Abstract
The avian embryo has been an important model system for studying enteric nervous system (ENS) development for over 50 years. Since the initial demonstration in chick embryos that the ENS is derived from the neural crest, investigators have used the avian model to reveal the cellular origins and migratory pathways of enteric neural crest-derived cells, with more recent work focusing on the molecular mechanisms regulating ENS development. Seminal contributions have been made in this field by researchers who have taken advantage of the strengths of the avian model system. These strengths include in vivo accessibility throughout development, ability to generate quail-chick chimeras, and the capacity to modulate gene expression in vivo in a spatially and temporally targeted manner. The recent availability of the chicken genome further enhances this model system, allowing investigators to combine classic embryologic methods with current genetic techniques. The strengths and versatility of the avian embryo continue to make it a valuable experimental system for studying the development of the ENS.
Keywords: Enteric nervous system, Chick, Avian embryo, Hirschsprung’s disease, Gut development
Investigations using the avian embryo have had a major impact on developmental biology for centuries. Seminal findings made in the chick include the discovery of the neural crest, the cellular movements of gastrulation, and the genetic control of left-right asymmetry (1). Each of the embryologic model organisms, including the fly, frog, worm, fish, chick, and mouse, has specific strengths that permit the investigator to ask a question tailored to the advantages of that model system. For the avian embryo, one of its major strengths is its easy accessibility throughout all developmental stages, permitting embryologic manipulations not easily performed in other species. The recent sequencing of the chick genome (2) further expands the power of this model system, opening the door to genetic manipulations that will help to unravel basic questions of embryogenesis. This review describes the work of investigators over the past 50 years using the avian embryo to study development of the enteric nervous system (ENS), illustrating how the strengths of the avian model have been exploited to yield important insights into the cellular and molecular origins of the ENS.
Fundamentals of the enteric nervous system
The ENS is the network of neurons and glial cells in the wall of the intestine responsible for regulating key intestinal functions, including motility, secretion and absorption. One of the unique features of this division of the autonomic nervous system is its ability to function independently of central nervous system input, an aspect that has led to its labeling as the “second brain” (3). The ENS is a complex nervous system, consisting of multiple subtypes of neurons that are highly interconnected and responsible for secreting at least 50 different substances (4). There are an estimated 100 million nerve cells in the mature ENS, regulating multiple aspects of gastrointestinal function (4). Given its complexity, it is not surprising that developmental anomalies of the ENS occur. The most common and well characterized of these is Hirschsprung’s disease (HSCR) (5). HSCR affects 1 in 5000 live births and is defined by the absence of enteric ganglia along a variable length of distal colon, most often limited to the rectosigmoid. The aganglionic segment becomes tonically contracted, resulting in severe functional obstruction that requires surgical resection for treatment. Many other functional intestinal disorders are associated with ENS abnormalities, including irritable bowel syndrome, chronic intestinal pseudo-obstruction, and slow transit constipation (6). These conditions produce severe intestinal dysmotility and significant morbidity in pediatric patients. Over the last several decades, much work has been done to elucidate the cellular and molecular events that control ENS development, and investigations using the avian embryo have been at the forefront of that progress.
Origins of enteric neural crest cells (ENCCs)
The Swiss embryologist, Wilhelm His, in 1868 discovered the neural crest in neurula-stage chick embryos, where he observed a strip of cells between the neural tube and dorsal ectoderm (7), thus initiating an entire field of study into this complex and fascinating vertebrate structure. Neural crest cells migrate throughout the embryo and give rise to a variety of cell types, including melanocytes, peripheral neurons and glial cells, bones and connective tissues of the head, and the ENS. Tracking the fate of neural crest cells to the gut was made possible by the avian embryo’s accessibility during development, allowing ablation of specific regions of the neural crest in vivo. Applying this technique in 1954, Yntema and Hammond (8) removed the dorsal neural tube, containing the neural crest, from 6-somite stage chick embryos from a region anterior to somite 1 extending posteriorly to somite 10. This resulted in the complete absence of enteric ganglia in the intestine, establishing for the first time the neural crest origin of the ENS.
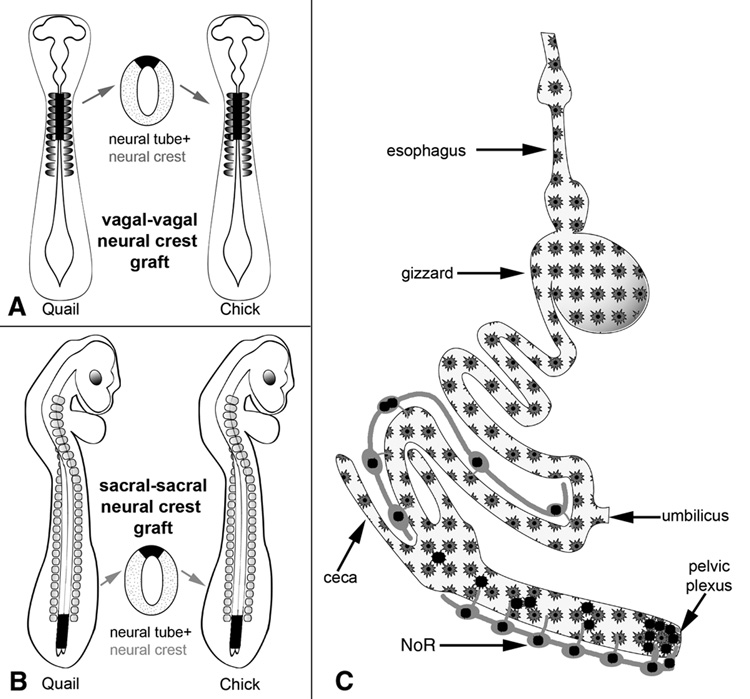
The exact axial level of the neural crest that gives rise to the ENS was identified nearly 20 years later following the development of quail-chick chimeras, an extraordinarily valuable technique in experimental embryology that has been extensively exploited to study neural crest cell development. The method was developed by Nicole Le Douarin (9), who made the important observation that the nuclei of quail cells have a large amount of condensed, heterochromatic nucleolar DNA, while chick nuclei do not. As a result, quail-chick chimeras made by transplanting tissues from one species to the other can be analyzed and the progeny cells from each easily recognized. The commercial availability of 8F3 and QCPN, antibodies specific for chick and quail, respectively, has made this even easier (10). By replacing precise segments of the chick neural tube with identical segments from stage-matched quail embryos, Le Dourain and Teillet (11) demonstrated that the majority of ENCCs arise from the vagal level of the neural tube, adjacent to somites 1 through 7 (Fig. 1). These cells migrate ventrally from the dorsal neural tube to the foregut, and then continue distally along the entire length of the gut, a remarkable migratory journey whose length is unparalleled in embryogenesis. Understanding the cellular and molecular mechanisms that drive this complex process has been the subject of much research over the last few decades, and the avian embryo has remained at the center of these efforts.
Figure 1. Quail-chick chimeras reveal the axial level of origin of ENCCs.
Vagal-level neural tube, adjacent to somites 1–7, is removed from a quail embryo and grafted into the equivalent position in a stage-matched chick following removal of the host vagal neural tube (A). Vagal-derived ENCCs are subsequently found along the length of the intestine, as schematically depicted (C, stars). When a similar chimera is made using the sacral neural tube posterior to somite 28 (B), sacral-derived ENCCs are found in the post-umbilical gut, primarily in the colorectum and Nerve of Remak (C, black circles).
