Abstract
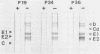
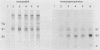
Immunoblot (IB) assays were developed for detection of rubella virus (RV)-specific immunoglobulin G (IgG), IgM, and IgA antibodies in human serum following natural infection or immunization. IB assays performed under nonreducing conditions were compared with those performed under reducing conditions and with immunoprecipitation assays. Significant loss of antigenicity (greater than 90%) of RV E1 and E2 proteins was observed when IB assays were performed in the presence of 2-mercaptoethanol as compared with assays under nonreducing conditions. In contrast, the antigenicity of RV capsid protein was not influenced by reducing agents. Sensitivity of IB for RV-specific IgG antibodies was determined to be 0.01 IU/ml under nonreducing conditions. In the determination of RV-specific IgM and IgA antibodies by IB, pretreatment of serum with protein G to remove competing high-affinity RV-specific IgG or rheumatoid factor significantly improved assay sensitivity. IB assays were observed to be superior to immunoprecipitation assays in their ability to better define the specificities of RV-specific antibodies and to detect antibodies of all immunoglobulin classes. However, the conformational sensitivity of RV protein antigenicity should be an important consideration in the interpretation of RV-specific antibodies by IB assays.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akerström B., Björck L. A physicochemical study of protein G, a molecule with unique immunoglobulin G-binding properties. J Biol Chem. 1986 Aug 5;261(22):10240–10247. [PubMed] [Google Scholar]
- Burman L. A., Norrby R., Trollfors B. Invasive pneumococcal infections: incidence, predisposing factors, and prognosis. Rev Infect Dis. 1985 Mar-Apr;7(2):133–142. doi: 10.1093/clinids/7.2.133. [DOI] [PubMed] [Google Scholar]
- Champsaur H., Fattal-German M., Arranhado R. Sensitivity and specificity of viral immunoglobulin M determination by indirect enzyme-linked immunosorbent assay. J Clin Microbiol. 1988 Feb;26(2):328–332. doi: 10.1128/jcm.26.2.328-332.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke D. M., Loo T. W., Hui I., Chong P., Gillam S. Nucleotide sequence and in vitro expression of rubella virus 24S subgenomic messenger RNA encoding the structural proteins E1, E2 and C. Nucleic Acids Res. 1987 Apr 10;15(7):3041–3057. doi: 10.1093/nar/15.7.3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cubie H., Edmond E. Comparison of five different methods of rubella IgM antibody testing. J Clin Pathol. 1985 Feb;38(2):203–207. doi: 10.1136/jcp.38.2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cusi M. G., Metelli R., Valensin P. E. Immune responses to wild and vaccine rubella viruses after rubella vaccination. Arch Virol. 1989;106(1-2):63–72. doi: 10.1007/BF01311038. [DOI] [PubMed] [Google Scholar]
- Cusi M. G., Rossolini G. M., Cellesi C., Valensin P. E. Antibody response to wild rubella virus structural proteins following immunization with RA 27/3 live attenuated vaccine. Arch Virol. 1988;101(1-2):25–33. doi: 10.1007/BF01314649. [DOI] [PubMed] [Google Scholar]
- Forghani B., Schmidt N. J., Lennette E. H. Demonstration of rubella IgM antibody by indirect fluorescent antibody staining, sucrose density gradient centrifugation and mercaptoethanol reduction. Intervirology. 1973;1(1):48–59. doi: 10.1159/000148832. [DOI] [PubMed] [Google Scholar]
