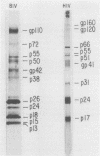
Abstract
The bovine lentivirus, known as bovine immunodeficiency-like virus (BIV), is genetically, structurally, and antigenically related to human immunodeficiency virus type 1 (HIV-1). It is not known whether sera from persons exposed to BIV proteins would show either positive or indeterminate reactivity on HIV-1 antibody tests. We used a BIV Western blot (immunoblot) analysis to examine human sera characterized as HIV-1 antibody positive, HIV-1 antibody negative, HIV-1 persistently indeterminate, HIV-1 p17 antibody positive only, HIV-1 p24 antibody positive only, human T-cell leukemia virus type 1 (HTLV-1) p19 antibody positive only, or HTLV-1 p24 antibody positive only. None of these sera were positive by Western blot to BIV-specific proteins. Many of these sera, however, displayed strong reactivities to bovine cell culture antigens on blots prepared from both mock-infected and BIV-infected cell cultures. The HIV-1 p17 and p24 antibody-positive and the HTLV-1 p19 and p24 antibody-positive sera were further examined by Western blot to bovine leukemia virus (BLV) and were found to be negative. We examined sera from laboratory personnel at risk for BIV exposure, including two laboratory workers who were exposed to BIV by accidental injection with BIV-infected cell culture material, and found no evidence of seroconversion to BIV-specific proteins. We tested 371 samples of fetal bovine sera, each sample representing serum pooled from one to three fetuses. All samples were negative by BIV Western blot. To date, we have not detected any human sera with antibody to BIV-specific proteins. Our data indicate that persistently indeterminate results on HIV-1 Western blot are not caused by a human antibody response to BIV proteins.
Full text
PDF

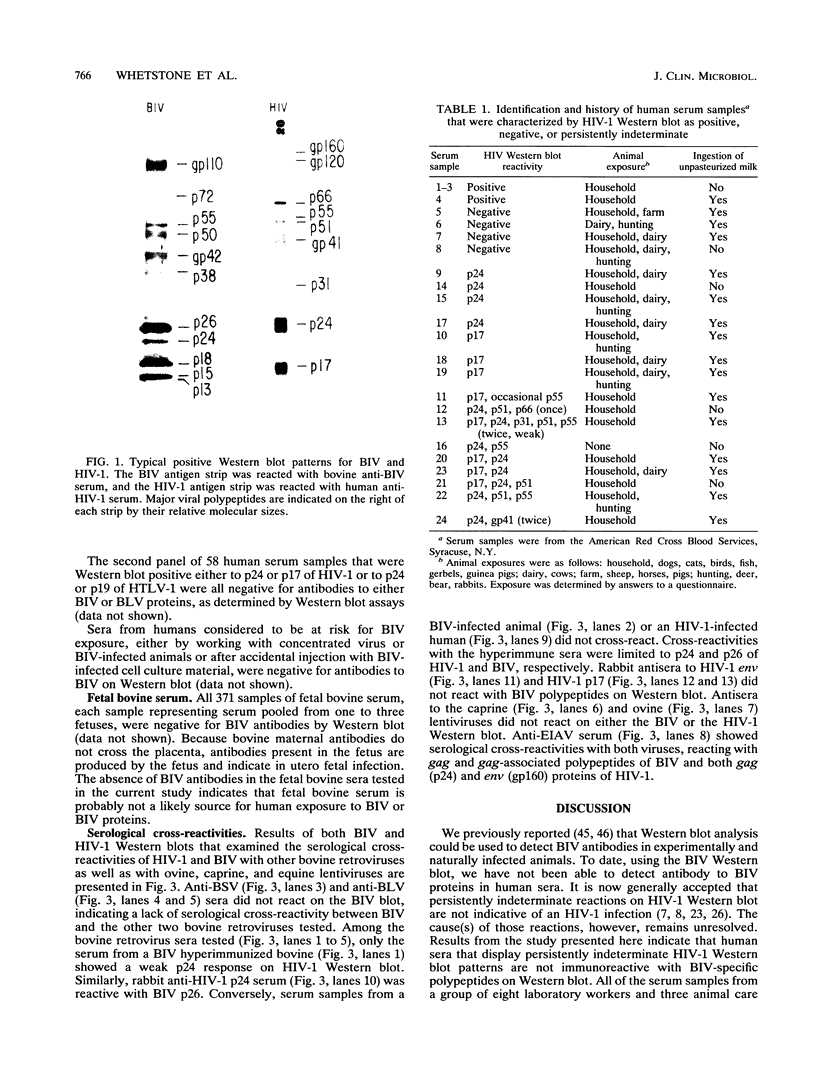
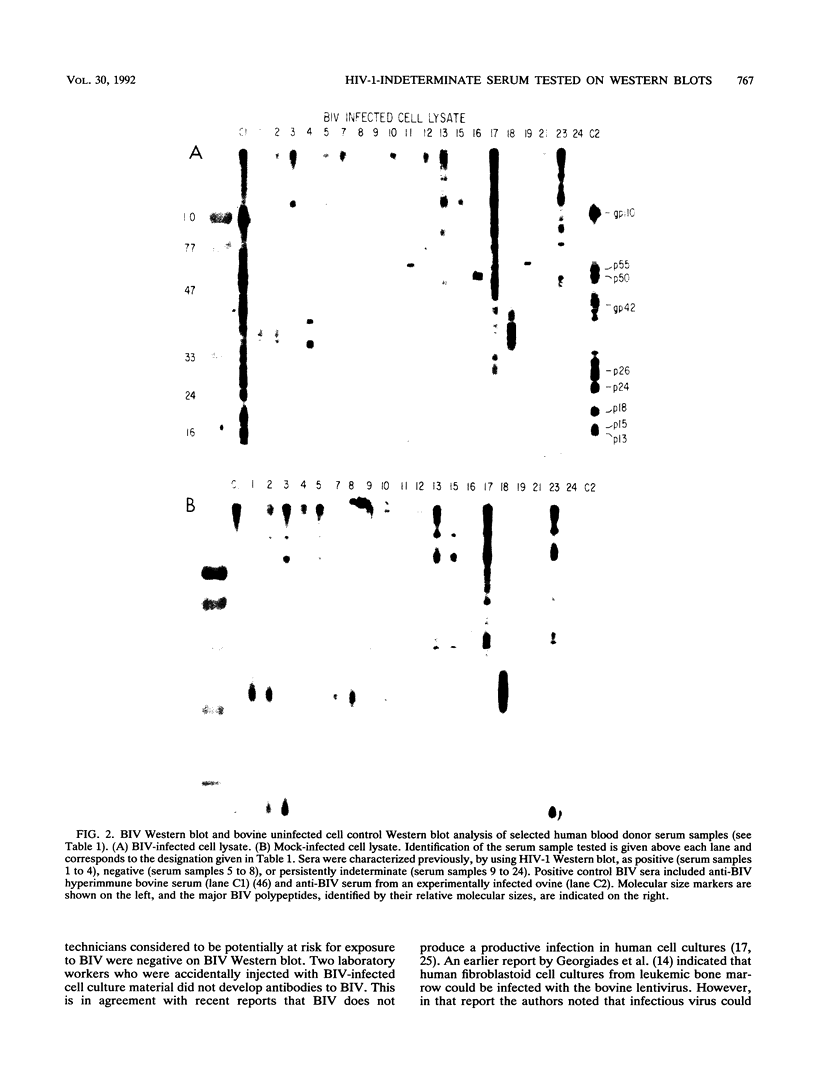


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blomberg J., Vincic E., Jönsson C., Medstrand P., Pipkorn R. Identification of regions of HIV-1 p24 reactive with sera which give "indeterminate" results in electrophoretic immunoblots with the help of long synthetic peptides. AIDS Res Hum Retroviruses. 1990 Dec;6(12):1363–1372. doi: 10.1089/aid.1990.6.1363. [DOI] [PubMed] [Google Scholar]
- Boothe A. D., Van der Maaten A. J. Ultrastructural studies of a visna-like syncytia-producing virus from cattle with lymphocytosis. J Virol. 1974 Jan;13(1):197–204. doi: 10.1128/jvi.13.1.197-204.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun M. J., Lahn S., Boyd A. L., Kost T. A., Nagashima K., Gonda M. A. Molecular cloning of biologically active proviruses of bovine immunodeficiency-like virus. Virology. 1988 Dec;167(2):515–523. [PubMed] [Google Scholar]