In order to define in greater detail the fate of neural crest-derived cells from specific axial levels of the neural tube, Burns et al. (12) replaced small segments of chick vagal neural tube with equivalent segments from quail. Quail neural tube grafts at the level of somites 1–2 gave rise mostly to pre-umbilical enteric ganglia, while neural crest cells from grafts at somite level 6–7 formed mostly post-umbilical ganglia, suggesting that the neural tube, or perhaps the surrounding microenvironment, is regionalized prior to neural crest cell migration. Neural crest adjacent to somites 3–5 was the most important segment, contributing large numbers of enteric ganglia to the entire gut (12). A similar observation was made by injecting lacZ-expressing retrovirus into individual somites, resulting in labeling of migrating neural crest cells and determination of their fate (13). However, when the neural tube adjacent to somites 3–5 (14) or 3–6 (12) was removed, only the hindgut remained aganglionic. Since this critical segment of vagal neural tube contributes ENCCs to the entire ENS, why does its ablation not result in more extensive aganglionosis? One possibility is that, although capable of forming ganglia in cultured hindgut (15), vagal crest cells from somite levels 1,2, and 7 can only compensate partially for the loss of segment 3–6, and cannot provide sufficient numbers of progenitor cells for complete colonization of the entire gut.
Recently, Barlow et al. (16) combined neural tube ablations and quail-chick chimeras to test this hypothesis. They ablated the neural crest at somite level 3–6 and found that ENCC migration terminated at the duodenum, a degree of aganglionosis much greater than previously seen with similar ablations (12). This discrepancy may be due to slight differences in the stage at which ablations were performed, with earlier ablations (16) resulting in a greater degree of aganglionosis, as some neural crest cells may have already delaminated and started migrating if ablations are performed later (12). Interestingly, grafting of a single somite-length of neural tube from any level of the neuraxis into those ablated embryos resulted in normal ENS colonization. Moreover, if the entire vagal neural tube was removed and only neural crest from the level of somite 3 was grafted back, normal ENS formation was observed (16), suggesting that neural crest cells originating from that axial level are highly proliferative. These results support the hypothesis that the size of the initial pool of ENCCs is critical for achieving complete intestinal colonization.
Migration and patterning in the ENS
Proximodistal migration of ENCCs along the gut is fundamental to ENS development and clinically relevant, as incomplete migration is the underlying defect in HSCR. As discussed above, work in avians demonstrates a critical link between the number of ENCCs and the length of gut colonized. Recent experimental and mathematical modeling suggests that this relationship between cell proliferation and migration is especially relevant at the migratory wavefront. The “wavefront” refers to that portion of migratory ENCCs located at or near the leading edge of migration. We have shown that if the chick hindgut is removed at E6, a stage when only a few cells are present at its proximal end, and then grafted onto the chorioallantoic membrane (CAM) for 7 days, those few cells will proliferate extensively and fully populate the colorectum (17). Simpson et al. (18) grafted segments of colonized quail gut into different regions of the chick intestine, either proximal or distal to the migratory wavefront, then determined the location of quail-derived (QCPN-immunoreactive) cells. Their experiments show that cells at the wavefront are highly proliferative and motile, while more proximal cells proliferate more slowly and are essentially stationary. Interestingly, if a segment of previously colonized quail gut proximal to the wavefront is grafted into the chick wavefront, the new environment will stimulate those quail cells to resume wavefront-like behavior, proliferating and migrating distally along the gut. Thus, proliferation of ENCCs at the wavefront is a key component driving intestinal colonization (19).
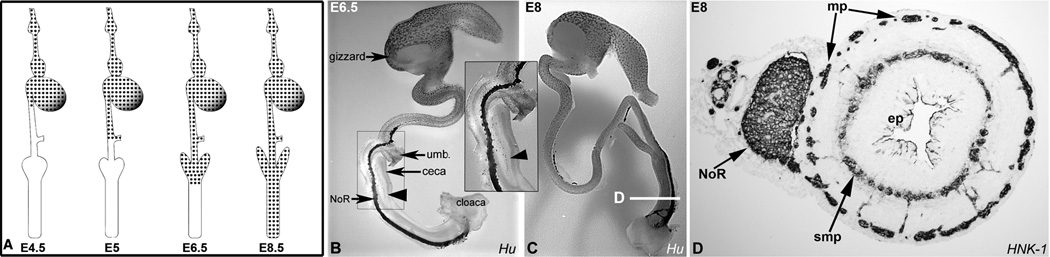
The migration of enteric neuronal precursor cells begins at the dorsal neural tube, from where vagal neural crest cells delaminate by about the 13-somite stage in the chick, corresponding to 45 hours of development (11, 12). These cells migrate ventrally to the developing pharynx and colonize the esophagus by embryonic day 3 (E3). Migrating at a rate of approximately 40 microns per hour (20), the wavefront reaches the distal duodenum at E4.5, the umbilicus at E5, and the ceca at E6. Over the next 2–3 days, colorectal colonization continues, with the distal rectum colonized at around E8 (20–22) (Fig. 2). Druckenbrod and Epstein (23) characterized the detailed movements of cells at the leading edge by electroporating the chick neural tube with a GFP-expressing construct and following the migratory path of individual labeled cells. They showed that ENCCs invade the mesenchyme as strands of cells (23, 24). The cells that undergo directional invasion originate from the wavefront, while more proximal cells exhibit little movement and virtually no directionality, similar to the findings of Simpson et al. (18). Furthermore, extension of the strands relies on the presence of a critical number of cells at the wavefront (24). Thus the continued directional migration of ENCCs relies on two critical components: (i) a sufficient pool of progenitor cells arising from the neural tube and (ii) robust ENCC proliferation within the gut at the migratory wavefront.
Figure 2. Migration and patterning in the chick ENS.
Vagal-derived ENCCs migrate proximodistally along the intestine, as shown schematically at different developmental stages (A). Hu-labeled whole-mount demonstrates the wavefront of enteric neurons at the level of the ceca at E6.5 (B, arrowhead, magnified in inset). At E8, colorectal colonization is complete (C). HNK-1-labeled cross-section through an E8 distal colorectum (D) at the level marked in (C) reveals the submucosal (smp) and myenteric (mp) plexuses, and the Nerve of Remak (NoR). The epithelium (ep) is labeled for orientation. umb.=umbilicus
The rate of ENCC migration is delayed in several mouse models of HSCR (25–27). This observation led to the suggestion that delayed migration causes distal aganglionosis because ENCCs encounter an intestinal environment that is more mature, and therefore less permissive, to ENCC colonization. Meijers et al (28) tested this by culturing preganglionic E4 chick hindgut on the CAM for 7 days, then subculturing onto a new CAM another 7 days, thereby generating aganglionic 18-day-old hindgut. When this more mature intestine was cocultured with E2 vagal neural tube, ENCC colonization still occurred, proving that the hindgut remains competent to support cell migration. Alternatively, the association between delayed migration and distal aganglionosis may be related to intestinal length rather than maturation. Since intestinal lengthening continues at the normal rate, delayed migration leaves the migratory wavefront progressively further from the distal colorectum. Thus, the slower migration of ENCCs in some HSCR models is compounded by a growing length of gut that is increasingly too long for the available pool of ENCCs to colonize, leaving the distal end aganglionic (29). ENCC number and migratory rate are therefore both critically important in ENS development.
