Abstract
L-Ascorbate (the reduced form of vitamin C) participates in diverse biological processes including pathogen defence mechanisms, and the modulation of plant growth and morphology, and also acts as an enzyme cofactor and redox status indicator. One of its chief biological functions is as an antioxidant. L-Ascorbate intake has been implicated in the prevention/alleviation of varied human ailments and diseases including cancer. To study the regulation of accumulation of this important nutraceutical in fruit, the expression of 24 tomato (Solanum lycopersicon) genes involved in the biosynthesis, oxidation, and recycling of L-ascorbate during the development and ripening of fruit have been characterized. Taken together with L-ascorbate abundance data, the results show distinct changes in the expression profiles for these genes, implicating them in nodal regulatory roles during the process of L-ascorbate accumulation in tomato fruit. The expression of these genes was further studied in the context of abiotic and post-harvest stress, including the effects of heat, cold, wounding, oxygen supply, and ethylene. Important aspects of the hypoxic and post-anoxic response in tomato fruit are discussed. The data suggest that L-galactose-1-phosphate phosphatase could play an important role in regulating ascorbic acid accumulation during tomato fruit development and ripening.
Keywords: Anoxic and post-anoxic stress, ascorbic acid, biosynthesis, ethylene, gene expression, stress, tomato, Solanum lycopersicon, Ailsa Craig
Introduction
Fruit ripening is a complex genetically programmed process that results in dramatic changes in colour, texture, flavour, and aroma of the fruit flesh (Giovannoni, 2001, 2007). It is well established that in order for ripening to proceed, expression of specific genes and subsequent synthesis of enzymes associated with normal ripening is required (Giovannoni, 2004). Part of these changes is initiated and progressed by the plant hormone ethylene (Alexander and Grierson, 2002). Furthermore, it was recently shown that ripening is under developmental control (Vrebalov et al., 2002; Adams-Phillips, et al., 2004; Giovannoni, 2004). Due to the economic importance of fruit crop species, these processes have been, and continue to be, studied extensively at both the biochemical and genetic levels. Fruit is a significant part of the human diet, supplying fibre, minerals, vitamins, and other chemopreventive agents such as antioxidants. Hence, in addition to research programmes directed to understanding and improving the organoleptic qualities of fruit, such as texture, palatability, taste, and aroma, significant efforts have also been devoted to increasing the content in fruit of molecules that promote human health. It is hoped that the ability to manipulate such traits through crop management, breeding, or biotechnology will provide better nutrition as well as improved fruit quality (Giovannoni, 2001). Tomato (Solanum lycopersicum Mill.) is a nutritious component of the Mediterranean diet containing antioxidants such as ascorbic acid (AA), β-carotene, lycopene, lutein, and zeaxanthin (Daood et al., 1990; Proteggente et al., 2002; Visioli et al., 2004). The Mediterranean diet, rich in plant-derived foods, has been associated with lower risk of certain cancers (Trichopoulou et al., 2000, 2003) and cardiovascular disease (Estruch et al., 2006). Understanding the role of AA in plant and fruit physiology provides us with opportunities to engineer the amount present in commercial crop varieties and to minimize losses due to post-harvest manipulation.
In plants, AA is highly abundant and accumulates in intracellular concentrations of 2–25 mM (Davey et al., 2000). It is primarily known for its antioxidant properties, but it also acts as a cofactor for various enzymes and further contributes to the regulation of cell division and expansion (Smirnoff and Wheeler, 2000). It is essential for plant growth (Alhagdow et al., 2007; Dowdle et al., 2007), participates in stress resistance, and seems to control flowering time and the commencement of senescence (Davey et al., 2000). In addition, AA and its oxidized form dehydroascorbic acid (DHA) can act as signalling agents (Pastori et al., 2003; Fotopoulos et al., 2008) participating in the interaction with the environment, for instance to ozone (Sanmartin et al., 2003), pathogens and oxidizing agents (Fotopoulos et al., 2006), and water loss (Fotopoulos et al., 2008). The benefits that arise from the increase of AA production in plants are profound. However, until recently, many aspects of AA biosynthesis, metabolism, and function remained unsettled (Davey et al., 2000; Smirnoff and Wheeler, 2000; Smirnoff et al., 2001; Conklin, 2001; Hancock and Viola, 2005; Ishikawa et al., 2006). The recent deciphering of the pathway of plant L-AA biosynthesis (see Fig. 1) (Wheeler, et al., 1998; Ishikawa et al., 2006), as well as the cloning of various enzymes of the pathway, has refocused attention on this important micronutrient, particularly as a potential route to improve nutritional quality and plant abiotic stress resistance. Ascorbate is synthesized from mannose-6-phosphate via GDP-mannose and GDP-L-galactose (GDP-L-Gal; Wheeler et al., 1998). The first part of the pathway also provides precursors for the synthesis of certain cell wall polysaccharides containing mannose, L-Gal, and L-fucose. The second part of the pathway is committed to ascorbate synthesis (Hancock and Viola, 2005). Free L-Gal is released from GDP-L-Gal via the action of GDP-L-Gal phosphorylase and L-Gal-1-phosphate phosphatase (GPP), and then is oxidized by L-Gal dehydrogenase to form L-galactono-1,4-lactone. L-Galactono-1,4-lactone is oxidized to ascorbate by L-galactono-1,4-lactone dehydrogenase which is located on the inner mitochondrial membrane. Apart from the last step, it is likely that all the other enzymes are cytosolic.
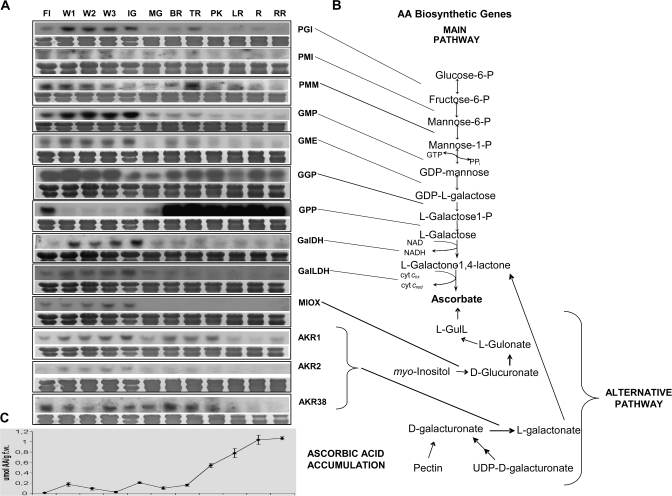
Fig. 1.
Expression of AA biosynthetic genes during tomato fruit ripening. (A) RNA blot analysis of AA biosynthetic genes. (B) The main and alternative AA biosynthetic routes. (C) Ascorbate levels during development and ripening. Total RNA (20 μg) isolated from different stages ranging from anthesis, the first, second, and third weeks from anthesis (FL) (W1, W2, and W3), IG (immature green), MG (mature green), BR (breaker), TR (turning), PK (pink), LR (light red), R (red), to RR (ripe red) was fractionated in formaldehyde denaturing agarose gels, transferred to nylon membranes, stained with 0.04% methylene blue (to observe the equality of loading), and hybridized with radiolabelled probes for AA biosynthetic genes. All experiments were performed in triplicate.
Interestingly, plants appear to possess alternative biosynthetic routes (Davey et al., 2000; Lorence et al., 2004; Valpuesta and Botella, 2004; Ishikawa et al., 2006), although the simultaneous operation of these pathways has to date only been demonstrated in Arabidopsis (Davey et al., 1999; Agius et al., 2003; Lorence et al., 2004). The existence of these alternative routes has recently gained some genetic support (Jain and Nessler, 2000) with the cloning of a D-galacturonate dehydrogenase (Agius et al., 2003) and myo-inositol oxygenase (MIOX) genes (Lorence et al., 2004), but the physiological relevance of these conversions still has to be demonstrated in vivo. Moreover, nothing is known about the existence or the nature of the de novo ascorbate pathways operating in ripening tomato fruit, while very little is known about the mechanisms governing ascorbate pool size in tomato fruit.
Therefore, additional work is needed to establish whether multiple biosynthetic routes co-exist within the cell, or whether different biosynthetic pathways are linked to specific tissues, developmental stages, or external influences. Understanding AA biosynthesis and metabolism in fruit tissues is required in order to develop plant foods with elevated AA content. The monitoring of gene expression of enzymes involved in ascorbate biosynthesis, oxidation, and recycling during tomato fruit ripening, in response to wounding, ethylene, heat and cold treatment, hypoxia, and post-anoxic injury is presented. Since, among all AA biosynthetic, recycling, and oxidizing genes tested, only GPP transcript abundance closely correlated with AA levels during fruit ripening, it is proposed that GPP could act as an important control point for ascorbate biosynthesis during tomato fruit development and ripening.
Materials and methods
Plant material and growing conditions
Tomato (S. lycopersicon Mill., cv. Ailsa Craig) plants were grown in the experimental field of the Agricultural School of the Aristotle University of Thessaloniki during the summer period. Flowers were hand pollinated and the developmental stages of the fruit were determined by date and colour and were harvested manually between 9 am and 10 am. The locular tissue was removed and the pericarp was immediately frozen in liquid nitrogen, and stored at –80 °C.
Stress treatments
Fruit at the mature green stage were used for all the stress treatments. To simulate wounding, fruit were wounded to a depth of 1–2 mm by making cuts 2 mm apart with a sterile scalpel, and maintained at 22 °C for 48 h. For cold stress, fruit were placed at 4 °C for 48 h. Fruit were subjected to heat stress, by incubating at 40 °C in a bench top incubator for 48 h. For ethylene treatment, fruit were dipped in 600 μL L−1 Ethrel (Ethrel 48, Phone-Poulenic, USA) and then kept at 22 °C for up to 48 h. For all treatments, samples for each time point (0, 3, 6, 12, 24, and 48 h) were collected.
