Abstract
The excitatory amino acid carrier EAAC1 belongs to a family of glutamate transporters that use the electrochemical transmembrane gradients of sodium and potassium to mediate uphill transport of glutamate into the cell. While the sites of cation interaction with EAAC1 are unknown, two cation binding sites were observed in the crystal structure of the bacterial glutamate transporter homologue GltPh. Although occupied by Tl+ in the crystal structure, these sites were proposed to be Na+ binding sites. Therefore, we tested whether Tl+ has the ability to replace Na+ also in the mammalian transporters. Our data demonstrate that Tl+ can bind to EAAC1 with high affinity and mediate a host of different functions. Tl+ can functionally replace potassium when applied to the cytoplasm and support glutamate transport current. When applied extracellularly, Tl+ induces some behavior that mimics that of the Na+-bound transporter, such as activation of the cation-induced anion conductance and creation of a substrate binding site, but it cannot replace Na+ in supporting glutamate transport current. Moreover, our data show a differential effect of mutations to two acidic amino acids potentially involved in cation binding (D367 and D454) on Na+ and Tl+ affinity. Overall, our results demonstrate that the ability of the glutamate transporters to interact with Tl+ is conserved between GltPh and a mammalian member of the transporter family. However, in contrast to GltPh, which does not bind K+, Tl+ is more efficient in mimicking K+ than Na+ when interacting with the mammalian protein.
The excitatory amino acid carrier 1 (EAAC1) belongs to a family of sodium-driven glutamate transporters, which in humans has five known members (1–6). In the central nervous system (CNS), these transporters are responsible for uptake of the excitatory neurotransmitter glutamate from the synaptic cleft, thus contributing to the control of glutamate concentration in the synaptic cleft (7, 8). A loss of control of this glutamate concentration is believed to be correlated with some severe CNS disorders, such as amyotrophic lateral sclerosis (ALS), Huntington’s disease, and Alzheimer’s disease and the pathophysiology of brain insults (e.g., ischemia, hypoxia, hypoglycemia, epilepsy). Thus, it is important to understand the molecular mechanisms underlying glutamate transport by these proteins.
Mammalian glutamate transporters take up glutamate against a transmembrane concentration gradient by cotransporting three sodium ions into the cell and one potassium ion out of cell down their own concentration gradient (7, 9–11). Moreover, one proton is cotransported with glutamate (7, 12). Therefore, the transport process is electrogenic, namely two positive charges are moved into the cell in each transport cycle (4). Although the sites of cation interaction are not known for the mammalian transporters, a recent crystal structure of a bacterial glutamate transporter homologue, the aspartate transporter Gltph from Pyrococcus horikoshii, showed the protein in complex with two thallium ions (13). Tl+ was used instead of Na+ because of its robust anomalous scattering signal. Based on this crystal structure and functional studies, GltPh cotransports one aspartate molecule with two sodium ions, but it is independent of potassium (13). Based on results from competition experiments, it was proposed that the two binding sites of GltPh for Tl+ are sodium binding sites. One of these two proposed sodium binding sites is in the vicinity, but not in direct contact with the bound substrate and is covered by reentrant-loop 2 (Na2 site). The other site (Na1 site) is located near the center part of transmembrane domain 8 (TM8) and the side chain of the highly conserved aspartate residue D405 (D454 in EAAC1) contributes to this site (13). However, in functional studies of mammalian EAAC1 no effect on Na+ binding affinity was observed upon mutagenesis of D454 (14), while a significant effect on Na+ affinity would be expected if this residue is a ligand of the bound Na+ ion. Another highly-conserved aspartate, D367 of EAAC1, was proposed to also be involved in sodium binding (14), but no bound cations were observed in the crystal structure near this residue (13).
Due to these discrepancies in interpretation of the cation effects from structural and functional studies, which may be caused by the use of the non-physiological Tl+ ion in the GltPh studies, we here investigated in detail the effects of Tl+ on the mammalian glutamate transporter EAAC1.
Materials and Methods
Molecular Biology and Transient Expression
Wild-type EAAC1 cloned from rat retina was subcloned into pBK-CMV (Stratagene) as described previously (15) and was used for site-directed mutagenesis according to the QuikChange protocol (Stratagene, La Jolla, CA) as described by the supplier. The primers for mutation experiments were obtained from the DNA core lab, Department of Biochemistry at the University of Miami School of Medicine. The complete coding sequences of mutated EAAC1 clones were subsequently sequenced. Wild-type and mutant EAAC1 constructs were used for transient transfection of sub-confluent human embryonic kidney cell (HEK293T/17, ATCC number CRL 11268) cultures using FuGENE 6 Transfection Reagent (Roche) according to the supplied protocol. Electrophysiological recordings were performed between days 1 to 3 post-transfection.