The ENS consists of two concentric rings of enteric ganglia located on either side of the circular muscle layer. The outer, myenteric plexus primarily regulates motor activity, while the inner, submucosal plexus mainly controls mucosal functions (4). The factors regulating this pattern formation are largely unknown. During ENCC migration through the avian foregut and midgut, the smooth muscle has not yet developed and neural crest-derived cells move within the outer intestinal mesenchyme (20, 30), where they are positioned to form the myenteric plexus once the circular muscle forms. In order to form the submucosal plexus, situated on the luminal side of the circular muscle, ENCCs must be migrate perpendicularly inward, toward the mucosa (31). Jiang et al. (32) found that the netrin family of axonal guidance factors plays a key role in this radial migration. Netrins are expressed by the intestinal epithelium in chick and mice, while ENCCs express the receptor, deleted in colorectal cancer (DCC). These investigators showed in cultured avian gut that ENCCs migrate toward a source of netrin, and that the inward migration of myenteric ENCCs toward the region occupied by the submucosal plexus is inhibited by antibodies to DCC.
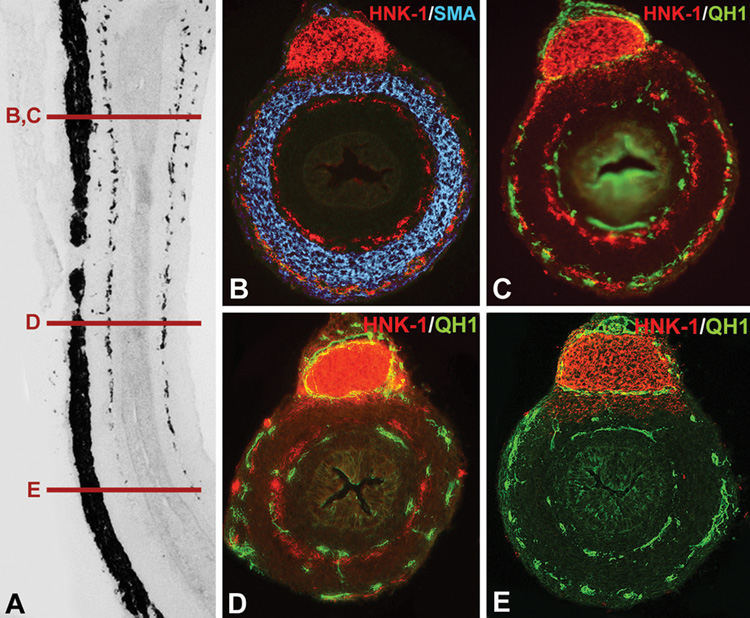
It is clear that these signaling molecules are not alone in patterning the highly conserved structure of the ENS. We recently identified an important role for intestinal blood vessels in this process. Examining serial sections of developing gut, we observed that endothelial cells develop in two concentric rings within the gut wall, similar to the ENS. However, endothelial cells are present and patterned prior to ENCC arrival. As shown in Fig. 3, sections through the proximal end of an E7 colorectum show QH1-labeled endothelial cells adjacent to HNK-1-labeled ENCCs (Fig. 3C). Distally in the same gut, where ENCCs have not yet colonized, endothelial cells are already arranged in two rings (Fig. 3E), presaging the patterning of arriving ENCCs. We also found that inhibition of blood vessel development in the gut leads to arrested ENCC migration (unpublished observations), suggesting that endothelial cells may be required for ENS development. The mechanisms underlying this neurovascular relationship are currently under investigation.
Figure 3. Endothelial cells presage ENCC patterning in the avian embryonic gut.
A longitudinal section of an E7 quail colorectum was labeled with the neuronal antibody, Hu (A), and multiple cross-sections taken at the levels marked (B–E). In a proximal section (B), two plexuses of ENCCs are seen on either side of the circular muscle, labeled with smooth muscle actin (SMA). Double-labeling with the endothelial cell marker, QH1, and the neural crest cell marker, HNK-1, shows that ENCCs are positioned adjacent to endothelial cells (C). In a more distal section through the mid-colorectum, ENCCs are present mostly in the submucosal plexus, with fewer cells in the myenteric plexus, while two rings of endothelial cells are present (D). In the distal colorectum, where ENCCs have not yet colonized, endothelial cells are already patterned into two concentric rings (E).
The path of migration through the avian colorectum is distinct from that observed in the midgut. At the stage when cells arrive at the proximal colon, the circular smooth muscle layer is already developing. Serial examination of the hindgut by immunohistochemistry shows two advancing waves of ENCCs, in the submucosal and myenteric layers, with the wavefront more advanced on the submucosal side (20, 22, 33, 34). Whether colorectal migration occurs as two independent wavefronts in each layer (33), or the myenteric cells arise from outward migration of submucosal cells (22), remains unclear, although our observations based on examining staged embryos supports the former hypothesis. The distinct migratory pattern in the avian colorectum is only one of several aspects of ENS development that are unique to this segment of the intestine. The contribution of sacral neural crest cells and the presence of the Nerve of Remak (NoR), both discussed below, add further complexity. Understanding colorectal ENS development is essential since most congenital neurointestinal diseases, such as HSCR, preferentially affect this portion of the gut.
Sacral neural crest contribution to the ENS
Using quail-chick neural tube chimeras, Le Douarin and Teillet (11) showed that the post-umbilical enteric nervous system contains neural crest cells derived from the sacral neural tube posterior to somite 28 (Fig. 1). Following that landmark observation, however, the contribution, timing, and migratory route of sacral neural crest cells to the gut have remained areas of controversy (35, 36). One subject of initial disagreement was whether sacral neural crest cells contributed to the ENS at all. Supporting such a contribution, studies in avians using NC-1 antibody as a neural crest cell marker (37) or neural tube injections with the vital fluorescent dye, DiI, (38) showed sacral neural crest cells entering the hindgut as early as E4, well before the arrival of vagal ENCCs. However, other investigators showed that the hindgut remains aganglionic following removal of the vagal neural crest (8), explantation of the hindgut prior to vagal ENCC arrival (20), or transection of the midgut distal to the wavefront of vagal-derived ENCCs (39). These latter experiments suggested that either (i) sacral neural crest simply does not contribute cells to the hindgut, or (ii) sacral neural crest cells require the presence of vagal-derived ENCCs to do so. Reconciling these apparently contradictory results is difficult. Different experimental approaches were used and each has limitations, as has been previously discussed (36).