To create hypoxic conditions, mature green fruit were placed in airtight containers and exposed to constant gas flow of 60–100 ml min−1 of a gas mix containing air, 97% N2 and 3% O2, 99.5% N2 and 0.5% O2, and 100% N2 for 1, 3, 6, 12, 24, 48, and 72 h. All treatments were performed at 22 °C.
For the post-hypoxic stress experiments, mature green fruit were placed in an airtight container under a constant gas flow of 60–100 ml min−1 of 100% N2 for 48 h. After this time, fruit were removed from the nitrogen atmosphere and were exposed to air. Samples were retrieved 0, 1, 3, 6, 12, 24, and 48 h after re-exposure to air. A second set of samples was exposed to air for 48 h and then samples were collected in parallel to those of the treatment group.
RNA gel-blot analysis
Total RNA from three separate tomato fruit was isolated according to Smith et al. (1986). Upon separation of total RNA (15–20 μg) on denaturing agarose–formaldehyde gels, the nucleic acids were transferred onto nylon membranes (Nytran® 0.45, Schleicher and Schuell) and hybridized to specific radiolabelled probes for ascorbate-related genes. The probes were labelled with [α-32P]dCTP using a random primer labelling kit (RadPrime DNA Labelling System, Invitrogen). Using known plant amino acid sequences coding for ascorbate-related genes, searches were conducted at the sgn.cornell.edu database and corresponding expressed sequence tag (EST) clones were obtained (Table 1). Gel-blot hybridization and membrane washing were performed as described by Church and Gilbert (1984). Staining with 0.04% methylene blue was performed to verify the uniformity of RNA loading on the blot.
Table 1.
Names of genes used as probes in the RNA blot analysis for ascorbate related genes. The clones or accession numbers of the ESTs and the corresponding unigenes are listed. For the analysis of GalLDH, a 1000bp fragment of the gene (AB080690) towards the 3’ end was used.
| Probed gene | Encoded enzyme | Clone or accession no | Unigene |
| GPI | Glucose-6-phosphate isomerase | cLEX-11-E13 | SGN-U317897 |
| PMI | Phosphomannose isomerase | cLEF-2-D24 | SGN-U325639 |
| GMP1 | GDP-mannose pyrophosphorylase | cLEM-18-K22 | SGN-U313112 |
| GMP2 | GDP-mannose pyrophosphorylase | cLEG-16-A23 | SGN-U313111 |
| GME | GDP-mannose-3′,5′- epimerase | cTOF-17-H22 | SGN-U314898 |
| GGP | GDP-L-galactose-1-phosphate phosphorylase | cTOD-24-H13 | SGN-U312646 |
| GPP | L-Galactose-1-phosphate phosphatase | cLEG-32-K19 | SGN-U317967 |
| GalDH | L-Galactose dehydrogenase | cLEG-10-C1 | SGN-U319047 |
| GalLDH | L-Galactono-1,4-lactone dehydrogenase | AB080690 | |
| AKR1 | Aldo/keto reductase | cLEN-18-J12 | SGN-U323295 |
| AKR2 | Aldo/keto reductase | cTOA-27-C18 | SGN-U316890 |
| AKR38 | Aldo/keto reductase | cLEL-1-B11 | SGN-U315474 |
| AO1 | Ascorbate oxidase | cLEC-76-O21 | SGN-U315984 |
| AO2 | Ascorbate oxidase | cLED-5-G11 | SGN-U319412 |
| AO3 | Ascorbate oxidase | cTOE-23-C21 | SGN-U319412 |
| MDHAR1 | Monodehydroascorbate reductase | cLEC-22-G22 | SGN-U320487 |
| MDHAR2 | Monodehydroascorbate reductase | cTOD-21-L24 | SGN-U315877 |
| DHAR | Dehydroascorbate reductase | cTOE-21-G3 | SGN-U313537 |
| APX | Thylakoid-bound ascorbate peroxidase | cTOF-27-J10 | SGN-U214170 |
| Cyt SOD | Cytoplasmic superoxide dismutase (CuZn) | cLEG-55-J1 | SGN-U314405 |
| Pl SOD | Plastid superoxide dismutase (Fe) | cTOF-10-G3 | SGN-U313819 |
| Chl SOD | Chloroplast superoxide dismutase (CuZn) | cLEI-2-I4 | SGN-U315384 |
| GR | Glutathione reductase | cLEG-27-C20 | SGN-U315455 |
| MIOX | Myo-inositol oxygenase | cLET-11-N2 | SGN-U315321 |
Ascorbic acid assay
AA was determined spectrophotometrically using the ascorbate oxidase enzyme as described in Pateraki et al. (2004).
Promoter analysis
For the promoter analysis, 21 Arabidopsis thaliana gene promoters were used. The promoter sequences for genes implicated in ascorbate biosynthesis, oxidation, and recycling were obtained with the help of the ‘Sequence Data Retrieval’ tool from Arabidopsis.org (http://www.arabidopsis.org/tools/bulk/sequences/). Promoter cis-acting regulatory element searches were conducted on all promoters using the PlantCARE software online at http://bioinformatics.psb.ugent.be/webtools/plantcare/html/ and all elements in each promoter were catalogued. The following gene promoters were used in the bioinformatic analyses: AT4G24620, glucose-6-phosphate isomerase; AT1G67070, phosphomannose isomerase; AT2G45790, phosphomannose mutase; AT2G39770, GDP-mannose pyrophosphorylase; AT5G28840, GDP-mannose-3,5-epimerase (GME), AT4G26850, GDP-L-galactose phosphorylase, AT3G02870, L-galactose-1-P phosphatase; AT4G33670, L-galactose dehydrogenase; AT3G47930, L-galactono-1,4-lactone dehydrogenase; AT1G60730, aldo/keto reductase; AT4G26260, myo-inositol oxygenase; AT1G19570, dehydroascorbate reductase; AT3G52880, monodehydroascorbate reductase 1; AT1G63940, monodehydroascorbate reductase 2; AT4G08390, chloroplastic stromal ascorbate peroxidase; AT1G77490, chloroplastic thylakoid ascorbate peroxidase; AT5G21105, ascorbate oxidase; AT3G54660, glutathione reductase; AT1G08830, cytoplasmic superoxide dismutase; AT4G25100, chloroplastic Fe superoxide dismutase; and AT2G28190, chloroplastic CuZn superoxide dismutase.
Results
L-AA content during fruit development and ripening
The levels of L-AA were determined from anthesis up to fruit development and ripening (Fig. 1C). Flowers exhibited a very low L-AA content, compared with young and green fruit, which displayed higher amounts, but still much lower than the ripe fruit that showed the highest L-AA levels. A substantial increase in L-AA content was recorded starting from the pink stage and persisted at the red and over-ripe stages, which are when the fruit are typically consumed.
Expression of AA biosynthetic genes during fruit development and ripening
The expression profiles of genes involved in the three ascorbic acid biosynthetic pathways (Fig. 1B) described in higher plants and deposited in the ‘http://www.sgn.cornell.edu’ database at the time of execution of the experiments were studied during fruit development and ripening (Fig. 1A). By RNA blot analysis, the expression of all the genes participating in the main ascorbate biosynthetic pathway, the so-called Smirnoff–Wheeler pathway (Wheeler et al., 1998) were examined; it should be noted that the first steps of the pathway which channel precursors for the formation of GDP-L-Gal, supply intermediates to a number of pathways, including cell wall polysaccharide synthesis and protein glycosylation (Smirnoff et al., 2001; Hancock and Viola, 2005). Although no available data directly correlate enzymes involved in the metabolism of glucose and phosphorylated D-mannose (D-man) intermediates with AA biosynthesis, it was deemed necessary to check the expression of these genes, since radiolabelled glucose and mannose lead to the production of labelled AA (for a review, see Loewus, 1999). In this context, the transcripts of PGI, which catalyses the production of fructose 6-phosphate, were only detected in flowers and during the tomato fruit expansion phase (Fig. 1), whereas transcript levels declined after the mature green stage and it was merely detected during ripening. The mRNA levels of the next enzyme in the pathway, PMI, which catalyses the first step in directing hexose phosphates into D-Man metabolism, were high during the first 3 weeks of fruit development and thereafter declined slightly to remain almost constant during ripening. PMM exhibited high expression during anthesis but decreased thereafter until the immature green stage; then it started increasing again up to the turning stage, where it showed the highest expression during development. However, PMM expression declined during ripening and no correlation was observed with elevated AA levels. Transcripts of GMP accumulated during the early stages of fruit development coinciding with fruit size increase and dropped dramatically when fruit expansion was complete at the mature green stage and during ripening. GME was expressed in flowers and green fruit, but transcript levels decreased throughout ripening. Accumulation of GGP mRNA was evident from anthesis up to 2 weeks of fruit development; it exhibited elevated levels up to the turning stage to decline thereafter. GPP mRNA was detected in flowers but not in the developing fruit. GPP was the only gene whose steady-state transcript levels increased dramatically with the onset of fruit ripening at the breaker stage and remained elevated throughout ripening. mRNA accumulation of L-GalDH, the enzyme, which commits L-Gal to the AA pathway, showed a higher expression during the early stages of fruit development compared with later stages of ripening. Transcripts of L-GalLDH, the final enzyme in the AA biosynthetic pathway, accumulated in all green tissues tested. During ripening, GalLDH mRNA abundance decreased, a pattern negatively correlating with AA levels (Fig. 1).
A recent study has provided the first molecular evidence for the use of myo-inositol as a precursor of AA biosynthesis in plants (Lorence et al., 2004). This pathway involves the oxidation of myo-inositol to D-glucuronic acid facilitated by the enzyme MIOX (EC 1.13.99). The search of the Tomato EST database (http://www.sgn.cornell.edu) for Arabidopsis MIOX homologues revealed one unigene (SGN-U315321). This unigene was 74.5% identical at the protein level to Arabidopsis MIOX4 (At4g26260). The EST used as a probe was cLET-11-N2 (Table 1). The tomato MIOX gene was only expressed in flowers and expanding green fruit but not in mature or ripening fruit (the clone showing similarity to Arabidopsis MIOX4 is depicted in Fig.1).