Electrophysiology
EAAC1 mediated currents were recorded with an Adams & List EPC7 amplifier under voltage-clamp conditions in the whole-cell current-recording configuration. The typical resistance of the recording electrode was 2–3 MΩ the series resistance was 5–8 MΩ In general, EAAC1-mediated currents were small (typically < 500 pA), so series resistance compensation had a negligible effect on the magnitude of the observed currents (< 4% error). Therefore, series resistance was not compensated. For transport currents recorded in the presence of sodium, the extracellular solution contained (in mM): 140 NaCl, 2 CaCl2, 2 MgCl2, and 30 4-(2-Hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), pH 7.4; for transport current recording in the presence of Tl+, the extracellular solution contained (in mM): 20 (or otherwise indicated in text) TlF and 30 HEPES, pH 7.4 (Ca2+ and Mg2+ were not used to avoid precipitation of their F− salts (Ca2+ and Mg2+ have no effect on the function of EAAC1), adjusted to a total cation concentration of 140 mM with N-methylglucamine+ (NMG+). Two different pipette solutions were used: one solution contained (in mM): 140 KCl, 2 MgCl2, 10 ethylene glycol-bis-(beta-aminoethyl ether)-N,N,N′,N′-tetracetic acid (EGTA), and 10 HEPES (pH 7.4/KOH); another one contained (in mM) 140 TlF, 10 EGTA and 10 HEPES (pH 7.4/KOH). The latter one was used to check if Tl+ can replace K+ to mediate transport current.
For the investigation of the effects of Tl+ applied to the extracellular side, we used two methods to ensure outward-facing cation binding sites. In one method, intracellular K+ was used to push the binding sites outward. In the second approach, we used high intracellular [Na+] and [glutamate] (16), which pushed cation binding sites to outward facing through reverse translocation. In this exchange mode (Na+/glutamate homoexchange, or Na+/glutamate/Tl+ heteroexchange) the pipette solution contained (in mM) 140 NaCl/NaSCN, 2 MgCl2, 10 EGTA, 10 glutamate, and 10 HEPES (pH 7.4/NaOH). Thiocyanate was used because it enhances glutamate transporter associated currents and allows the detection of the EAAC1 anion-conducting mode (17). In this mode, concentrations of glutamate are used on the intra- and extracellular side, which saturate their respective binding site.
The currents were low pass filtered at 1–10 kHz (Krohn-Hite 3200), and digitized with a digitizer board (Axon, Digidata 1200) at a sampling rate of 10–50 kHz, which was controlled by software (Axon PClamp). All the experiments were performed at room temperature.
Rapid Solution Exchange
Rapid solution exchange was performed as described previously (15). Briefly, substrates and/or cations (Na+, Tl+) were applied to the EAAC1-expressing cell by means of a quartz tube (opening diameter: 350 µm) positioned at a distance of ~0.5 mm to the cell. The linear flow rate of the solutions emerging from the opening of the tube was ~5–10 cm/s, resulting in typical rise times of the whole-cell current of 30–50 ms (10–90%).
Data Analysis
Non-linear regression fits of experimental data were performed with Origin (Microcal software, Northampton, MA) or Clampfit (pClamp8 software, Axon Instruments, Foster City, CA). Dose response relationships of currents were fitted with a Michaelis-Menten-like equation, yielding Km and Imax. Charge movement—voltage relationship is fitted to Boltzmann function Q(Vm) = Qmin + Qmax · [1 + exp(zQ · (VQ−Vm) · F/(RT))]−1 where F is the Faraday constant, R is the molar gas constant, and T is the temperature. VQ is the midpoint potential of the charge movement, Qmin and Qmax are the minimum and maximum values of the charge movement, respectively, and zQ is the apparent valence. Errors in kinetic parameters are given as standard deviation and were determined from at least 4 independent experiments from at least 3 different cells.