The controversy surrounding the sacral neural crest contribution was clarified in a series of elegant experiments by Burns and Le Douarin (22). These investigators used quail-chick sacral neural tube chimeras and followed the fate of the transplanted cells using the quail-specific antibody, QCPN. They identified QCPN+ enteric neurons and glial cells in the post-umbilical intestine of the host chick embryo, proving their sacral neural crest origin (Fig. 1). The density of sacral-derived ENCCs decreased in a distal-to-proximal gradient within the hindgut, accounting for 17% of enteric neurons distally and only 0.3% proximally. They noted that sacral neural crest cells did not enter the colorectum in large numbers until E10, 2–3 days after arrival of vagal ENCCs. This delayed entry of sacral-derived cells raises the possibility suggested above, that sacral neural crest cells require the presence of vagal neural crest cells in order to colonize the intestine. Burns et al. (12) again used the avian embryo to test that hypothesis by ablating the vagal neural crest of chick embryos and then replacing the sacral neural tube with an equivalent portion from a quail embryo. QCPN immunohistochemistry demonstrated that quail sacral neural crest cells contribute enteric neurons and glial cells to the chick hindgut, even in the absence of vagal-derived ENCCs. Interestingly, the number and size of those sacral-derived ganglia was much smaller than when the vagal neural crest is present. Hearn and Newgreen (40) cultured aneural chick hindgut, isolated prior to vagal ENCC arrival, together with quail sacral neural tube. These intestinal grafts developed enteric ganglia, also confirming that the sacral neural crest can contribute enteric neurons in the absence of vagal-derived ENCCs. We reached a similar conclusion by isolating aneural E5 hindgut together with the peri-cloacal mesenchyme, which contains the pelvic plexus, a sacral neural crest-derived structure. After 9 days of culture on the CAM of a host chick embryo, the explant contained enteric neurons, all of which were of sacral crest origin, since the hindgut remained aganglionic if the pelvic plexus was not included with the graft (10).
Another area of controversy surrounds how sacral neural crest cells enter the gut. Burns and Le Douarin (22) showed that these cells migrate ventrally to form the NoR by E4. Named after the German embryologist and physician, Robert Remak (1815–1865) (41), the NoR is an avian-specific structure that consists of a chain of ganglia located within the intestinal mesentery and extending from the cloaca to the beginning of the midgut (42). These investigators found that neural crest cells reside in the NoR for 3 days, at which time they begin to enter the gut by migrating along nerve fibers extending from the NoR into the intestine. An alternative migratory route for sacral-derived neural crest cells is suggested by our recent results using chimeric organ cultures (10). We cultured chick NoR with quail hindgut on the CAM for 7 days and found no chick-derived cells in the quail intestine, suggesting that NoR does not contribute cells to the gut, although axonal projections did extend from the NoR into the intestine. However, when the chick cloaca and peri-cloacal mesenchyme were cultured with quail hindgut, many chick-derived cells entered the colorectum to become enteric ganglion cells. These results suggest that the pelvic plexus, and not the NoR, serves as the staging area for sacral neural crest-derived cells to enter the hindgut.
The NoR is not technically part of the ENS, as its cell bodies are found outside the gut wall. Rather, it is a component of the extrinsic autonomic innervation of the avian intestine, projecting axons into the distal gut (42). While the NoR forms at E4 (22), its axons do not extend into the gut until E7.5 (22). Shepherd and Raper (43) offered an explanation for this delay by showing that the axonal repellent, collapsin-1, a member of the semaphorin family of axon guidance molecules, is expressed in the outer colorectal mesenchyme at E6. At E8, collapsin-1 expression retreats to the inner mesenchyme and NoR axons begin to enter the outer layer. The authors show that collapsin-1 is a chemorepellent to NoR neurites, which express the collapsin-1 receptor. The timing of extrinsic neurite extension into the gut appears to be at least partly regulated by expression of this semaphorin.
The experiments described above suggest the presence of intrinsic differences between vagal and sacral ENCCs. For example, while vagal ENCCs normally colonize the entire intestinal tract, sacral ENCCs contribute only a small percentage of enteric progenitors to the distal gut (22). When the chick sacral neural tube is replaced by the quail vagal neural tube, the vagal neural crest cells retain their invasiveness and migrate into the hindgut in larger numbers and at earlier stages than sacral neural crest cells normally do (21, 44). To determine the reasons for this difference in invasiveness, Delalande et al (45)compared gene expression in avian vagal and sacral neural crest cells using microarray analysis. These investigators found that the expression of the Ret receptor was fourfold greater in vagal, as compared to sacral, neural crest cells. Furthermore, overexpression of Ret in the sacral neural tube increased colonization of the hindgut by sacral neural crest cells, offering a molecular explanation for the differential invasive potential of these two sources of ENCCs. Ret activation by its ligand, glial-derived neurotrophic factor (Gdnf), promotes ENCC migration (17, 46), potentially accounting for the increased invasiveness of Ret-overexpressing sacral neural crest cells.
Molecular factors regulating avian ENS development
The critical role of the Ret and endothelin receptor B signaling pathways in ENS development was initially discovered in mice with spontaneous or targeted mutations in those genes (47), and those findings have led to important observations in humans with HSCR. While a powerful genetic model, however, the mouse is not easily amenable to experimental manipulation during embryogenesis, and creating mouse strains to study the effects of targeted mutagenesis is labor-intensive and expensive. The strengths of the avian embryo can be exploited to study the molecular regulation of ENS development by taking advantage of the accessibility of the embryo throughout development and the ability to upregulate or downregulate gene expression in vivo. Chicken eggs are also plentiful, inexpensive, and relatively simple to maintain. The availability of speciesspecific antibodies relevant to the ENS further facilitates this research (Table 1 (48–51)). When combined with classic avian embryologic methods, such as quail-chick chimeras, coelomic transplantation, CAM grafting, and tissue recombination, molecular studies in the avian embryo can yield important insights into ENS development. A great deal is known about the molecular control of ENS development, and this topic has been reviewed recently (52). We will discuss here specifically how the avian embryo has contributed to that body of work.
Table 1.
Antibodies used to study the avian ENS.