A second alternative pathway for AA biosynthesis starts from D-galacturonic acid (GalU), which leads to the formation of AA through L-galactonic acid (Agius et al., 2003; Valpuesta and Botella, 2004). GalUR (E.C. 1.1.1.203), which belongs to the family of aldo/keto reductases (AKRs), catalyses the conversion of GalU to L-galactonic acid (Agius et al., 2003). Bioinformatic analyses revealed the existence of a number of AKRs in the tomato EST database. The present work was focused on three unigenes annotated as SGN-U323295, SGN-U316890 and SGN-U315474 in the tomato EST database (http://www.sgn.cornell.edu) with cLEN-18J12 (AKR1), cTOA-27-C18 (AKR2), and cLEL-1-B11 (AKR38) as their respective ESTs (Table 1). cLEL-1-B11 or AKR38 showed ∼36% similarity with strawberry GalUR, similar to what was reported by Zou et al. (2006). AKR1 is 58% identical to At1G60680, AKR2 is 78% identical to At1G60730, and AKR38 is 50% identical to At1G59960. AKR1 and AKR38 transcripts gradually increased during fruit development up to the immature green stage. Expression levels declined at the later stages of ripening. Transcripts of the third clone for AKR2 were barely detectable during fruit expansion. Thus no AKR clones correlated with increased levels of AA during ripening, suggesting that the GalU alternative pathway or AKR1, 2, or 38 may not participate in AA biosynthesis during tomato fruit ripening.
Expression of AA oxidation and recycling genes during fruit development and ripening
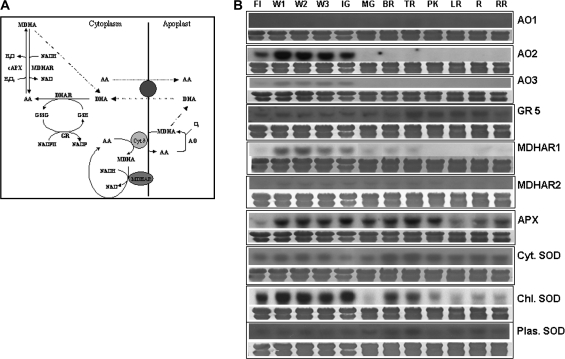
In plant tissues, AA is oxidized in the apoplast and regenerated in the cytoplasm through the action of a battery of enzymes. In the apoplast, AA is oxidized to DHA via the monodehydroascorbate radical (MDHA) with the concomitant reduction of molecular oxygen to water by ascorbate oxidase (AO) (Fig. 2A). Three distinct tomato EST clones (suggesting the presence of three different genes) for AO, showing the highest similarities to known AOs, were included in the expression studies. The AO1, AO2, and AO3 genes show 70, 67 and 71% identity to the Arabidopsis genes At4G39830, At5G21105, and At5G21105, respectively. Concomitant with fruit expansion, transcript accumulation of two AOs, AO2 and AO3, was only detected in young developing immature green fruit, but not in mature and ripening stages, where fruit growth is completed. The third AO1 clone was not detected in the tissues studied (Fig. 2B). The enzyme consuming the majority of AA is ascorbate peroxidase (APX) (Conklin, 2001), which reduces hydrogen peroxide to water with the concurrent oxidation of AA. The expression profile of a thylakoid-bound APX was also studied since this enzyme is involved in AA oxidation. The presence of APX was first detected in developing fruit 1 week post-anthesis and the expression level remained constant until the pink stage, after which it declined (Fig. 2B). After oxidation to MDHA, via APX, ascorbate can be recycled enzymatically by the enzyme monodehydroascorbate reductase (MDHAR). Two unigenes (SGN-U231949 and SGN-U215267) were used to probe the expression of MDHAR, one showing expression only during fruit development and the other showing low but constant expression during fruit development and the early stages of ripening (Fig. 2B). DHA produced in the apoplast via AO action is transported to the cytoplasm where it is recycled to AA by DHAR. DHAR transcript expression was not detected even after repeated hybridizations (data not shown). Since glutathione is used as the reductant in the recycling reaction of DHA through DHAR, the expression of a GR was also studied. The mRNA levels of this enzyme remained constant throughout fruit development and ripening (Fig. 2B).
Fig. 2.
Expression of AA oxidation and recycling genes during tomato fruit ripening. (A) Schematic representation of the AA–GSH cycle responsible for AA oxidation in the apoplast and recycling in the symplast (modified from Smirnoff, 2000). (B) RNA blot analysis of AA oxidation and recycling genes, as well as antioxidant enzymes (SOD). Total RNA (20 μg) was fractionated in formaldehyde denaturing agarose gels, transferred to nylon membranes, stained with 0.04% methylene blue (to observe the equality of loading), and hybridized with radiolabelled probes for AA oxidation and recycling genes. (For sampling details, see legend to Fig. 1.) All experiments were performed in triplicate.
It was decided to explore any correlations which may exist between other antioxidant enzymes and ascorbate-related genes. A key enzyme for reactive oxygen species (ROS) detoxification in the chloroplast is superoxide dismutase (SOD). This enzyme catalyses the dismutation of two molecules of superoxide anion radical into oxygen and hydrogen peroxide. Transcript levels of a chloroplastic SOD were high in flowers and during fruit development, decreased in the mature green stage, and increased with the onset of ripening at the breaker stage, gradually decreasing thereafter. The presence of a plastid SOD and a cytoplasmic SOD transcript was constant in all tissues studied (Fig. 2B).
Expression of AA biosynthetic genes in response to ethylene and stress
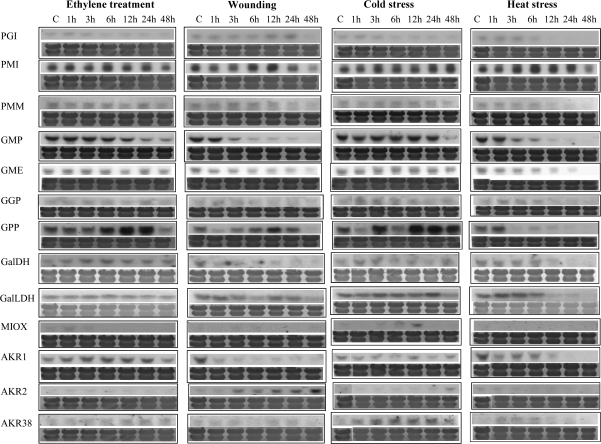
To investigate the effect of various stress conditions on the expression of AA biosynthetic enzymes, RNA blot analysis was performed on total RNA extracted from mature green tomatoes that were exposed to ethylene and each individual stress for a set period of time (Fig. 3). Total RNA was isolated from mature green tomatoes 0, 1, 3, 6, 12, 24, and 48 h after dipping in 600 μL L−1 Ethrel. Most biosynthetic genes showed either no change in expression (PGI, PMI, PMM, GME, GGR, L-GalDH, and L-GalLDH) or a gradual decline in expression during the time course of the experiment (GMP). GPP was the only mRNA that was induced by this treatment. As illustrated in Fig. 3, transcript levels gradually increased after 3 h of exposure, peaking at 24 h, and were barely detectable at 48 h post-exposure. AKR1, AKR2, and MIOX transcripts were not detected during this treatment, whereas AKR38 showed some induction after 12 h.
Fig. 3.
Expression of AA biosynthetic genes in mature green fruit subjected to ethylene and various stresses. Tomatoes were grown in the field and harvested at the mature green stage. After subjecting fruit to various stresses, total RNA was isolated, fractionated in denaturing agarose gels, transferred to Hybond N-membranes, and hybridized with specific 32P-radiolabelled probes. Staining with 0.04% methylene blue was performed to verify the uniformity of RNA loading on the gel. All experiments were performed in triplicate.
Wounding of mature green tomatoes also induced the expression of GPP, with a distinct peak at 12 h after treatment. Accumulation of the AKR2 mRNA increased gradually starting at 3 h upon the implementation of the stress, with the highest levels at 48 h after wounding. Transcript levels of other biosynthetic genes, namely GPI, PMI, GME, and L-GalLDH, remained unaffected by wounding. GMP transcripts declined sharply 1 h post-wounding and L-GalDH transcripts showed similar behaviour 3 h post-wounding. The MIOX transcript was not detected in these studies (Fig. 3).
Subjecting mature green tomatoes to cold stress resulted in an increase in the expression of GPP that commenced at 3 h after exposure, peaked at 12 h, and remained constant up to 48 h post-treatment. Other enzymes either remained unaffected by the treatment (PGI, PMI, PMM, GME, L-GalDH, L-GalLDH, or AKR1) or gradually decreased during the course of the cold stress treatment (GMP). AKR38 expression was triggered by cold 3 h after treatment (Fig. 3).
The majority of AA biosynthetic genes were down-regulated by exposing mature green tomatoes to heat stress (40 °C). These include GPP whose expression was reduced 1 h after exposure to heat stress and then remained almost undetectable. Levels of PMI transcript remained constant throughout the time course of the stress treatment. AKR2 and MIOX transcripts were not detected.