- Di Guardo G. Potential human health risks associated with animal retroviruses: some hypotheses. Med Hypotheses. 1989 Jul;29(3):195–198. doi: 10.1016/0306-9877(89)90194-1. [DOI] [PubMed] [Google Scholar]
- Dock N. L., Lamberson H. V., Jr, O'Brien T. A., Tribe D. E., Alexander S. S., Poiesz B. J. Evaluation of atypical human immunodeficiency virus immunoblot reactivity in blood donors. Transfusion. 1988 Sep-Oct;28(5):412–418. doi: 10.1046/j.1537-2995.1988.28588337326.x. [DOI] [PubMed] [Google Scholar]
- Dodd R. Y., Fang C. T. The western immunoblot procedure for HIV antibodies and its interpretation. Arch Pathol Lab Med. 1990 Mar;114(3):240–245. [PubMed] [Google Scholar]
- Donham K. J., Burmeister L. F., VanLier S. F., Greiner T. C. Relationships of bovine leukemia virus prevalence in dairy herds and density of dairy cattle to human lymphocytic leukemia. Am J Vet Res. 1987 Feb;48(2):235–238. [PubMed] [Google Scholar]
- Egberink H. F., Ederveen J., Montelaro R. C., Pedersen N. C., Horzinek M. C., Koolen M. J. Intracellular proteins of feline immunodeficiency virus and their antigenic relationship with equine infectious anaemia virus proteins. J Gen Virol. 1990 Mar;71(Pt 3):739–743. doi: 10.1099/0022-1317-71-3-739. [DOI] [PubMed] [Google Scholar]
- Evermann J. F. Comparative features of retroviral infections of livestock. Comp Immunol Microbiol Infect Dis. 1990;13(3):127–136. doi: 10.1016/0147-9571(90)90275-x. [DOI] [PubMed] [Google Scholar]
- Garry R. F., Fermin C. D., Hart D. J., Alexander S. S., Donehower L. A., Luo-Zhang H. Detection of a human intracisternal A-type retroviral particle antigenically related to HIV. Science. 1990 Nov 23;250(4984):1127–1129. doi: 10.1126/science.1701273. [DOI] [PubMed] [Google Scholar]
- Garvey K. J., Oberste M. S., Elser J. E., Braun M. J., Gonda M. A. Nucleotide sequence and genome organization of biologically active proviruses of the bovine immunodeficiency-like virus. Virology. 1990 Apr;175(2):391–409. doi: 10.1016/0042-6822(90)90424-p. [DOI] [PubMed] [Google Scholar]
- Georgiades J. A., Billiau A., Vanderschueren B. Infection of human cell cultures with bovine visna virus. J Gen Virol. 1978 Feb;38(2):375–381. doi: 10.1099/0022-1317-38-2-375. [DOI] [PubMed] [Google Scholar]
- Gonda M. A., Braun M. J., Carter S. G., Kost T. A., Bess J. W., Jr, Arthur L. O., Van der Maaten M. J. Characterization and molecular cloning of a bovine lentivirus related to human immunodeficiency virus. 1987 Nov 26-Dec 2Nature. 330(6146):388–391. doi: 10.1038/330388a0. [DOI] [PubMed] [Google Scholar]