| Antibody | Cell type identified | Specificity | Species specificity | Antibody subtype | Source |
|---|---|---|---|---|---|
| 8F3 | Chicken cells | unknown cytoplasmic antigen | C | mouse IgG1 | DSHB |
| QCPN | Quail cells | unknown perinuclear epitope | Q | mouse IgG1 | DSHB |
| HNK-1 | Neural crest cells | 3-sulphoglucuronosyl epitope | C, Q | mouse IgM | NeoMarkers |
| Ret | Neural crest cells | Ret receptor | C,Q | Goat IgG | Neuromics |
| Ret | Neural crest cells | Ret receptor | C, Q | rabbit polyclonal | IBL, Japan |
| CSAT | Neural crest cells | β-1 integrin | C, Q | mouse IgG2b | DSHB |
| GFAP (Z0334) | Enteric glia | glial fibrillary acidic protein | C, Q | rabbit polyclonal | DAKO |
| Bfabp | Enteric glia | brain fatty acid binding protein | C, Q | rabbit polyclonal | Dr. C. Birchmeier Ref (48) |
| TuJ1 (B1195) | Neuron | TuJ-1, class III β-tubulin | C, Q | mouse IgG2a | R&D Systems |
| CN | Neuron | unknown | C | mouse IgG1 | Dr. H. Tanaka Ref (50) |
| QN | Neuron | unknown | Q | mouse IgG1 | Dr. H. Tanaka Ref (50) |
| 8D9 | Neuron | L1-like CAM | C | mouse IgG1 | DSHB |
| 4H6 | Neuron | neurofilament | C, Q | mouse IgG | DSHB |
| HuC/HuD (16A11) | Neuron | neuronal RNA-binding protein | C, Q | mouse IgG2b | Molecular Probes Inc. |
| 2F11 | Neuron | neurofilament | C, Q | mouse IgG1 | NeoMarkers |
| 5e | Neuron | N-CAM | C | mouse IgG1 | DSHB |
| MEP-21 | Endothelial cells | thrombomucin | C | mouse IgG1 | Dr. K. McNagny Ref (49) |
| QH1 | Endothelial cells | alpha-macroglobulin | Q | mouse IgG1 | DSHB |
| 1A4 | Smooth muscle | alpha-smooth muscle actin | C, Q | mouse IgG2a | NeoMarkers |
| GIIC9 | Smooth muscle | gamma-smooth muscle actin | C, Q | mouse IgG1 | Ref (51) |
Abbr: DSHB, Developmental Studies Hybridoma Bank; C, chicken; Q, quail
One of the most important molecules in ENS development is Gdnf, a protein expressed in the intestine that binds and activates the receptor tyrosine kinase, Ret, present on migrating ENCCs (53). Null mutations of Gdnf or Ret in rodents result in intestinal aganglionosis (54), and Ret mutations are a major cause of HSCR (55). Gdnf-Ret signaling has multiple roles during ENS development, including promoting the survival, proliferation, and differentiation of ENCCs, effects which have been demonstrated in rodent models (56, 57). Gdnf is also a potent chemoattractant for ENCCs in vitro. When the midgut is explanted from an avian embryo and cultured in the presence of exogenous Gdnf, robust migration of ENCCs occurs out of the gut and into the surrounding collagen matrix (17). Whether a similar chemoattractive role is played by Gdnf in vivo is unclear. Gdnf expression is initially limited to the ceca and cloaca in the E5 avian embryo (17), a stage when vagal-derived ENCCs are migrating toward the ceca, and sacral-derived ENCCs are forming the pelvic plexus. Thus, the spatiotemporal expression of Gdnf is consistent with a chemoattractive role. However, unlike the midgut, addition of Gdnf does not stimulate ENCC migration out of the colorectum (unpublished observations). Moreover, by E6, when ENCCs are at the ceca, Gdnf is already expressed uniformly throughout the intestinal mesenchyme (17). These observations suggest either that the chemoattractive role of Gdnf may be specific to the midgut or that Gdnf is not a chemoattractant in vivo.
Endothelin-3 (Edn3) is another key gene in ENS development. Edn3 is a 21 amino acid peptide expressed in the gut wall, while its receptor, endothelin receptor B (EdnrB), is present on ENCCs. Mutations in either gene lead to distal colorectal aganglionosis in mice (58, 59) and humans (55), but the reasons for the aganglionosis are incompletely understood. Similar to mice, avians express Edn3 in the intestinal mesenchyme (17, 60) and EdnrB on ENCCs (17, 61). Hearn et al. (62) isolated ENCCs from the quail intestine and cultured them in the presence of Edn3 and Gdnf. They found that Edn3 inhibited the neuronal differentiation normally induced by Gdnf. In doing so, Edn3 maintains ENCCs in a progenitor state, consistent with the idea first proposed by Michael Gershon (63) that Edn3 acts to maintain an adequate pool of undifferentiated precursor cells in the gut. We tested this hypothesis by adding the EdnrB antagonist, BQ788, to cultured avian intestine at E5 and found that ENCCs stopped migrating at the level of the ceca. Addition of Edn3 protein to cultured intestine led to marked colorectal hyperganglionosis (17). To confirm this finding in vivo, we used a new method for studying ENS development using quail-chick intestinal chimeras (64). The method is based on the observation that when the preganglionic quail hindgut is grafted into the coelomic cavity of a chick embryo, vagal crest cells from the chick host migrate to the graft and form two ganglionated plexuses of neurons and glial cells, indistinguishable from a normal ENS (64). The method is useful for manipulating either the intestinal mesenchymal environment of the graft, or the neural crest cells of the host embryo, in order to assess the effect on ENS development. When the grafted hindgut was pretreated with Edn3 prior to coelomic transplantation, significant hyperganglionosis resulted, associated with a significant increase in ENCC proliferation and inhibition of neuronal differentiation (17). These results support the idea that Edn3-EdnrB signaling is important for maintaining a sufficient pool of undifferentiated ENCC precursors, thereby promoting colonization of the distal intestine.
In addition to its influence on ENCC proliferation and differentiation, EdnrB signaling also regulates ENCC migration. Both Edn3 and Gdnf are highly expressed in the ceca and cloaca just prior to the arrival of vagal and sacral neural crest-derived cells, respectively, to those regions of the gut (17). If Gdnf is chemoattractive, then how are ENCCs able to migrate past regions of Gdnf expression? The answer may be related to the observation that Edn3 inhibits this chemoattractive effect (17, 65, 66). As a result, Edn3 can counteract the chemoattraction to Gdnf at both the proximal and distal ends of the colorectum in order to allow ENCCs to advance past these Gdnf-expressing zones. Further studies on the role of these pathways in the colorectum are necessary to enhance our understanding of their actions. We are currently using replication-competent retroviral vectors that express Edn3, Gdnf, or small interfering RNAs targeting these genes, in order to modulate the activity of these pathways in vivo in the developing avian intestine.
The completion in 2004 of a physical map of the chicken genome (67) and a draft sequence (2), have opened up opportunities for expanding avian molecular genetic research. These resources facilitate the design of experiments to test the function of genes or their regulatory elements in the developing avian embryo. One opportunity afforded by the easy access to the chick embryo is the use of the genomic data together with RNA interference technology to perform large-scale screens of gene function during gut development (68). By combining the strengths of classic avian embryology with newer technologies to manipulate gene expression, significant progress can be made in understanding the molecular regulation of ENS development.
Conclusions
In HSCR, enteric ganglia are absent specifically from the distal intestine. One explanation for this is that the distal bowel is most susceptible simply because of the distance that vagal ENCCs travel. By interfering with normal ENCC development, genetic mutations of members of the Ret and EdnrB pathways reduce the number of progenitor cells available to populate the bowel, leaving the distal end aganglionic (69). Unique aspects of colorectal ENS development may also contribute to the susceptibility of this intestinal segment. Over the last few decades, we have learned a great deal about the neural crest origins of ENCCs, the spatiotemporal pattern of their migration, and the cellular and molecular factors controlling their survival, proliferation, and differentiation. However, many questions remain unanswered. One of the major challenges in studying ENS development is that it is a dynamic process, with ENCC proliferation, migration, and differentiation occurring simultaneously at different levels along the intestine. For example, while ENCCs at the wavefront are highly proliferative and migratory, those immediately proximal show less proliferation and more differentiation. Therefore, studies on the effects of specific factors on ENS development need to consider the possibility that the role of a given factor may vary depending on the specific part of the intestine being studied or the developmental stage being examined. The avian embryo has several strengths that make it an excellent model system to address these issues: accessibility throughout embryogenesis, multiple methodologies for creating chimeras, ability to modulate signaling pathways in a temporally and spatially specific manner, and recent availability of the chicken genome combined with multiple techniques for upregulating or silencing gene expression. Over the last several decades, avians have provided important insights to the ENS field, answering fundamental questions regarding its origins and its development. We are confident that the avian will continue to enhance our knowledge of ENS development and our understanding of human neurointestinal disease.