The effect of low oxygen environments on the expression profile of AA biosynthetic genes was next investigated (Supplementary Fig. 1S available at JXB online). Mature green fruit were subjected to 3, 0.5, and 0% oxygen for 72 h, with a control group being exposed to ambient air in parallel. Lowering the oxygen concentration to 3% led to a gradual decrease of the expression of PGI, PMI, PMM, GMP, GME, L-GalLDH, and AKR1, 6 h after exposure to the modified atmosphere. L-GalDH mRNA levels remained unaffected by the treatment. Interestingly, 6 h after the application of all low oxygen treatments, GPP transcript exhibited a sharp peak in expression, which decreased equally sharply at 24 h and remained unchanged up to 72 h. It should be noted that GPP in samples exposed to air, which served as controls, did show a substantial increase after 6 h upon harvest and peaked at 72 h. The transcripts of the three AKR genes showed a different but noticeable expression profile under the three low oxygen regimes. AKR1 was suppressed at all oxygen levels after 6 h into the treatment. AKR2 was only detected after 48 h in 0% oxygen, and AKR38 was also induced only in 0% oxygen (1 h after onset of the treatment). This suggests that both AKR2 and AKR38 might be anoxia-responsive genes. The expression of the gene encoding MIOX accumulated 1 h after transfer to 3% oxygen and remained at almost constant levels through the course of the experiment. A similar profile was observed for tomato samples exposed to 0.5% and 0% oxygen levels. These oxygen concentrations (0.5% and 0%) had a more pronounced effect on the expression of MIOX, as its mRNA levels gradually increased to peak at 72 h after treatment. This pattern of expression implies that MIOX might be another anoxia-responsive gene. It should be noted that all fruit samples held for 48 h in low oxygen regimes and transferred and sampled after 24 h in air exhibited elevated levels of all genes tested, indicating normal ripening.
Expression of AA recycling and oxidation genes in response to ethylene and stress
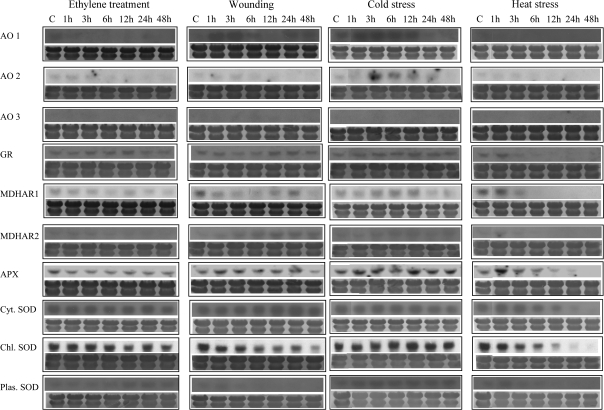
To investigate the effect of various stresses on the expression of AA oxidation and recycling enzymes, RNA blot analysis was performed on total RNA extracted from mature green tomatoes that were exposed to each stress for a set period of time (Fig. 4). Ethylene treatment did not affect the expression of AA oxidation and recycling enzymes. In terms of the ROS-scavenging enzymes, a gradual decrease in message accumulation was observed for the cytoplasmic SOD, with the lowest levels present in fruit 48 h after wounding.
Fig. 4.
Expression of AA oxidation and recycling enzymes in response to ethylene and various stresses. Tomatoes were grown in the field and harvested at the mature green stage. After subjecting fruit to various stresses, total RNA was isolated, fractionated in denaturing agarose gels, transferred to Hybond N-membranes, and hybridized with specific 32P-radiolabelled probes. Staining with 0.04% methylene blue was performed to verify the uniformity of RNA loading on the gel. All experiments were performed in triplicate.
The expression patterns of AA oxidation and recycling enzymes were not different from the untreated control for the duration of the cold stress treatment, except for AO which exhibited an increased transcription starting at 3 h until 24 h after exposure to cold (Fig. 4). Subjecting mature green tomatoes to heat stress had a more pronounced effect on gene expression. MDHAR transcript was at control levels 1 h after the treatment and then dropped sharply at 4 h to become undetectable thereafter. Thylakoid-bound APX showed an initial induction at 1 h after exposure at 40 °C and then returned to control levels at 3 h and 6 h, with a progressive reduction subsequently. The mRNA levels of the three SOD genes exhibited a gradual decrease and became undetectable at 48 h. An induction after 1 h at 40 °C followed by a sharp decrease from then on was observed for GR.
A similar effect of the different oxygen levels was observed for the AA oxidation and recycling enzymes (Supplementary Fig. 2S at JXB online). Exposure of mature green tomatoes to low oxygen, regardless of concentration, resulted in a decrease of MDHAR transcript levels after 1 h. Among the antioxidant enzymes, the message of thylakoid-bound APX increased at 1 h, decreased at 6 h, and became undetectable after 24 h. The three SOD genes exhibited a similar response; their transcript became undetectable after 6 h into the treatment. The mRNA levels of GR remained constant for the duration of the treatments at all oxygen levels.
Expression of AA biosynthetic genes and AA content in response to post-anoxic stress
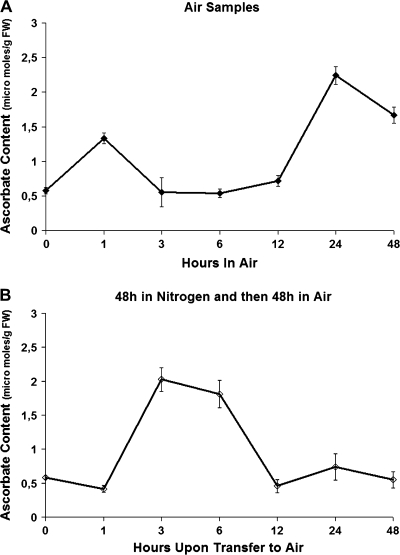
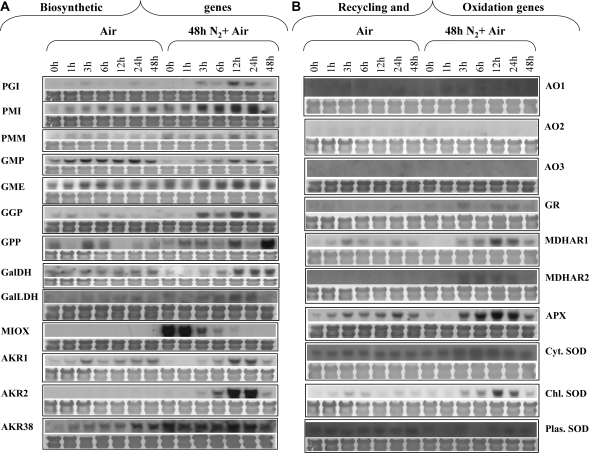
Mature green tomatoes were subjected to a 100% nitrogen atmosphere (anoxia) for 48 h and subsequently returned to air for 48 h, and AA content and the expression profiles of ascorbate biosynthetic, oxidizing, and recycling genes, as well as APX and SOD were studied (see below). As illustrated in Fig. 5A and B, the post-anoxic stress caused the AA levels to increase ∼8-fold at 3–6 h after re-exposure to air, suggesting an increased need for this antioxidant in order to compensate for the presumed increased amounts of ROS. However, a subsequent dramatic and continuous decrease in AA content was observed after 6 h and up to 48 h, suggesting the participation of ascorbate in the ROS scavenging activity. At the same time, AA in control mature green fruit markedly increased during the first hour and then declined before increasing again after 12 h. Regarding the expression profiles of the AA genes, it is intriguing to note that this stress triggered the induction of gene expression of all enzymes participating in AA biosynthesis (Fig. 6A). The transcript levels of most of the genes were evident after 3 h and peaked at 12 h upon re-exposure to air, coinciding with the elevated content of AA. Specifically, the steady-state level of GPI and PMM mRNAs peaked 12 h after re-exposure to air. Similarly, PMI transcript peaked at 12–24 h upon re-exposure to air. A peak at 12 h after exposure to air was also observed in the expression of GMP, GME, L-GalDH L-GalLDH, and AKR1 and 2. AKR38 was already highly expressed while in anoxia and remained unchanged up to 24 h in air, and then decreased. GPP transcript was present at low levels at 1 h and 3 h post-hypoxia, increased at 12 h, and peaked at 48 h after re-exposure to air. The level of these transcripts in the control group that was only exposed to air exhibited a rhythmicity during the time course of the experiment; expression was high at 0 h and dropped at 1 h, but was high again at 3 h and 6 h; expression was again low at 12 h and high at 24 h and 48 h. In fact, such rhythmicity is also seen in the expression of Arabidopsis GPP (unpublished microarray results available at Affymetrix online, experiment #149 conducted by Kieron Edwards and Andrew Millar). The Arabidopsis gene also showed sharp increases and declines within 6 h time frames. The transcripts of tomato GGP increased dramatically 3 h after transfer to air from the anoxia treatment. Expression levels dropped 24 h after transfer to air. The air controls exhibited a low steady-state level of expression which peaks at 1, 24, and 48 h after transfer to air, again indicating a rhythmic expression pattern for this gene. Transcripts showing homology with MIOX showed high accumulation of their mRNA after 48 h in anoxia, but decreased sharply as soon as the fruit were transferred back to air, and were almost undetectable after 12 h.
Fig. 5.
Effect of post-anoxic stress on ascorbate levels. Tomatoes were grown in the field and harvested at the mature green stage. Fruit were incubated either in air (Air) or in 100% nitrogen (N2) (anoxia) for 48 h and then were transferred to air for an additional 48 h and sampled after 1, 3, 6, 12, 24, and 48 h. (A) Ascorbate levels of mature green tomatoes held in air for 48 h. (B) Ascorbate levels of mature green tomatoes previously kept in anoxia for 48 h and then transferred to air for an additional 48 h. Results are shown from 0 h to 48 h ‘after’ the initial 48 h treatment in air or nitrogen. Hence the fruits used for ascorbate quantification are actually closer to the ‘turning’ stage in developmental terms and have been off the vine for at least 48 h. This is important when comparing the air control results in this figure with those in Fig. 1. All experiments were performed in triplicate.
Fig. 6.
Expression of AA biosynthetic, oxidation, and recycling genes in response to post-anoxic conditions. Total RNA isolated from the fruit shown in Fig. 5 was isolated, fractionated in denaturing agarose gels, transferred to Hybond N-membranes, and hybridized with specific 32P-radiolabelled probes corresponding to ascorbate biosynthetic genes (A) and oxidation and recycling genes (B). Staining with 0.04% methylene blue was performed to verify the uniformity of RNA loading on the gel. All experiments were performed in triplicate.