Results
Tl+ interacts with EAAC1 with high affinity
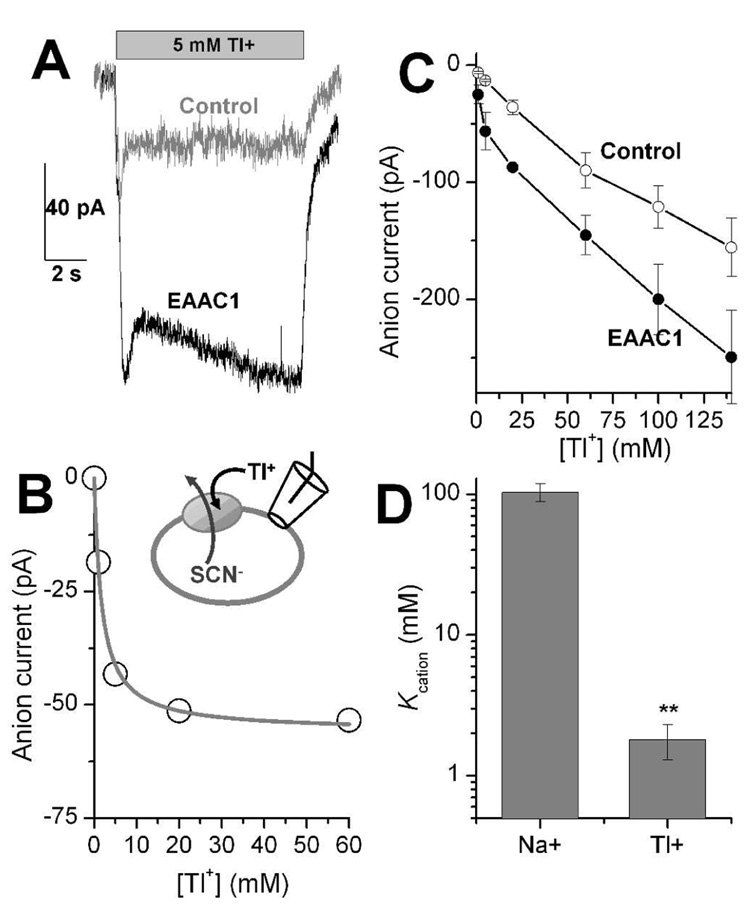
Application of Tl+ to EAAC1-WT expressing cells (in the absence of glutamate) induced an anion current under conditions that favor outward-facing cation and substrate binding sites (140 mM NaSCN and 10 mM glutamate in the pipette solution, see Materials and Methods for details), as shown in Fig. 1A. Only little current was observed upon Tl+ application to non-transfected cells (Fig. 1A, grey trace), indicating that the current is specific for EAAC1. The anion current was abolished when permeable anions were omitted from the intracellular solution. We have used this cation-induced anion current, which was previously also observed for Na+ as the cation (16, 18, 19), as a tool to determine the apparent dissociation constant (Kcation) of the glutamate-free form of EAAC1-WT for Tl+. As shown in Fig. 1B, specific stationary anion currents increased with increasing Tl+ concentration, saturating with an apparent Kcation of 1.8 ± 0.5 mM. EAAC1-specific anion currents were obtained by subtracting unspecific stationary currents, which were measured in control cells from the total currents (containing both specific and unspecific components) measured in EAAC-WT-transfected cells (Fig. 1C, n = 6). Although a current increasing almost linearly with [Tl+] was also seen in control cells, the high-affinity component characteristic of the specific interaction of Tl+ with EAAC1 was missing in the control-cell response (Fig. 1C, open circles). The exact origin of this unspecific current, which is most likely an anion current, was not determined here, but it could be either induced by the unspecific binding of Tl+ to the membrane, or by an unspecific effect of Tl+ on EAAC1-independent anion conductances. Notably, Tl+ interaction with EAAC1 is of much higher affinity than that with Na+, with an apparent dissociation constant obtained from the sodium-induced anion current of 103 ± 14 mM (summarized in Fig. 1D, n = 4), in agreement with previous results (14). Although Na+ interacts with EAAC1 with lower affinity, the specific anion current (SCN− in the pipette) induced by 140 mM Na+, which is only about half saturating concentration, is −220 ± 10 pA (n = 3) which is about 2.5 folds larger than that induced by saturating Tl+, indicating that the EAAC1:Tl+ complex is less anion conducting than the EAAC1:Na+ complex.
Figure 1.