Acknowledgments
We thank Olive Mwizerwa for her technical assistance, David Molnar for help with the illustrations, Drucilla Roberts for her guidance, and Alan Burns and Amanda Barlow for their critical reading of the manuscript.
Financial Support: A.M.G is supported by NIH K08HD46655 and the American Pediatric Surgical Association Foundation. N.N. is supported by a grant from the Hungarian Scientific Research Fund (K-69061) and a Young Investigator Award from Semmelweis University.
Abbreviations
- Bmp
bone morphogenetic protein
- CAM
chorioallantoic membrane
- Edn3
endothelin-3
- EdnrB
endothelin receptor B
- ENCC
enteric neural crest cell
- ENS
enteric nervous system
- Gdnf
glial-derived neurotrophic factor
- HSCR
Hirschsprung’s disease
- NoR
Nerve of Remak
Footnotes
Publisher's Disclaimer: Pediatric Research Articles Ahead of Print contains articles in unedited manuscript form that have been peer-reviewed and accepted for publication. As a service to our readers, we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting and review of the resulting proof before it is published in its final definitive form. Please note that during the production process errors may be discovered, which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Stern CD. The chick; a great model system becomes even greater. Dev Cell. 2005;8:9–17. doi: 10.1016/j.devcel.2004.11.018. [DOI] [PubMed] [Google Scholar]
- 2.ICGS. Sequence and comparative analysis of the chicken genome provide unique perspectives on vertebrate evolution. Nature. 2004;432:695–716. doi: 10.1038/nature03154. [DOI] [PubMed] [Google Scholar]
- 3.Gershon MD. The enteric nervous system: a second brain. Hosp Pract (Minneap) 1999;34:31–32. doi: 10.3810/hp.1999.07.153. 35–38, 41-32 passim. [DOI] [PubMed] [Google Scholar]
- 4.Schemann M. Control of gastrointestinal motility by the "gut brain"--the enteric nervous system. J Pediatr Gastroenterol Nutr. 2005;41:S4–S6. doi: 10.1097/01.scs.0000180285.51365.55. [DOI] [PubMed] [Google Scholar]
- 5.Kapur RP. Developmental disorders of the enteric nervous system. Gut. 2000:iv81–iv83. doi: 10.1136/gut.47.suppl_4.iv81. discussion iv87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wood JD. Neuropathophysiology of functional gastrointestinal disorders. World J Gastroenterol. 2007;13:1313–1332. doi: 10.3748/wjg.v13.i9.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Le Douarin N, Kalcheim C. The Neural Crest. Cambridge: Cambridge University Press; 1999. [Google Scholar]
- 8.Yntema CL, Hammond WS. The origin of intrinsic ganglia of trunk viscera from vagal neural crest in the chick embryo. J Comp Neurol. 1954;101:515–541. doi: 10.1002/cne.901010212. [DOI] [PubMed] [Google Scholar]
- 9.Le Douarin N. A biological cell labeling technique and its use in expermental embryology. Dev Biol. 1973;30:217–222. doi: 10.1016/0012-1606(73)90061-4. [DOI] [PubMed] [Google Scholar]
- 10.Nagy N, Brewer KC, Mwizerwa O, Goldstein AM. Pelvic plexus contributes ganglion cells to the hindgut enteric nervous system. Dev Dyn. 2007;236:73–83. doi: 10.1002/dvdy.20933. [DOI] [PubMed] [Google Scholar]
- 11.Le Douarin NM, Teillet MA. The migration of neural crest cells to the wall of the digestive tract in avian embryo. J Embryol Exp Morphol. 1973;30:31–48. [PubMed] [Google Scholar]
- 12.Burns AJ, Champeval D, Le Douarin NM. Sacral neural crest cells colonise aganglionic hindgut in vivo but fail to compensate for lack of enteric ganglia. Dev Biol. 2000;219:30–43. doi: 10.1006/dbio.1999.9592. [DOI] [PubMed] [Google Scholar]
- 13.Epstein ML, Mikawa T, Brown AM, McFarlin DR. Mapping the origin of the avian enteric nervous system with a retroviral marker. Dev Dyn. 1994;201:236–244. doi: 10.1002/aja.1002010307. [DOI] [PubMed] [Google Scholar]
- 14.Peters-van der Sanden MJ, Kirby ML, Gittenberger-de Groot A, Tibboel D, Mulder MP, Meijers C. Ablation of various regions within the avian vagal neural crest has differential effects on ganglion formation in the fore-, mid- and hindgut. Dev Dyn. 1993;196:183–194. doi: 10.1002/aja.1001960305. [DOI] [PubMed] [Google Scholar]
- 15.Peters-van der Sanden MJ, Luider TM, van der Kamp AW, Tibboel D, Meijers C. Regional differences between various axial segments of the avian neural crest regarding the formation of enteric ganglia. Differentiation. 1993;53:17–24. doi: 10.1111/j.1432-0436.1993.tb00641.x. [DOI] [PubMed] [Google Scholar]
- 16.Barlow AJ, Wallace AS, Thapar N, Burns AJ. Critical numbers of neural crest cells are required in the pathways from the neural tube to the foregut to ensure complete enteric nervous system formation. Development. 2008;135:1681–1691. doi: 10.1242/dev.017418. [DOI] [PubMed] [Google Scholar]
- 17.Nagy N, Goldstein AM. Endothelin-3 regulates neural crest cell proliferation and differentiation in the hindgut enteric nervous system. Dev Biol. 2006;293:203–217. doi: 10.1016/j.ydbio.2006.01.032. [DOI] [PubMed] [Google Scholar]
- 18.Simpson MJ, Zhang DC, Mariani M, Landman KA, Newgreen DF. Cell proliferation drives neural crest cell invasion of the intestine. Dev Biol. 2007;302:553–568. doi: 10.1016/j.ydbio.2006.10.017. [DOI] [PubMed] [Google Scholar]
- 19.Landman KA, Simpson MJ, Newgreen DF. Mathematical and experimental insights into the development of the enteric nervous system and Hirschsprung' disease. Dev Growth Differ. 2007;49:277–286. doi: 10.1111/j.1440-169X.2007.00929.x. [DOI] [PubMed] [Google Scholar]
- 20.Allan IJ, Newgreen DF. The origin and differentiation of enteric neurons of the intestine of the fowl embryo. Am J Anat. 1980;157:137–154. doi: 10.1002/aja.1001570203. [DOI] [PubMed] [Google Scholar]
- 21.Burns AJ, Le Douarin NM. Enteric nervous system development: analysis of the selective developmental potentialities of vagal and sacral neural crest cells using quail-chick chimeras. Anat Rec. 2001;262:16–28. doi: 10.1002/1097-0185(20010101)262:1<16::AID-AR1007>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 22.Burns AJ, Le Douarin NM. The sacral neural crest contributes neurons and glia to the post-umbilical gut: spatiotemporal analysis of the development of the enteric nervous system. Development. 1998;125:4335–4347. doi: 10.1242/dev.125.21.4335. [DOI] [PubMed] [Google Scholar]