Expression of AA oxidation and recycling genes in response to post-anoxic stress
Enzymes involved in AA recycling and ROS-scavenging activity responded similarly to the biosynthetic enzymes, i.e. post-anoxic conditions provoked an induction of MDHAR1, MDHAR2, thylakoid-bound APX, all tested SOD genes, and GR (Fig. 6B). Their expression was evident at ∼3 h with a peak at 12 h post-anoxia. After this time point, the expression of these genes was progressively reduced. Levels of cytoplasmic and plastid SOD and AO remained unaffected. On the other hand, AO genes were suppressed in the post-anoxic environment.
Promoter analysis of ascorbate-related genes
To see whether DNA elements present in ascorbate-related gene promoters might lend a clue as to the regulation of ascorbate accumulation in plants, 21 promoter sequences of ascorbate-related genes were analysed. Out of all promoter motifs detected in the promoters studied, 10 motifs were represented in ascorbate-related gene promoters at a high frequency (Supplementary Fig. 3S and Table S1 at JXB online). Light-responsive promoter elements were the most predominant promoter motif present in the promoter sequences. Four different types of light-responsive elements were detected. When all four elements are taken together, 17 out of 21 gene promoters from the data set have at least one light-responsive element. The exceptions are GPP, SOD, chloroplastic Fe superoxide dismutase, and APX. Abscisic acid (ABA)-, gibberellin-, heat shock-, wounding-, fungal elicitor-, and endosperm-responsive elements were also detected in the promoters.
Discussion
Gene expression during development and ripening
Although AA is a highly abundant metabolite in many plant tissues and involved in many metabolic pathways in plants, very little is known about the mechanism and control of its accumulation in fruit. During fruit ripening, AA acts as a cofactor for 1-aminocyclopropane-1-carboxylic acid (ACC) oxidase, a key enzyme in the biosynthetic pathway of ethylene (Ververidis and John, 1991). Levels of AA increased during tomato fruit ripening starting at the turning stage, implying that cellular antioxidant status may play a fundamental role in this process (Jimenez et al., 2002; Carrari et al., 2006). An associated role of AA in ripening has been proposed through the generation of hydroxyl radicals in the apoplast (Fry, 1998; Green and Fry, 2005a,b). Ascorbate-generated hydroxyl radicals can cause non-enzymatic degradation of polysaccharides in vitro, leading to natural fruit softening (Dumville and Fry, 2003).
The AA biosynthetic pathway was recently fully elucidated through an amalgamation of biochemical, genetic, and transgenic approaches. These studies illustrated that the L-Man/L-Gal pathway is the main one in plants tissues (Linster and Clarke, 2008). However, the understanding of the regulation of this pathway is far from complete especially in ripening tomato fruit in which little is also known about the AA operational biosynthetic routes. To address this issue, the expression profiles of all genes involved in ascorbate biosynthesis, oxidation, and recycling during tomato fruit development and ripening were investigated.
The results suggest that the D-Man/L-Gal pathway operates and could be the major one functioning during tomato fruit ripening in accordance with in vivo labelling and feeding experiments (IM, AKK, and MD Davey, unpublished data). A similar conclusion was reached in ripening fruit of blackcurrant (Ribes nigrum) in which AA accumulation was under strong developmental, metabolic, and genetic control. (Hancock et al., 2007).
An interesting observation from the present work was the low expression of all AA biosynthetic genes except GPP during the pink and red stages of tomato fruit development. The same genes exhibited higher expression in green fruit tissues, a pattern that antiparallels the AA accumulation during tomato development and ripening (Fig. 1). The AA biosynthesis can be characterized by non-committed and committed steps. Surprisingly, among the non-committed genes, both PMI and PMM were detected in tomato fruit, in contrast to recent controversy regarding the involvement of these genes in the L-Gal pathway (Wolucka and Van Montagu, 2007; Linster and Clarke, 2008). Thus, this observation further favours the operation of the D-Man/L-Gal pathway in tomato fruit, with the D-mannose-6-P serving as a functional precursor.
The committed enzyme, GalDH, is present, albeit exhibiting low expression during fruit ripening, corroborating with findings by Laing et al. (2004b), who found that this gene exhibited similar expression profile and enzyme activity in ripening kiwifruit, suggesting a minor role for GalDH in regulating AA levels during ripening. Further, GalDH overexpression in tobacco resulted in a 3.5-fold increase in extractable activity, but without a concomitant increase in leaf ascorbate concentration (Gatzek et al., 2002), again pointing to a minor regulatory role for GalDH in AA biosynthesis. This can be said for most AA biosynthetic, recycling, and oxidizing genes, given their low expression pattern in ripening tomato fruit (Figs 1, 2). However, the important finding from this work is that only GPP steady-state transcript levels, among all AA biosynthetic and recycling genes, increased dramatically with the onset of fruit ripening and remained elevated throughout the ripening period, closely correlating with elevated AA levels, suggesting a regulatory role for GPP in AA biosynthesis during tomato fruit ripening. This notion is supported by in vivo labelling experiments in Arabidopsis cell suspension cultures, suggesting that the rate-limiting step in ascorbate biosynthesis was operating between D-Man and L-Gal (Davey et al., 1999). GPP was also induced during ripening off-the-vine (Fig. 5B, Supplementary Fig. S1 at JXB online) and in response to ethylene (Fig. 3), implying that this gene is ripening and ethylene regulated. Previous studies based on the strong correlation between ascorbate levels and GME activity in the colourless microalga Prototheca moriformis proposed that GMEs, which catalyse the synthesis of both GDP-L-gulose and GDP-L-galactose from GDP-D-mannose (Wolucka and Van Montagu, 2003, 2007; Watanabe et al., 2006), are possibly catalysing the rate-limiting step in this pathway (Running et al., 2003). A recent quantitative trait locus (QTL) study has also implicated GME in regulating carbon flux and hence ascorbate levels (Stevens et al., 2007). In addition, jasmonates induced the accumulation of AA coinciding with elevated transcript levels of GME in plant cell suspensions (Wolucka et al., 2005). However, the present data do not favour this suggested role for GMEs in tomato ripe fruit. Neither does our study support recent claims (Badejo et al., 2007, 2008) that GMP activity promoter and gene expression are tightly related to high ascorbate levels in Malpighia glabra and tobacco. In fact, the present study show the reverse effect, with expression levels of this gene being negatively correlated with ascorbate levels (Fig. 1). However, one cannot rule out the possibility of different regulatory mechanisms controlling the expression of GMP in tomato versus M. glabra, which shows dramatically high GMP transcript levels in the fruits when compared with tomato (Badejo et al., 2008).
Laing et al. (2004a) have found that the EST for the putative group 1 MIOX 1 (the group with demonstrated GPP activity) mostly came from libraries made from fruit tissues both in apple and in kiwifruit (frequency of 0.014% in apple and 0.017% in kiwifruit). This finding lends further support to the hypothesis that the L-Gal pathway is operational in fruit. L-Gal is also found in the side chain A of the cell wall polymer rhamnogalacturonan II (Conklin et al., 2006). The Arabidopsis vtc4 mutant, which is impaired in AA biosynthesis, was shown to possess a copy of the GPP gene with a point mutation at the enzyme's predicted active site (Conklin et al., 2006). In addition to reduced AA content, this mutant also exhibited increased L-Gal accumulation in polysaccharides of the cell wall. These findings, taken together with the observation of increased transcript accumulation of GPP during fruit ripening, led to the speculation that this enzyme plays an important role in regulating ascorbate accumulation in ripening tomato fruit. The expression profile of MIOX and the three putative AKR genes assessed in this work did not show any specific accumulation during fruit ripening. These transcripts were mostly present in the expanding fruit phase and declined during ripening, indicating that the biosynthetic pathway through myo-inositol and GalU acid are probably not induced by ripening in tomato fruit. However, one cannot exclude the possibility that GPP may also utilize myo-inositol-1-P as a substrate in tomato fruit. Laing et al. (2004a) demonstrated that the Escherichia coli-expressed kiwifruit GPP hydrolysed myo-inositol phosphatase, but it was much more efficient against L-Gal-1-P than myo-inositol-1-P. Further support for this hypothesis comes from the finding that tomato fruit discs from mature green, pink, and ripe fruit were able to convert myo-inositol into AA (IM, AKK, and MD Davey, unpublished data).
The recent studies on GGP (VTC2/GDP-L-Gal phosphorylase) proposed that this gene might play a major role in regulating AA biosynthesis in leaves (Dowdle et al., 2007; Laing et al., 2007; Linster et al., 2007; Linster and Clarke, 2008). This gene was expressed at a higher level in green tissues and was light induced. In addition, the VTC2 protein seemed to be detected only in green tissues and especially in both cytosol and nucleus, implicating a dual function protein (Dowdle et al., 2007). Figure 1 showed a rather constitutive but high expression of GGP during fruit development and ripening, and no induction by ethylene or by any other stress tested (Fig. 3), thus the proposed regulatory role of GGP needs to be shown in ripening fruit.
The last step in the AA pathway is catalysed by GalLDH, an inner mitochondrial membrane enzyme, which could play an important role in the regulation of cell growth-related processes in plants (Alhagdow et al., 2007). Its gene expression in tomato fruit followed an opposite profile when compared with the corresponding melon gene, which accumulated during melon fruit ripening, coinciding with elevated levels of AA (Pateraki et al., 2004), indicating different means of regulating AA synthesis in these two fruit.
