim M, et al. D-glucose releases 5-hydroxytryptamine from human BON cells as a model of enterochromaffin cells. Gastroenterology. 2001;121:1400–1406. doi: 10.1053/gast.2001.29567. [DOI] [PubMed] [Google Scholar]
- 28.Kim M, Javed NH, Yu JG, Christofi F, Cooke HJ. Mechanical stimulation activates Galphaq signaling pathways and 5-hydroxytryptamine release from human carcinoid BON cells. J Clin Invest. 2001;108:1051–1059. doi: 10.1172/JCI12467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spiller RC. Effects of serotonin on intestinal secretion and motility. Curr Opin Gastroenterol. 2001;17:99–103. doi: 10.1097/00001574-200103000-00001. [DOI] [PubMed] [Google Scholar]
- 30.Patapoutian A, Macpherson L. Channeling pain. Nat Med. 2006;12:506–507. doi: 10.1038/nm0506-506. Channeling pain. [DOI] [PubMed] [Google Scholar]
- 31.Braun T, Voland P, Kunz L, Prinz C, Gratzl M. Enterochromaffin cells of the human gut: sensors for spices and odorants. Gastroenterology. 2007;132:1890–1901. doi: 10.1053/j.gastro.2007.02.036. [DOI] [PubMed] [Google Scholar]
- 32.Matia I, et al. Serotonin levels in the small bowel mucosa as a marker of ischemic injury during small bowel preservation. Ann Transplant. 2004;9:48–51. [PubMed] [Google Scholar]
- 33.Kaihara S, et al. Serotonin as a useful parameter for cold and warm ischemic injury in small bowel transplantation. Transplantation. 1997;64:405–410. doi: 10.1097/00007890-199708150-00005. [DOI] [PubMed] [Google Scholar]
- 34.Zhang XF, Chen J, Faltynek CR, Moreland RB, Neelands TR. Transient receptor potential A1 mediates an osmotically activated ion channel. Eur J Neurosci. 2008;27:605–611. doi: 10.1111/j.1460-9568.2008.06030.x. [DOI] [PubMed] [Google Scholar]
- 35.Berthoud HR, Kressel M, Raybould HE, Neuhuber WL. Vagal sensors in the rat duodenal mucosa: distribution and structure as revealed by in vivo DiI-tracing. Anat Embryol (Berl) 1995;191:203–212. doi: 10.1007/BF00187819. [DOI] [PubMed] [Google Scholar]
- 36.Hansen MB. Neurohumoral control of gastrointestinal motility. Physiol Res. 2003;52:1–30. [PubMed] [Google Scholar]
- 37.Hendriks R, Bornstein JC, Furness JB. Evidence for two types of 5-hydroxytryptamine receptor on secretomotor neurons of the guinea-pig ileum. Naunyn Schmiedebergs Arch Pharmacol. 1989;339:409–414. doi: 10.1007/BF00736055. [DOI] [PubMed] [Google Scholar]
- 38.Yamano M, Miyata K. Investigation of 5-HT3 receptor-mediated contraction in guinea-pig distal colon. Eur J Pharmacol. 1996;317:353–359. doi: 10.1016/s0014-2999(96)00754-6. [DOI] [PubMed] [Google Scholar]
- 39.Fox A, Morton IK. An examination of the 5-HT3 receptor mediating contraction and evoked [3H]-acetylcholine release in the guinea-pig ileum. British J Pharmacol. 1990;101:553–558. doi: 10.1111/j.1476-5381.1990.tb14119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Foxx-Orenstein AE, Kuemmerle JF, Grider JR. Distinct 5-HT receptors mediate the peristaltic reflex induced by mucosal stimuli in human and guinea pig intestine. Gastroenterology. 1996;111:1281–1290. doi: 10.1053/gast.1996.v111.pm8898642. [DOI] [PubMed] [Google Scholar]
- 41.Gregory RE, Ettinger DS. 5-HT3 receptor antagonists for the prevention of chemotherapy-induced nausea and vomiting. A comparison of their pharmacology and clinical efficacy. Drugs. 1998;55:173–189. doi: 10.2165/00003495-199855020-00002. [DOI] [PubMed] [Google Scholar]
- 42.Brock N, Stekar J, Pohl J, Niemeyer U, Scheffler G. Acrolein, the causative factor of urotoxic side-effects of cyclophosphamide, ifosfamide, trofosfamide, and sufosfamide. Arzneimittel-Forschung. 1979;29:659–661. [PubMed] [Google Scholar]
- 43.Kloppel G, Heitz PU. Pancreatic endocrine tumors. Pathol Res Pract. 1988;183:155–168. doi: 10.1016/S0344-0338(88)80043-8. [DOI] [PubMed] [Google Scholar]
- 44.Bargsten G. Cytological and immunocytochemical characterization of the insulin secreting insulinoma cell line RINm5F. Arch Histol Cytol. 2004;67:79–94. doi: 10.1679/aohc.67.79. [DOI] [PubMed] [Google Scholar]
- 45.Bhathena SJ, et al. Insulin, glucagon, and somatostatin receptors on cultured cells and clones from rat islet cell tumor. Diabetes. 1982;31:521–531. doi: 10.2337/diab.31.6.521. [DOI] [PubMed] [Google Scholar]
- 46.Rindi G, et al. Targeted ablation of secretin-producing cells in transgenic mice reveals a common differentiation pathway with multiple enteroendocrine cell lineages in the small intestine. Development (Cambridge, England) 1999;126:4149–4156. doi: 10.1242/dev.126.18.4149. [DOI] [PubMed] [Google Scholar]
- 47.Schafermeyer A, et al. Isolation and receptor profiling of ileal enterochromaffin cells. Acta Physiol Scand. 2004;182:53–62. doi: 10.1111/j.1365-201X.2004.01299.x. [DOI] [PubMed] [Google Scholar]
- 48.Andrade EL, Ferreira J, Andre E, Calixto JB. Contractile mechanisms coupled to TRPA1 receptor activation in rat urinary bladder. Biochem Pharmacol. 2006;72:104–114. doi: 10.1016/j.bcp.2006.04.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.