- 23.Druckenbrod NR, Epstein ML. Behavior of enteric neural crest-derived cells varies with respect to the migratory wavefront. Dev Dyn. 2007;236:84–92. doi: 10.1002/dvdy.20974. [DOI] [PubMed] [Google Scholar]
- 24.Druckenbrod NR, Epstein ML. The pattern of neural crest advance in the cecum and colon. Dev Biol. 2005;287:125–133. doi: 10.1016/j.ydbio.2005.08.040. [DOI] [PubMed] [Google Scholar]
- 25.Natarajan D, Marcos-Gutierrez C, Pachnis V, de Graaff E. Requirement of signalling by receptor tyrosine kinase RET for the directed migration of enteric nervous system progenitor cells during mammalian embryogenesis. Development. 2002;129:5151–5160. doi: 10.1242/dev.129.22.5151. [DOI] [PubMed] [Google Scholar]
- 26.Lee HO, Levorse JM, Shin MK. The endothelin receptor-B is required for the migration of neural crest-derived melanocyte and enteric neuron precursors. Dev Biol. 2003;259:162–175. doi: 10.1016/s0012-1606(03)00160-x. [DOI] [PubMed] [Google Scholar]
- 27.Maka M, Stolt CC, Wegner M. Identification of Sox8 as a modifier gene in a mouse model of Hirschsprung disease reveals underlying molecular defect. Dev Biol. 2005;277:155–169. doi: 10.1016/j.ydbio.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 28.Meijers JH, Tibboel D, van der Kamp AW, Van Haperen-Heuts IC, Kluck P, Molenaar JC. The influence of the stage of differentiation of the gut on the migration of neural cells: an experimental study of Hirschsprung's disease. Pediatr Res. 1987;21:466–470. doi: 10.1203/00006450-198705000-00009. [DOI] [PubMed] [Google Scholar]
- 29.Newgreen DF, Southwell B, Hartley L, Allan IJ. Migration of enteric neural crest cells in relation to growth of the gut in avian embryos. Acta Anat (Basel) 1996;157:105–115. doi: 10.1159/000147871. [DOI] [PubMed] [Google Scholar]
- 30.Connor PJ, Focke PJ, Noden DM, Epstein ML. Appearance of neurons and glia with respect to the wavefront during colonization of the avian gut by neural crest cells. Dev Dyn. 2003;226:91–98. doi: 10.1002/dvdy.10219. erratum 727. [DOI] [PubMed] [Google Scholar]
- 31.Payette RF, Bennett GS, Gershon MD. Neurofilament expression in vagal neural crest-derived precursors of enteric neurons. Dev Biol. 1984;105:273–287. doi: 10.1016/0012-1606(84)90285-9. [DOI] [PubMed] [Google Scholar]
- 32.Jiang Y, Liu MT, Gershon MD. Netrins and DCC in the guidance of migrating neural crest-derived cells in the developing bowel and pancreas. Dev Biol. 2003;258:364–384. doi: 10.1016/s0012-1606(03)00136-2. [DOI] [PubMed] [Google Scholar]
- 33.Conner PJ, Focke PJ, Noden DM, Epstein ML. Appearance of neurons and glia with respect to the wavefront during colonization of the avian gut by neural crest cells. Dev Dyn. 2003;226:91–98. doi: 10.1002/dvdy.10219. [DOI] [PubMed] [Google Scholar]
- 34.Doyle AM, Roberts DJ, Goldstein AM. Enteric nervous system patterning in the avian hindgut. Dev Dyn. 2004;229:708–712. doi: 10.1002/dvdy.20011. [DOI] [PubMed] [Google Scholar]
- 35.Young HM, Newgreen D. Enteric neural crest-derived cells: origin, identification, migration, and differentiation. Anat Rec. 2001;262:1–15. doi: 10.1002/1097-0185(20010101)262:1<1::AID-AR1006>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 36.Burns AJ. Migration of neural crest-derived enteric nervous system precursor cells to and within the gastrointestinal tract. Int J Dev Biol. 2005;49:143–150. doi: 10.1387/ijdb.041935ab. [DOI] [PubMed] [Google Scholar]
- 37.Pomeranz HD, Gershon MD. Colonization of the avian hindgut by cells derived from the sacral neural crest. Dev Biol. 1990;137:378–394. doi: 10.1016/0012-1606(90)90262-h. [DOI] [PubMed] [Google Scholar]
- 38.Serbedzija GN, Burgan S, Fraser SE, Bronner-Fraser M. Vital dye labelling demonstrates a sacral neural crest contribution to the enteric nervous system of chick and mouse embryos. Development. 1991;111:857–866. doi: 10.1242/dev.111.4.857. [DOI] [PubMed] [Google Scholar]
- 39.Meijers JH, Tibboel D, van der Kamp AW, van Haperen-Heuts IC, Molenaar JC. A model for aganglionosis in the chicken embryo. J Pediatr Surg. 1989;24:557–561. doi: 10.1016/s0022-3468(89)80505-6. [DOI] [PubMed] [Google Scholar]
- 40.Hearn C, Newgreen D. Lumbo-sacral neural crest contributes to the avian enteric nervous system independently of vagal neural crest. Dev Dyn. 2000;218:525–530. doi: 10.1002/1097-0177(200007)218:3<525::AID-DVDY1003>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 41.Pearce JM. Remak, father and son. Lancet. 1996;347:1669–1670. doi: 10.1016/s0140-6736(96)91492-0. [DOI] [PubMed] [Google Scholar]
- 42.Suzuki M, Ohmori Y, Watanabe T. Projections of neurons in the intestinal nerve of Remak to the chicken intestine. J Auton Nerv Syst. 1996;61:79–86. doi: 10.1016/0165-1838(96)00061-6. [DOI] [PubMed] [Google Scholar]
- 43.Shepherd IT, Raper JA. Collapsin-1/semaphorin D is a repellent for chick ganglion of Remak axons. Dev Biol. 1999;212:42–53. doi: 10.1006/dbio.1999.9294. [DOI] [PubMed] [Google Scholar]
- 44.Burns AJ, Delalande JM, Le Douarin NM. In ovo transplantation of enteric nervous system precursors from vagal to sacral neural crest results in extensive hindgut colonisation. Development. 2002;129:2785–2796. doi: 10.1242/dev.129.12.2785. [DOI] [PubMed] [Google Scholar]
- 45.Delalande JM, Barlow AJ, Thomas AJ, Wallace AS, Thapar N, Erickson CA, Burns AJ. The receptor tyrosine kinase RET regulates hindgut colonization by sacral neural crest cells. Dev Biol. 2008;313:279–292. doi: 10.1016/j.ydbio.2007.10.028. [DOI] [PubMed] [Google Scholar]
- 46.Young HM, Hearn CJ, Farlie PG, Canty AJ, Thomas PQ, Newgreen DF. GDNF is a chemoattractant for enteric neural cells. Dev Biol. 2001;229:503–516. doi: 10.1006/dbio.2000.0100. [DOI] [PubMed] [Google Scholar]