Increased ascorbate content in ripening fruit may also be the result of the combined action of oxidizing and recycling enzymes. However, based on the expression profile of recycling genes, this possibility gains little support. AO transcripts were high during fruit early development, indicating a role for this enzyme in fruit expansion. AO, by controlling the ascorbate redox state, has been proposed to play a role in regulating cell division and expansion (Davey et al., 2000; Potters et al., 2000; Tabata et al., 2001; Sanmartin et al., 2007). Its transcript declined massively during fruit ripening, which may partially contribute to elevated levels of reduced AA. In contrast and similar to the GalLDH pattern (see above), AO1 transcripts levels increased substantially in melon ripening fruit (Sanmartin et al., 2007), again suggesting that the two fruit possess a different mode of controlling AA pool sizes during ripening. On the flip side, a recent study (Stevens et al., 2008) shows that MDHAR activity levels correlate with reduced AA levels in tomato fruit under chilling stress. Another study has shown that the MDHAR gene is implicated in the increment of dehydroascorbate (Bermudez et al., 2008) as a fruit metabolite locus (QML). The results lend further support to these observations by the fact that the MDHAR1 transcript levels are negatively correlated with AA levels (Fig. 2) during tomato fruit development. This is most prominent during the later stages of fruit development, when AA levels peak significantly and MDHAR1 transcript levels are at their lowest level. A similar expression profile is seen for the MDHAR2 gene, albeit at much lower magnitude (Fig. 2).
Post-anoxic injury
A role for AA has also been proposed in many stress-induced oxidative processes (Noctor and Foyer, 1998; Smirnoff and Wheeler, 2000). Its protective effects against photoxidative stress and ozone are well documented (Sanmartin et al., 2003; Müller-Moulé et al., 2004). Post-hypoxic and/or post-anoxic conditions are among the stresses in which ROS are implicated as the principle cause of injury (Crawford and Braendle, 1996; Blokhina et al., 2003). When aerobic conditions are re-established, a burst of ROS takes place, resulting in post-hypoxic or post-anoxic injury to the tissues (Babior, 1987). Plant enzymes such as APX, SOD, DHAR, and catalase, along with water-soluble (ascorbate, glutathione, flavonoids) and fat-soluble (tocopherols, carotenoids) antioxidants are recruited to scavenge ROS (Noctor and Foyer, 1998). Ascorbate plays a role both in the detoxification from ROS and in the maintenance of ROS levels that are required for signalling (Foyer and Noctor, 2005). Therefore, it was deemed necessary to investigate the expression profiles of AA biosynthetic, oxidizing, and recycling enzymes during post-anoxic injury in mature green tomatoes that were subjected to anoxia for 48 h and subsequently returned to air.
Interestingly, post-anoxic stress caused mature green tomatoes to respond by inducing the transcript accumulation of all committed and non-committed AA biosynthetic genes as early as 3–6 h after return to air, coinciding with elevated levels of AA. Similarly, enzymes involved in AA recycling responded to the post-anoxic stress by increasing their mRNA steady-state levels upon return to air. This is an indication of the magnitude of the oxidative damage and the crucial role of AA in scavenging ROS under these conditions. Not only was there an induction of AA biosynthetic genes, but there was also an induction of AA recycling genes, suggesting that this massive activation of transcription is needed to increase the reduced AA pool in order to compensate for the oxidative stress. The 3–6 h delay in this induction might indicate that there is a threshold AA concentration that induces its biosynthesis and recycling and suppresses its oxidation. No other stress tested in this study (namely wounding, low and high temperature, hypoxia, or anoxia) or ethylene was able to induce all the ascorbate-related genes coordinately. Current experiments are aimed at exploring the regulatory gene network underlying this specific massive induction of both biosynthesis and regeneration of ascorbate, which seems to be unique to post-anoxic stress.
Ethylene and stress
Ascorbate has also been implicated in hormone signalling (Pastori et al., 2003). Ethylene levels increase during tomato fruit ripening and may influence the expression of AA biosynthetic enzymes. In fact, it was found that out of all genes probed, only GPP mRNA levels increased in response to ethylene. This transcript was also found to accumulate at high levels during fruit ripening on- and off-the-vine, indicating that ethylene may regulate the transcription of this gene.
A potential role for AA in wounding was proposed since AA is required by peptidyl-prolyl-4-hydroxylase (P4H). This protein catalyses the post-translational modification of proline that is incorporated in the synthesis of hydroxyproline-rich proteins. These proteins are synthesized in response to wounding (Arrigoni and De Tullio, 2002). However, in the present experiments, wounding did not induce the transcription of AA-related enzymes except for GPP whose steady-state mRNA levels increased relative to control 12 h after wounding, and AKR2 that presented an increase in mRNA accumulation starting at 3 h after treatment. Tissue damage caused by mechanical sources, such as wounding, or by pathogen invasion leads to cell wall breakdown and release of oligosaccharides including GalU. Hence, it is possible that the AA biosynthetic pathway through GalU may be induced by wounding. Ascorbate recycling enzymes remained unaffected by this treatment.
Antioxidative systems were also found to respond to temperature stress (Gechev et al., 2003; Suzuki and Mittler, 2006). Various metabolic processes in the cells respond to temperature stress differently since these may have diverse temperature optima and the enzymes involved may be more or less heat labile. Temperature stress can lead to ROS production and to induction of ROS detoxification systems (Suzuki and Mittler, 2006). Ascorbate biosynthetic genes were not influenced by cold stress, except for GPP whose transcript accumulated at high levels starting at 3 h after exposure to 4 °C. This may also indicate that when increased AA levels are required, the cell compensates by increasing the GPP transcript levels so that more L-Gal 1-P is channelled for AA production. The origin of this L-Gal 1-P pool is still to be determined.
All the above considerations in terms of AA accumulation and the expression of AA-related genes in response to various stresses and during fruit ripening, and the presence of heat shock and wounding-responsive promoter elements (see below) indicate the complex involvement of AA metabolism in post-harvest fruit life and quality, thus emphasizing the demand for understanding AA pool size in fruit. The present data provide evidence pointing to GPP as a possible control gene regulating AA levels in tomato fruit during ripening and post-harvest stress conditions such as mechanical injury, cold storage (chilling injury), ethylene effects, and low oxygen stress.
Promoter analysis
Promoter analysis of the genes studied in this work suggests that light plays an important role in the expression of ascorbate-related genes. This observation is based on the fact that four different types of light-responsive promoter motifs are found in a high percentage of ascorbate-related genes. An interesting proposal has been made recently (Wolucka and Van Montagu, 2007) which links photosynthesis, cell wall metabolism, and vitamin C biosynthesis with VTC2. In this context, recent studies have already demonstrated strong evidence for the light regulation of VTC2 (Müller-Moulé, 2008), PMI (Maruta et al., 2008), GalDH (Gatzek et al., 2002), and GalLDH (Tamaoki et al., 2003) in Arabidopsis. Additionally, expression analysis of Arabidopsis ascorbate-related genes using online data from GeneInvestigator (https://www.genevestigator.ethz.ch/gv/index.jsp) shows that the transcripts for VTC2 are up-regulated in response to white light (data not shown). Combined with the results reported in the present study, the light regulation of AA biosynthesis and/or accumulation deserves further attention. ABA and gibberellins may also play roles in the regulation of ascorbate biosynthesis and/or accumulation. Interestingly, Lopez-Carbonell et al. (2006) recently observed a correlation between DHA content and ABA accumulation in foliage of the AA-deficient vtc1 mutant of Arabidopsis. This mutant, defective in the activity of the AA biosynthetic enzyme GDP-mannose pyrophosphorylase, is characterized by low AA content (∼30% of that of the wild-type) and a low AA/DHA ratio (Conklin et al., 1996). The presence of heat shock and wounding-responsive promoter motifs in the present data set underlines the significance of ascorbate when plants are under these stresses. Further studies aimed at characterizing comprehensive ascorbate-related gene expression profiles under varying light conditions and treatment with gibberellins and ABA may confirm the putative results suggested here.
Supplementary data
Supplementary data are available at JXB online.
Supplementary Material
Acknowledgments
This work was supported by EU-FOOD-CT-2006-016214 and (GSRT-GR-UK joint program in Agricultural Biotechnology).