- Gispen R., Nagel J., Brand-Saathof B., De Graaf S. Immunofluorescence test for IgM rubella antibodies in whole serum after absorption with anti-gammaFc. Clin Exp Immunol. 1975 Dec;22(3):431–437. [PMC free article] [PubMed] [Google Scholar]
- Grahame R., Armstrong R., Simmons N., Wilton J. M., Dyson M., Laurent R., Millis R., Mims C. A. Chronic arthritis associated with the presence of intrasynovial rubella virus. Ann Rheum Dis. 1983 Feb;42(1):2–13. doi: 10.1136/ard.42.1.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green K. Y., Dorsett P. H. Rubella virus antigens: localization of epitopes involved in hemagglutination and neutralization by using monoclonal antibodies. J Virol. 1986 Mar;57(3):893–898. doi: 10.1128/jvi.57.3.893-898.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harcourt G. C., Best J. M., Banatvala J. E. Rubella-specific serum and nasopharyngeal antibodies in volunteers with naturally acquired and vaccine-induced immunity after intranasal challenge. J Infect Dis. 1980 Aug;142(2):145–155. doi: 10.1093/infdis/142.2.145. [DOI] [PubMed] [Google Scholar]
- Heggie A. D., Robbins F. C. Natural rubella acquired after birth. Clinical features and complications. Am J Dis Child. 1969 Jul;118(1):12–17. doi: 10.1001/archpedi.1969.02100040014003. [DOI] [PubMed] [Google Scholar]
- Ho-Terry L., Cohen A. Degradation of rubella virus envelope components. Arch Virol. 1980;65(1):1–13. doi: 10.1007/BF01340535. [DOI] [PubMed] [Google Scholar]
- Ho-Terry L., Terry G. M., Cohen A., Londesborough P. Immunological characterisation of the rubella E 1 glycoprotein. Brief report. Arch Virol. 1986;90(1-2):145–152. doi: 10.1007/BF01314152. [DOI] [PubMed] [Google Scholar]
- Katow S., Sugiura A. Antibody response to individual rubella virus proteins in congenital and other rubella virus infections. J Clin Microbiol. 1985 Mar;21(3):449–451. doi: 10.1128/jcm.21.3.449-451.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katow S., Sugiura A. Conformational change of rubella virus spike proteins induced by 2-mercaptoethanol. Jpn J Med Sci Biol. 1988 Jun;41(3):109–115. doi: 10.7883/yoken1952.41.109. [DOI] [PubMed] [Google Scholar]
- Kurtz J. B., Anderson M. J. Cross-reactions in rubella and parvovirus specific IgM tests. Lancet. 1985 Dec 14;2(8468):1356–1356. doi: 10.1016/s0140-6736(85)92647-9. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leinikki P. O., Shekarchi I., Dorsett P., Sever J. L. Determination of virus-specific IgM antibodies by using ELISA: elimination of false-positive results with protein A-Sepharose absorption and subsequent IgM antibody assay. J Lab Clin Med. 1978 Dec;92(6):849–857. [PubMed] [Google Scholar]
- Levine J. B., Berkowitz C. D., St Geme J. W., Jr Rubella virus reinfection during pregnancy leading to late-onset congenital rubella syndrome. J Pediatr. 1982 Apr;100(4):589–591. doi: 10.1016/s0022-3476(82)80761-0. [DOI] [PubMed] [Google Scholar]
- Lindmark R., Thorén-Tolling K., Sjöquist J. Binding of immunoglobulins to protein A and immunoglobulin levels in mammalian sera. J Immunol Methods. 1983 Aug 12;62(1):1–13. doi: 10.1016/0022-1759(83)90104-7. [DOI] [PubMed] [Google Scholar]
- Loo T. W., MacDonald I., Clarke D. M., Trudel M., Tingle A., Gilam S. Detection of antibodies to individual proteins of rubella virus. J Virol Methods. 1986 May;13(2):149–159. doi: 10.1016/0166-0934(86)90083-2. [DOI] [PubMed] [Google Scholar]
- Maller R., Frydén A., Sörén L. Mitogen stimulation and distribution of T- and B-lymphocytes during natural rubella infection. Acta Pathol Microbiol Scand C. 1978 Jun;86C(3):93–98. doi: 10.1111/j.1699-0463.1978.tb02564.x. [DOI] [PubMed] [Google Scholar]