- 47.Gershon MD. Lessons from genetically engineered animal models. II. Disorders of enteric neuronal development: insights from transgenic mice. Am J Physiol. 1999;277:G262–G267. doi: 10.1152/ajpgi.1999.277.2.G262. [DOI] [PubMed] [Google Scholar]
- 48.Kurtz A, Zimmer A, Schnutgen F, Bruning G, Spener F, Muller T. The expression pattern of a novel gene encoding brain-fatty acid binding protein correlates with neuronal and glial cell development. Development. 1994;120:2637–2649. doi: 10.1242/dev.120.9.2637. [DOI] [PubMed] [Google Scholar]
- 49.McNagny KM, Pettersson I, Rossi F, Flamme I, Shevchenko A, Mann M, Graf T. Thrombomucin, a novel cell surface protein that defines thrombocytes and multipotent hematopoietic progenitors. J Cell Biol. 1997;138:1395–1407. doi: 10.1083/jcb.138.6.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tanaka H, Kinutani M, Agata A, Takashima Y, Obata K. Pathfinding during spinal tract formation in the chick-quail chimera analysed by species-specific monoclonal antibodies. Development. 1990;110:565–571. doi: 10.1242/dev.110.2.565. [DOI] [PubMed] [Google Scholar]
- 51.Nagy N, Magyar A, Olah I. A novel monoclonal antibody identifies all avian embryonic myogenic cells and adult smooth muscle cells. Anat Embryol (Berl) 2001;204:123–134. doi: 10.1007/s004290100192. [DOI] [PubMed] [Google Scholar]
- 52.Heanue TA, Pachnis V. Enteric nervous system development and Hirschsprung's disease: advances in genetic and stem cell studies. Nat Rev Neurosci. 2007;8:466–479. doi: 10.1038/nrn2137. [DOI] [PubMed] [Google Scholar]
- 53.Homma S, Oppenheim RW, Yaginuma H, Kimura S. Expression pattern of GDNF, c-ret, and GFRalphas suggests novel roles for GDNF ligands during early organogenesis in the chick embryo. Dev Biol. 2000;217:121–137. doi: 10.1006/dbio.1999.9543. [DOI] [PubMed] [Google Scholar]
- 54.Schuchardt A, D'gati V, Larsson-Blomberg L, Costantini F, Pachnis V. Defects in the kidney and enteric nervous system of mice lacking the tyrosine kinase receptor Ret. Nature. 1994;367:380–383. doi: 10.1038/367380a0. [DOI] [PubMed] [Google Scholar]
- 55.Amiel J, Lyonnet S. Hirschsprung disease, associated syndromes, and genetics: a review. J Med Genet. 2001;38:729–739. doi: 10.1136/jmg.38.11.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chalazonitis A, Rothman TP, Chen J, Gershon MD. Age-dependent differences in the effects of GDNF and NT-3 on the development of neurons and glia from neural crest-derived precursors immunoselected from the fetal rat gut: expression of GFRalpha-1 in vitro and in vivo. Dev Biol. 1998;204:385–406. doi: 10.1006/dbio.1998.9090. [DOI] [PubMed] [Google Scholar]
- 57.Taraviras S, Pachnis V. Development of the mammalian enteric nervous system. Curr Opin Genet Dev. 1999;9:321–327. doi: 10.1016/s0959-437x(99)80048-3. [DOI] [PubMed] [Google Scholar]
- 58.Baynash AG, Hosoda K, Giaid A, Richardson JA, Emoto N, Hammer RE, Yanagisawa M. Interaction of endothelin-3 with endothelin-B receptor is essential for development of epidermal melanocytes and enteric neurons. Cell. 1994;79:1277–1285. doi: 10.1016/0092-8674(94)90018-3. [DOI] [PubMed] [Google Scholar]
- 59.Hosoda K, Hammer RE, Richardson JA, Baynash AG, Cheung JC, Giaid A, Yanagisawa M. Targeted and natural (piebald-lethal) mutations of endothelin-B receptor gene produce megacolon associated with spotted coat color in mice. Cell. 1994;79:1267–1276. doi: 10.1016/0092-8674(94)90017-5. [DOI] [PubMed] [Google Scholar]
- 60.Nataf V, Amemiya A, Yanagisawa M, Le Douarin NM. The expression pattern of endothelin 3 in the avian embryo. Mech Dev. 1998;73:217–220. doi: 10.1016/s0925-4773(98)00048-3. [DOI] [PubMed] [Google Scholar]
- 61.Nataf V, Lecoin L, Eichmann A, Le Douarin NM. Endothelin-B receptor is expressed by neural crest cells in the avian embryo. Proc Natl Acad Sci USA. 1996;93:9645–9650. doi: 10.1073/pnas.93.18.9645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hearn CJ, Murphy M, Newgreen D. GDNF and ET-3 differentially modulate the numbers of avian enteric neural crest cells and enteric neurons in vitro. Dev Biol. 1998;197:93–105. doi: 10.1006/dbio.1998.8876. [DOI] [PubMed] [Google Scholar]
- 63.Gershon MD. Endothelin and the development of the enteric nervous system. Clin Exp Pharmacol Physiol. 1999;26:985–988. doi: 10.1046/j.1440-1681.1999.03176.x. [DOI] [PubMed] [Google Scholar]
- 64.Nagy N, Goldstein AM. Intestinal coelomic transplants: a novel method for studying enteric nervous system development. Cell Tissue Res. 2006;326:43–55. doi: 10.1007/s00441-006-0207-3. [DOI] [PubMed] [Google Scholar]
- 65.Barlow A, de Graaff E, Pachnis V. Enteric nervous system progenitors are coordinately controlled by the G protein-coupled receptor EDNRB and the receptor tyrosine kinase RET. Neuron. 2003;40:905–916. doi: 10.1016/s0896-6273(03)00730-x. [DOI] [PubMed] [Google Scholar]
- 66.Kruger GM, Mosher JT, Tsai YH, Yeager KJ, Iwashita T, Gariepy CE, Morrison SJ. Temporally distinct requirements for endothelin receptor B in the generation and migration of gut neural crest stem cells. Neuron. 2003;40:917–929. doi: 10.1016/s0896-6273(03)00727-x. [DOI] [PubMed] [Google Scholar]
- 67.Wallis JW, Aerts J, Groenen MA, Crooijmans RP, Layman D, Graves TA, Scheer DE, Kremitzki C, Fedele MJ, Mudd NK, Cardenas M, Higginbotham J, Carter J, McGrane R, Gaige T, Mead K, Walker J, Albracht D, Davito J, Yang SP, Leong S, Chinwalla A, Sekhon M, Wylie K, Dodgson J, Romanov MN, Cheng H, de Jong PJ, Osoegawa K, Nefedov M, Zhang H, McPherson JD, Krzywinski M, Schein J, Hillier L, Mardis ER, Wilson RK, Warren WC. A physical map of the chicken genome. Nature. 2004;432:761–764. doi: 10.1038/nature03030. [DOI] [PubMed] [Google Scholar]
- 68.Brown WR, Hubbard SJ, Tickle C, Wilson SA. The chicken as a model for large-scale analysis of vertebrate gene function. Nat Rev Genet. 2003;4:87–98. doi: 10.1038/nrg998. [DOI] [PubMed] [Google Scholar]
- 69.Heuckeroth RO. Finding your way to the end: a tale of GDNF and endothelin-3. Neuron. 2003;40:871–873. doi: 10.1016/s0896-6273(03)00763-3. [DOI] [PubMed] [Google Scholar]