References
- Adams-Phillips L, Barry C, Giovannoni J. Signal transduction systems regulating fruit ripening. Trends in Plant Science. 2004;9:331–338. doi: 10.1016/j.tplants.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Agius F, Gonzalez-Lamothe R, Caballero JL, Muñoz-Blanco J, Botella MA, Valpuesta V. Engineering increased vitamin C levels in plants by overexpressing of a D-galacturonic acid reductase. Nature Biotechnology. 2003;21:177–181. doi: 10.1038/nbt777. [DOI] [PubMed] [Google Scholar]
- Alexander L, Grierson D. Ethylene biosynthesis and action in tomato: a model for climacteric fruit ripening. Journal of Experimental Botany. 2002;53:2039–2055. doi: 10.1093/jxb/erf072. [DOI] [PubMed] [Google Scholar]
- Alhagdow M, Mounet F, Gilbert L, et al. Silencing of the mitochondrial ascorbate synthesizing enzyme L-galactono-1,4-lactone dehydrogenase affects plant and fruit development in tomato. Plant Physiology. 2007;145:1408–1422. doi: 10.1104/pp.107.106500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrigoni O, De Tullio MC. Ascorbic acid: much more than just an antioxidant. Biochimica et Biophysica Acta. 2002;1569:1–9. doi: 10.1016/s0304-4165(01)00235-5. [DOI] [PubMed] [Google Scholar]
- Babior BM. The respiratory burst oxidase. Trends in Biochemical Sciences. 1987;12:241–243. [Google Scholar]
- Badejo AA, Jeong ST, Goto-Yamamoto N, Esaka M. Cloning and expression of GDP-D-mannose pyrophosphorylase gene and ascorbic acid content of acerola (Malpighia glabra L.) fruit at ripening stages. Plant Physiology and Biochemistry. 2007;45:665–672. doi: 10.1016/j.plaphy.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Badejo AA, Tanaka N, Esaka M. Analysis of GDP-D-mannose pyrophosphorylase gene promoter from acerola (Malpighia glabra) and increase in ascorbate content of transgenic tobacco expressing the acerola gene. Plant and Cell Physiology. 2008;49:126–132. doi: 10.1093/pcp/pcm164. [DOI] [PubMed] [Google Scholar]
- Bermudez L, Urias U, Milstein D, Kamenetzky L, Asis R, Fernie AR, Van Sluys MA, Carrari F, Rossi M. A candidate gene survey of quantitative trait loci affecting chemical composition in tomato fruit. Journal of Experimental Botany. 2008;59:2875–2890. doi: 10.1093/jxb/ern146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blokhina O, Virolainen E, Fagerstedt KV. Antioxidants, oxidative damage and oxygen deprivation stress: a review. Annals of Botany. 2003;91:179–194. doi: 10.1093/aob/mcf118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrari F, Baxter C, Usadel B, et al. Integrated analysis of metabolite and transcript levels reveals the metabolic shifts that underlie tomato fruit development and highlight regulatory aspects of metabolic network behavior. Plant Physiology. 2006;142:1380–1396. doi: 10.1104/pp.106.088534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church GM, Gilbert W. Genomic sequencing. Proceedings of the National Academy of Sciences, USA. 1984;81:1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conklin PL, Williams E, Last RL. Environmental stress sensitivity of an ascorbic acid-deficient Arabidopsis mutant. Proceedings of the National Academy of Sciences, USA. 1996;93:9970–9974. doi: 10.1073/pnas.93.18.9970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conklin PL. Recent advances in the role and biosynthesis of ascorbic acid in plants. Plant Cell and Environment. 2001;24:383–394. [Google Scholar]
- Conklin PL, Gatzek S, Wheeler GL, Dowdle J, Raymond MJ, Rolinski S, Isupov M, Littlechild JA, Smirnoff N. Arabidopsis thaliana VTC4 encodes L-galactose-1-P phosphatase, a plant ascorbic acid biosynthetic enzyme. Journal of Biological Chemistry. 2006;281:15662–15670. doi: 10.1074/jbc.M601409200. [DOI] [PubMed] [Google Scholar]
- Crawford RMM, Braendle R. Oxygen deprivation stress in a changing environment. Journal of Experimental Botany. 1996;47:145–159. [Google Scholar]
- Daood HG, Al-Qitt MA, Bshenah KA, Bouragba M. Varietal and chemical aspect of tomato processing. Acta Alimentaria. 1990;19:347–357. [Google Scholar]
- Davey MW, Persiau Géstergaard J, Gilot C, Han Y, Bauw G, Van Montagu M. Ascorbate biosynthesis in Arabidopsis cell suspension culture. Plant Physiology. 1999;121:535–544. doi: 10.1104/pp.121.2.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey MD, Van Montagu M, Inze D, Sanmartin M, Kanellis AK, Smirnoff N, Benzie IJJ, Strain JJ, Favell D, Fletcher J. Plant L-ascorbic acid: chemistry, function, metabolism, bioavailability and effects of processing. Journal of the Science of Food and Agriculture. 2000;80:825–860. [Google Scholar]
- Dowdle J, Ishikawa T, Gatzek S, Rolinski S, Smirnoff N. Two genes in Arabidopsis thaliana encoding GDP-L-galactose phosphorylase are required for ascorbate biosynthesis and seedling viability. The Plant Journal. 2007;52:673–689. doi: 10.1111/j.1365-313X.2007.03266.x. [DOI] [PubMed] [Google Scholar]
- Dumville JC, Fry SC. Solubilization of tomato fruit pectins by ascorbate: a possible non-enzymatic mechanism of fruit softening. Planta. 2003;217:951–961. doi: 10.1007/s00425-003-1061-0. [DOI] [PubMed] [Google Scholar]
- Estruch R, Martínez-González MA, Corella D, et al. Effects of a Mediterranean-style diet on cardiovascular risk factors. Annals of Internal Medicine. 2006;145:1–11. doi: 10.7326/0003-4819-145-1-200607040-00004. [DOI] [PubMed] [Google Scholar]
- Fotopoulos V, Sanmartin M, Kanellis AK. Effect of ascorbate oxidase over-expression on ascorbate recycling gene expression in response to agents imposing oxidative stress. Journal of Experimental Botany. 2006;57:3933–3943. doi: 10.1093/jxb/erl147. [DOI] [PubMed] [Google Scholar]
- Fotopoulos V, De Tullio MC, Barnes J, Kanellis AK. Altered stomatal dynamics in ascorbate oxidase over-expressing tobacco plants suggest a role for dehydroascorbate signalling. Journal of Experimental Botany. 2008;59:729–737. doi: 10.1093/jxb/erm359. [DOI] [PubMed] [Google Scholar]
- Foyer CH, Noctor G. Redox homeostasis and antioxidant signaling: a metabolic interface between stress perception and physiological responses. The Plant Cell. 2005;17:1866–1875. doi: 10.1105/tpc.105.033589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry SC. Oxidative scission of plant cell wall polysaccharides by ascorbate-induced hydroxyl radicals. Biochemical Journal. 1998;332:507–515. doi: 10.1042/bj3320507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatzek S, Wheeler GL, Smirnoff N. Antisense suppression of L-galactose dehydrogenase in Arabidopsis thaliana provides evidence for its role in ascorbate synthesis and reveals light modulated L-galactose synthesis. The Plant Journal. 2002;30:541–553. doi: 10.1046/j.1365-313x.2002.01315.x. [DOI] [PubMed] [Google Scholar]
- Gechev T, Willekens H, Van Montagu M, Inzé D, Van Camp W, Toneva V, Minkov L. Different responses of tobacco antioxidant enzymes to light and chilling stress. Journal of Plant Physiology. 2003;160:509–515. doi: 10.1078/0176-1617-00753. [DOI] [PubMed] [Google Scholar]
- Giovannoni J. Molecular biology of fruit maturation and ripening. Annual Review of Plant Physiology and Plant Molecular Biology. 2001;52:725–749. doi: 10.1146/annurev.arplant.52.1.725. [DOI] [PubMed] [Google Scholar]
- Giovannoni J. Genetic regulation of fruit development and ripening. The Plant Cell. 2004;16:S170–S180. doi: 10.1105/tpc.019158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovannoni J. Fruit ripening mutants yield insights into ripening control. Current Opinion in Plant Biology. 2007;10:283–289. doi: 10.1016/j.pbi.2007.04.008. [DOI] [PubMed] [Google Scholar]
- Green MA, Fry SC. Vitamin C degradation in plant cells via enzymatic hydrolysis of 4-O-oxalyl-L-threonate. Nature. 2005a;433:83–88. doi: 10.1038/nature03172. [DOI] [PubMed] [Google Scholar]
- Green MA, Fry SC. Apoplastic degradation of ascorbate: novel enzymes and metabolites permeating the plant cell wall. Plant Biosystems. 2005b;139:2–7. [Google Scholar]
- Hancock RD, Viola R. Improving the nutritional value of crops through enhancement of L-ascorbic acid (vitamin C) content: rationale and biotechnological opportunities. Journal of Agriculture and Food Chemistry. 2005;53:5248–5257. doi: 10.1021/jf0503863. [DOI] [PubMed] [Google Scholar]
- Hancock RD, Walker PG, Simon DA, Pont SDA, Marqui N, Vivera S, Gordon SL, Brennan RM, Viola R. L-Ascorbic acid accumulation in fruit of Ribes nigrum occurs by in situ biosynthesis via the L-galactose pathway. Functional Plant Biology. 2007;34:1080–1091. doi: 10.1071/FP07221. [DOI] [PubMed] [Google Scholar]
- Ishikawa T, Dowdle J, Smirnoff N. Progress in manipulating ascorbic acid biosynthesis and accumulation in plants. Physiology Plantarum. 2006;126:343–355. [Google Scholar]
- Jain AK, Nessler CL. Metabolic engineering of an alternative pathway for ascorbic acid biosynthesis in plants. Molecular Breeding. 2000;6:73–78. [Google Scholar]
- Jimenez A, Creissen G, Kular B, Firmin J, Robinson S, Verhoeyen M, Mullineaux P. Changes in oxidative processes and components of the antioxidant system during tomato fruit ripening. Planta. 2002;214:751–758. doi: 10.1007/s004250100667. [DOI] [PubMed] [Google Scholar]
- Laing WA, Bulley S, Wright M, Cooney J, Jensen D, Barraclough D, MacRae E. A highly specific L-galactose-1-phosphate phosphatase on the path to ascorbate biosynthesis. Proceedings of the National Academy of Sciences, USA. 2004a;101:16976–16981. doi: 10.1073/pnas.0407453101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laing WA, Fearson N, Bulley S, MacRae E. Kiwifruit L-galactose dehydrogenase: molecular, biochemical and physiological aspects of the enzyme. Functional Plant Biology. 2004b;31:1015–1025. doi: 10.1071/FP04090. [DOI] [PubMed] [Google Scholar]