- Gonda M. A., Oberste M. S., Garvey K. J., Pallansch L. A., Battles J. K., Pifat D. Y., Bess J. W., Jr, Nagashima K. Development of the bovine immunodeficiency-like virus as a model of lentivirus disease. Dev Biol Stand. 1990;72:97–110. [PubMed] [Google Scholar]
- Goudsmit J., Houwers D. J., Smit L., Nauta I. M. LAV/HTLV-III gag gene product p24 shares antigenic determinants with equine infectious anemia virus but not with visna virus or caprine arthritis encephalitis virus. Intervirology. 1986;26(3):169–173. doi: 10.1159/000149697. [DOI] [PubMed] [Google Scholar]
- Grote J. Bovine visna virus and the origin of HIV. J R Soc Med. 1988 Oct;81(10):620–620. doi: 10.1177/014107688808101036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huet T., Dazza M. C., Brun-Vézinet F., Roelants G. E., Wain-Hobson S. A highly defective HIV-1 strain isolated from a healthy Gabonese individual presenting an atypical western blot. AIDS. 1989 Nov;3(11):707–715. doi: 10.1097/00002030-198911000-00004. [DOI] [PubMed] [Google Scholar]
- Kashanchi F., Liu Z. Q., Atkinson B., Wood C. Comparative evaluation of bovine immunodeficiency-like virus infection by reverse transcriptase and polymerase chain reaction. J Virol Methods. 1991 Feb-Mar;31(2-3):197–209. doi: 10.1016/0166-0934(91)90158-v. [DOI] [PubMed] [Google Scholar]
- Kleinman S. The significance of HIV-1-indeterminate western blot results in blood donor populations. Arch Pathol Lab Med. 1990 Mar;114(3):298–303. [PubMed] [Google Scholar]
- Lucas M. H., Roberts D. H. Bovine visna virus and the origin of HIV. J R Soc Med. 1989 May;82(5):317–317. doi: 10.1177/014107688908200528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama K., Fukushima T., Mochizuki S. Cross-reactive antibodies to BLV and HTLV in bovine and human hosts with retrovirus infection. Vet Immunol Immunopathol. 1989 Oct;22(3):265–273. doi: 10.1016/0165-2427(89)90013-5. [DOI] [PubMed] [Google Scholar]
- Miller J. M., van der Maaten M. J. Bovine leukosis--its importance to the dairy industry in the United States. J Dairy Sci. 1982 Nov;65(11):2194–2203. doi: 10.3168/jds.S0022-0302(82)82482-X. [DOI] [PubMed] [Google Scholar]
- Montelaro R. C., Robey W. G., West M. D., Issel C. J., Fischinger P. J. Characterization of the serological cross-reactivity between glycoproteins of the human immunodeficiency virus and equine infectious anaemia virus. J Gen Virol. 1988 Jul;69(Pt 7):1711–1717. doi: 10.1099/0022-1317-69-7-1711. [DOI] [PubMed] [Google Scholar]