- Meurman O. Detection of antiviral IgM antibodies and its problems--a review. Curr Top Microbiol Immunol. 1983;104:101–131. doi: 10.1007/978-3-642-68949-9_7. [DOI] [PubMed] [Google Scholar]
- Morris G. E., Coleman R. M., Best J. M., Benetato B. B., Nahmias A. J. Persistence of serum IgA antibodies to herpes simplex, varicella-zoster, cytomegalovirus, and rubella virus detected by enzyme-linked immunosorbent assays. J Med Virol. 1985 Aug;16(4):343–349. doi: 10.1002/jmv.1890160407. [DOI] [PubMed] [Google Scholar]
- Partanen P., Seppänen H., Suni J., Vaheri A. Selective reactivity of antibodies to human immunoglobulins G, M, and A with rubella virus proteins. J Clin Microbiol. 1985 May;21(5):800–802. doi: 10.1128/jcm.21.5.800-802.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partridge J. W., Flewett T. H., Whitehead J. E. Congenital rubella affecting an infant whose mother had rubella antibodies before conception. Br Med J (Clin Res Ed) 1981 Jan 17;282(6259):187–188. doi: 10.1136/bmj.282.6259.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattison J. R., Jackson C. M., Hiscock J. A., Cradock-Watson J. E., Ridehalgh M. K. Comparison of methods for detecting specific IgM antibody in infants with congenital rubella. J Med Microbiol. 1978 Nov;11(4):411–418. doi: 10.1099/00222615-11-4-411. [DOI] [PubMed] [Google Scholar]
- Salonen E. M., Vaheri A., Suni J., Wager O. Rheumatoid factor in acute viral infections: interference with determination of IgM, IgG, and IgA antibodies in an enzyme immunoassay. J Infect Dis. 1980 Aug;142(2):250–255. doi: 10.1093/infdis/142.2.250. [DOI] [PubMed] [Google Scholar]
- Serdula M. K., Halstead S. B., Wiebenga N. H., Herrmann K. L. Serological response to rubella revaccination. JAMA. 1984 Apr 20;251(15):1974–1977. [PubMed] [Google Scholar]
- Sever J. L. Immunoglobulin determinations for the detection of perinatal infections. J Pediatr. 1969 Dec;75(6):1111–1115. doi: 10.1016/s0022-3476(69)80365-3. [DOI] [PubMed] [Google Scholar]
- Tingle A. J., Chantler J. K., Pot K. H., Paty D. W., Ford D. K. Postpartum rubella immunization: association with development of prolonged arthritis, neurological sequelae, and chronic rubella viremia. J Infect Dis. 1985 Sep;152(3):606–612. doi: 10.1093/infdis/152.3.606. [DOI] [PubMed] [Google Scholar]
- Tingle A. J., Pot K. H., Yong F. P., Puterman M. L., Hancock E. J. Kinetics of isotype-specific humoral immunity in rubella vaccine-associated arthropathy. Clin Immunol Immunopathol. 1989 Nov;53(2 Pt 2):S99–106. doi: 10.1016/0090-1229(89)90075-5. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxham M. N., Wolinsky J. S. Detailed immunologic analysis of the structural polypeptides of rubella virus using monoclonal antibodies. Virology. 1985 May;143(1):153–165. doi: 10.1016/0042-6822(85)90104-7. [DOI] [PubMed] [Google Scholar]
- Waxham M. N., Wolinsky J. S. Immunochemical identification of rubella virus hemagglutinin. Virology. 1983 Apr 15;126(1):194–203. doi: 10.1016/0042-6822(83)90471-3. [DOI] [PubMed] [Google Scholar]
- Wolinsky J. S. Subacute sclerosing panencephalitis, progressive rubella panencephalitis, and multifocal leukoencephalopathy. Res Publ Assoc Res Nerv Ment Dis. 1990;68:259–268. [PubMed] [Google Scholar]
- de Mazancourt A., Waxham M. N., Nicolas J. C., Wolinsky J. S. Antibody response to the rubella virus structural proteins in infants with the congenital rubella syndrome. J Med Virol. 1986 Jun;19(2):111–122. doi: 10.1002/jmv.1890190203. [DOI] [PubMed] [Google Scholar]