Tl+ interacts with EAAC1 with higher affinity than sodium. (A) Typical traces of anion current (SCN− efflux) induced by 5 mM Tl+ in a control (non-transfected) cell (grey) and an EAAC1-WT expressing cell (black) in the absence of glutamate. (B) Tl+ concentration dependence of specific anion currents carried by EAAC1-WT. The specific component of the current was determined by subtracting the Tl+-induced stationary current measured in control (non-transfected) cells (open circles) from that measured in EAAC1-transfected cells (closed circles), as shown in (C). (D) Comparison of the Kcation determined for Tl+ and Na+ activation of anion current in the absence of glutamate. The stars indicate statistical significance at the p<0.005 level (Student’s t-test). All experiments were done under exchange conditions with 140 mM NaSCN and 10 mM glutamate in the pipette (V = 0 mV).
Tl+ interaction with EAAC1 is associated with charge movement
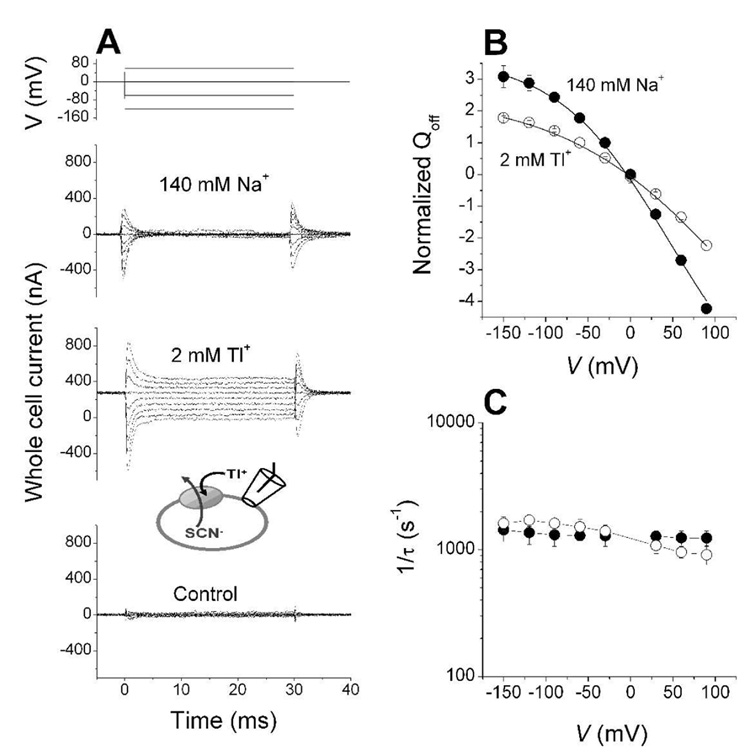
A transient capacitive current was observed in response to voltage jumps applied to EAAC1-expressing cells in the presence of Na+ and in the absence of glutamate (Fig. 2A), consistent with previous reports (20, 21). This transient current was proposed to be caused by the electrogenic binding of sodium to the glutamate-free form of transporter (or a conformational change associated with it). In order to determine if the transporter displays the same behavior in the presence of Tl+, we repeated the voltage jump experiment after replacing 140 mM Na+ with 2 mM Tl+. Permeable anions such as Cl− or SCN− were replaced with the impermeable anion methanesulfonate (Mes−) to avoid contamination of the signal with anion current. Current traces specific for EAAC1 were obtained by subtracting currents recorded in the presence of TBOA from those in its absence (TBOA binds to EAAC1 in the presence of Tl+, as discussed below). As shown in Fig. 2A, transient currents were observed in addition to a steady-state current component, which was absent in the experiment performed in Na+ solution and which was not further examined here. Both transient and steady-state currents were absent in non-transfected cells (Fig. 2A, bottom panel).
Figure 2.
Tl+ interaction with the glutamate transporter in the absence of glutamate is associated with transmembrane charge movement. (A) Time dependence of currents in response to voltage jumps (voltage protocol shown in the upper panel) in the presence of 140 mM Na+ and 2 mM Tl+. Control (non-transfected) cells did not respond to voltage jumps in the presence of Tl+ (lower panel). The signals shown are the TBOA-sensitive components of the currents, obtained by subtraction of the signals in the absence and presence of TBOA (100 µM). (B) Voltage dependence of the normalized charge movement (obtained by integrating the transient component of the off current over time) in the presence of 140 mM Na+ (closed circles) and 2 mM Tl+ (open circles, off charge movement). The lines represent calculations according to the Boltzmann equation with zQ = 0.38 and Kcation=70 mM at 140 mM Na+ and zQ = 0.25 and Kcation = 0.6 mM at 2 mM Tl+. (C) Rate constants of the decay of the transient current (on current) induced by 2 mM Tl+ (open circles) and 140 mM Na+ (closed circles). All experiments were done under exchange conditions with 140 mM NaMes and 10 mM glutamate in the pipette. The low-pass filter used was 3 kHz, resulting in an effective time resolution of about 5-times the measured decay rates.