- Laing WA, Wright MA, Cooney J, Bulley SM. The missing step of the L-galactose pathway of ascorbate biosynthesis in plants, an L-galactose guanyltransferase, increases leaf ascorbate content. Proceedings of the National Academy of Sciences, USA. 2007;104:9534–9539. doi: 10.1073/pnas.0701625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linster CL, Gomez TA, Christensen KC, Adler LN, Young BD, Brenner C, Clarke SG. Arabidopsis VTC2 encodes a GDP-L-galactose phosphorylase, the last unknown enzyme in the Smirnoff–Wheeler pathway to ascorbic acid in plants. Journal of Biological Chemistry. 2007;282:18879–18885. doi: 10.1074/jbc.M702094200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linster CL, Clarke SG. L-Ascorbate biosynthesis in higher plants: the rope of VTC2. Trends in Plant Sciences. 2008;13:567–573. doi: 10.1016/j.tplants.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewus FA. Biosynthesis and metabolism of ascorbic acid in plants and of analogs of ascorbic acid in fungi. Phytochemistry. 1999;52:193–210. [Google Scholar]
- López-Carbonell M, Munné-Bosch S, Alegre L. The ascorbate-deficient vtc-1 Arabidopsis mutant shows altered ABA accumulation in leaves and chloroplasts. Journal of Plant Growth Regulation. 2004;25:137–144. [Google Scholar]
- Lorence A, Chevone BI, Mendes P, Nessler GL. Myo-inositol oxygenase offers a possible entry point into plant ascorbate biosynthesis. Plant Physiology. 2004;134:1200–1205. doi: 10.1104/pp.103.033936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruta T, Yonemitsu M, Yabuta Y, Tamoi M, Ishikawa T, Shigeru Shigeoka S. Arabidopsis phosphomannose isomerase 1, but not phosphomannose isomerase 2, is essential for ascorbic acid biosynthesis. Journal of Biological Chemistry. 2008;283:28842–28851. doi: 10.1074/jbc.M805538200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller-Moulé P, Golan T, Niyogi KK. Ascorbate deficient mutants of Arabidopsis grow in high light despite chronic photooxidative stress. Plant Physiology. 2004;134:1163–1172. doi: 10.1104/pp.103.032375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller-Moulé P. An expression analysis of the ascorbate biosynthesis enzyme VTC2. Plant Molecular Biology. 2008;68:31–41. doi: 10.1007/s11103-008-9350-4. [DOI] [PubMed] [Google Scholar]
- Noctor G, Foyer CH. Ascorbate and glutathione: keeping active oxygen under control. Annual Review of Plant Physiology and Plant Molecular Biology. 1998;49:249–279. doi: 10.1146/annurev.arplant.49.1.249. [DOI] [PubMed] [Google Scholar]
- Pastori GM, Kiddle G, Antoniw J, Bernard S, Veljovic-Jovanovic S, Verrier PJ, Noctor G, Foyer CH. Leaf vitamin C contents modulate plant defense transcripts and regulate genes controlling development through hormone signalling. The Plant Cell. 2003;15:1212–1226. doi: 10.1105/tpc.010538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pateraki I, Sanmartin M, Kalamaki MS, Gerasopoulos D, Kanellis AK. Molecular characterization and expression studies during fruit development and ripening of L-galactono-1,4 lactone dehydrogenase. Journal of Experimental Botany. 2004;55:1623–1633. doi: 10.1093/jxb/erh186. [DOI] [PubMed] [Google Scholar]
- Potters G, Horemans N, Caubergs RJ, Asard H. Ascorbate and dehydroascorbate influence cell cycle progression in tobacco cell suspension. Plant Physiology. 2000;124:17–20. doi: 10.1104/pp.124.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proteggente AR, Pannala AS, Paganga G, Van Buren L, Wagner E, Wiseman S, Van De Put F, Dacombe C, Rice-Evans CA. The antioxidant activity of regularly consumed fruit and vegetables reflects their phenolic and vitamin C composition. Free Radical Research. 2002;36:217–233. doi: 10.1080/10715760290006484. [DOI] [PubMed] [Google Scholar]
- Running JA, Burlingame RP, Berry A. The pathway of L-ascorbic acid biosynthesis in the colourless microalga Prototheca moriformis. Journal of Experimental Botany. 2003;54:1841–1849. doi: 10.1093/jxb/erg207. [DOI] [PubMed] [Google Scholar]
- Sanmartin M, Drogoudi PD, Lyons T, Pateraki I, Barnes J, Kanellis AK. Over-expression of ascorbate oxidase in the apoplast of transgenic tobacco results in altered ascorbate and glutathione redox states and increased sensitivity to ozone. Planta. 2003;216:918–928. doi: 10.1007/s00425-002-0944-9. [DOI] [PubMed] [Google Scholar]
- Sanmartin AM, Pateraki I, Chatzopoulou F, Kanellis AK. Differential expression of melon ascorbate oxidase multigene family during fruit development and in response to stress. Planta. 2007;225:873–885. doi: 10.1007/s00425-006-0399-5. [DOI] [PubMed] [Google Scholar]
- Smirnoff N. Ascorbic acid: metabolism and functions of a multi-facetted molecule. Current Opinion of Plant Biology. 2000;3:229–235. [PubMed] [Google Scholar]
- Smirnoff N, Wheeler GL. Ascorbic acid in plants: biosynthesis and function. Critical Reviews in Biochemistry and Molecular Biology. 2000;35:291–314. doi: 10.1080/10409230008984166. [DOI] [PubMed] [Google Scholar]
- Smirnoff N, Conklin PL, Loewus FA. Biosynthesis of ascorbic acid in plants: a renaissance. Annual Review of Plant Physiology and Plant Molecular Biology. 2001;52:437–467. doi: 10.1146/annurev.arplant.52.1.437. [DOI] [PubMed] [Google Scholar]
- Smith CJS, Slater A, Grierson D. Rapid appearance of an mRNA correlated with ethylene synthesis encoding a protein of molecular weight 35000. Planta. 1986;168:94–100. doi: 10.1007/BF00407014. [DOI] [PubMed] [Google Scholar]
- Stevens R, Buret M, Duffe P, Garchery C, Baldet P, Rothan C, Causse M. Candidate genes and quantitative trait loci affecting fruit ascorbic acid content in three tomato populations. Plant Physiology. 2007;143:1943–1953. doi: 10.1104/pp.106.091413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens R, Page D, Gouble B, Garchery C, Zamir D, Causse M. Tomato fruit ascorbic acid content is linked with monodehydroascorbate reductase activity and tolerance to chilling stress. Plant, Cell and Environment. 2008;31:1086–1096. doi: 10.1111/j.1365-3040.2008.01824.x. [DOI] [PubMed] [Google Scholar]
- Suzuki N, Mittler R. Reactive oxygen species and temperature stresses: a delicate balance between signalling and destruction. Physiologia Plantarum. 2006;126:45–51. [Google Scholar]
- Tabata K, Oba K, Suzyki K, Esaka M. Generation and properties of ascorbic acid-deficient transgenic tobacco cells expressing antisense RNA for L-galactono-1,4-lactone dehydrogenase. The Plant Journal. 2001;27:139–148. doi: 10.1046/j.1365-313x.2001.01074.x. [DOI] [PubMed] [Google Scholar]
- Tamaoki M, Mukai F, Asai N, Nalajima N, Kubo A, Aono M, Sajim MH. Light-controlled expression of a gene encoding L-galactono-gamma-lactone dehydrogenase which affects ascorbate pool size in Arabidopsis thaliana. Plant Science. 2003;164:1111–1117. [Google Scholar]
- Trichopoulou A, Lagiou P, Kuper H, Trichopoulos D. Cancer and Mediterranean dietary traditions. Cancer Epidemiology Biomarkers and Prevention. 2000;9:869–873. [PubMed] [Google Scholar]
- Trichopoulou A, Naska A, Antoniou A, Friel S, Trygg K, Turrini A. Vegetable and fruit: the evidence in their favour and the public health perspective. International Journal for Vitamin and Nutrition Research. 2003;73:63–69. doi: 10.1024/0300-9831.73.2.63. [DOI] [PubMed] [Google Scholar]
- Valpuesta V, Botella MA. Biosynthesis of L-ascorbic acid in plants: new pathways for an old antioxidant. Trends in Plant Science. 2004;9:573–577. doi: 10.1016/j.tplants.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Ververidis P, John P. Complete recovery in vitro of ethylene forming enzyme activity. Phytochemistry. 1991;30:725–727. [Google Scholar]
- Visioli F, Grande S, Bogani P, Galli C. The role of antioxidants in the Mediterranean diets: focus on cancer. European Journal of Cancer Prevention. 2004;13:337–343. doi: 10.1097/01.cej.0000137513.71845.f6. [DOI] [PubMed] [Google Scholar]
- Vrebalov J, Ruezinsky D, Padmanabhan V, White R, Medrano D, Drake R, Schuch W, Giovannoni J. A MADS-box gene necessary for fruit ripening at the tomato ripening-inhibitor (rin) locus. Science. 2002;296:343–346. doi: 10.1126/science.1068181. [DOI] [PubMed] [Google Scholar]
- Watanabe K, Suzuki K, Kitamura S. Characterization of a GDP-D-mannose 3′,5′-epimerase from rice. Phytochemistry. 2006;67:338–346. doi: 10.1016/j.phytochem.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Wheeler GL, Jones MA, Smirnoff N. The biosynthetic pathway of vitamin C in higher plants. Nature. 1998;393:365–369. doi: 10.1038/30728. [DOI] [PubMed] [Google Scholar]
- Wolucka BA, Goossens A, Inze D. Methyl jasmonate stimulates the de novo biosynthesis of vitamin C in plant cell suspensions. Journal of Experimental Botany. 2005;56:2527–2538. doi: 10.1093/jxb/eri246. [DOI] [PubMed] [Google Scholar]
- Wolucka BA, Van Montagu M. GDP-mannose 3′,5′-epimerase forms GDP-L-gulose, a putative intermediate for the de novo biosynthesis of vitamin C in plants. Journal of Biological Chemistry. 2003;278:47483–47490. doi: 10.1074/jbc.M309135200. [DOI] [PubMed] [Google Scholar]
- Wolucka BA, Van Montagu MV. The VTC2 cycle and the de novo biosynthesis pathways for vitamin C in plants: an opinion. Phytochemistry. 2007;68:2602–2613. doi: 10.1016/j.phytochem.2007.08.034. [DOI] [PubMed] [Google Scholar]
- Zou L, Li H, Ouyang B, Zhang J, Ye Z. Cloning and mapping of genes involved in tomato ascorbic acid biosynthesis and metabolism. Plant Science. 2006;170:120–127. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.