- Olmsted R. A., Barnes A. K., Yamamoto J. K., Hirsch V. M., Purcell R. H., Johnson P. R. Molecular cloning of feline immunodeficiency virus. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2448–2452. doi: 10.1073/pnas.86.7.2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagata N., Yasunaga T., Tsuzuku-Kawamura J., Ohishi K., Ogawa Y., Ikawa Y. Complete nucleotide sequence of the genome of bovine leukemia virus: its evolutionary relationship to other retroviruses. Proc Natl Acad Sci U S A. 1985 Feb;82(3):677–681. doi: 10.1073/pnas.82.3.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindzielorz A. H., Belshe R. B., Mufson M. A. Occurrence, characteristics, and patterns of HIV-1 and HIV-2 western blot indeterminate sera in low risk populations in West Virginia and pre-AIDS Africa. Am J Trop Med Hyg. 1990 May;42(5):460–464. doi: 10.4269/ajtmh.1990.42.460. [DOI] [PubMed] [Google Scholar]
- Schneider J., Kaaden O., Copeland T. D., Oroszlan S., Hunsmann G. Shedding and interspecies type sero-reactivity of the envelope glycopolypeptide gp120 of the human immunodeficiency virus. J Gen Virol. 1986 Nov;67(Pt 11):2533–2538. doi: 10.1099/0022-1317-67-11-2533. [DOI] [PubMed] [Google Scholar]
- Stephens R. M., Casey J. W., Rice N. R. Equine infectious anemia virus gag and pol genes: relatedness to visna and AIDS virus. Science. 1986 Feb 7;231(4738):589–594. doi: 10.1126/science.3003905. [DOI] [PubMed] [Google Scholar]
- Thiry L., Sprecher-Goldberger S., Jacquemin P., Cogniaux J., Burny A., Bruck C., Portetelle D., Cran S., Clumeck N. Bovine leukemia virus-related antigens in lymphocyte cultures infected with AIDS-associated viruses. Science. 1985 Mar 22;227(4693):1482–1484. doi: 10.1126/science.2579433. [DOI] [PubMed] [Google Scholar]
- Van der Maaten M. J., Boothe A. D., Seger C. L. Isolation of a virus from cattle with persistent lymphocytosis. J Natl Cancer Inst. 1972 Dec;49(6):1649–1657. doi: 10.1093/jnci/49.6.1649. [DOI] [PubMed] [Google Scholar]
- Van der Maaten M. J., Whetstone C. A., Khramtsov V. V., Miller J. M. Experimentally-induced infections with bovine immunodeficiency-like virus, a bovine lentivirus. Dev Biol Stand. 1990;72:91–95. [PubMed] [Google Scholar]
- Whetstone C. A., VanDerMaaten M. J., Black J. W. Humoral immune response to the bovine immunodeficiency-like virus in experimentally and naturally infected cattle. J Virol. 1990 Jul;64(7):3557–3561. doi: 10.1128/jvi.64.7.3557-3561.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whetstone C. A., VanDerMaaten M. J., Miller J. M. A western blot assay for the detection of antibodies to bovine immunodeficiency-like virus in experimentally inoculated cattle, sheep, and goats. Arch Virol. 1991;116(1-4):119–131. doi: 10.1007/BF01319236. [DOI] [PubMed] [Google Scholar]