The voltage dependence of the voltage jump-induced charge movement, Q, which was obtained by integrating the transient current over time, is shown in Fig. 2B (n = 5). At 2 mM Tl+, a concentration close to the Kcation for Tl+, the charge movement starts saturating at very negative potential, but it is far from saturating at positive potentials, making a full Boltzmann-type analysis from fitting the data difficult. However, the data are consistent with a Boltzmann curve with the parameters zQ = 0.25 and Kcation = 0.6 mM for Tl+ association with EAAC1, as shown by the solid line in Fig. 2B. For Na+ as the cation, the data are consistent with previous reports (20, 21) with zQ = 0.38 and Kcation = 70 mM. The Kcation for Tl+ is three times smaller than that obtained by Tl+ concentration jump experiment discussed above. This difference is not significant mainly because of the lack of saturation of the charge movement at positive potentials, which leads to increased uncertainty of the parameters obtained from the Boltzmann analysis. With that said, comparison of both of these two Kcation values for Tl+ with that for Na+ indicate clearly that the affinity of EAAC1 for Tl+ is much higher than that for Na+.
At 2 mM Tl+, the rate constants of the decay of the transient currents were 1700 ± 100 s−1(Fig. 2C, n = 6). Like in Na+ (16), these rate constants were virtually voltage independent (Fig. 2C, n = 6).
Mutations of two conserved acidic amino acid residues have differential effects on Tl+ and Na+ affinity
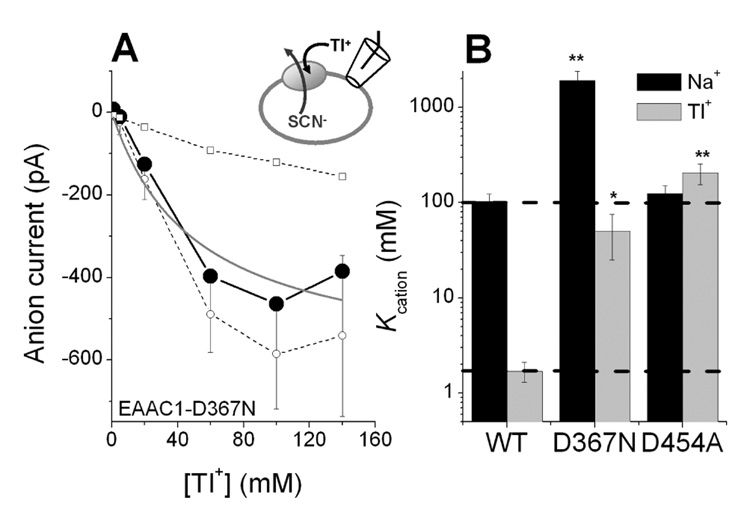
It was previously shown that neutralization of D367, which is highly conserved among all members of the solute carrier 1 (SLC1) family, and which is located in the middle of transmembrane domain seven (TM7), results in a dramatically decreased affinity of the transporter for Na+, suggesting a potential involvement of this residue in cation binding (14). Therefore, we performed the analogous concentration jump experiments with Tl+ as the cation, in order to determine if mutation of this residue has the same effect on Tl+ affinity of the glutamate-free form of the transporter. As shown in Fig. 3A, application of Tl+ to an EAAC1-D367N expressing cell induced [Tl+]-dependent inward anion currents (SCN− outflow). This inward anion current saturated at Tl+ concentrations higher than 60 mM (Fig. 3A, filled circles). The Tl+-induced currents in EAAC1-D367N were significantly larger than those observed in control cells (Fig. 3A, open squares). They were also much larger than the currents induced by Tl+ in EAAC1-WT (60 mM Tl+ induced anion current in EAAC1-WT was −53 ± 33 pA; 60 mM Tl+ induced anion current in EAAC1-D367N was −400 ± 90 pA, which is 7 fold larger than that of wild type; see also Fig. 1), consistent with previous results that showed larger Na+-induced anion currents in EAAC1-D367N than in the wild-type transporter (14). The current-[Tl+] relationship can be fitted with a Hill equation with a Hill coefficient of n = 3.6 and Kcation = 25 ± 5 mM. The Hill coefficient of n = 3.6 is somewhat unexpected, because there is only one Tl+ binding site observed in the substrate-free form of GltPh. Therefore, and because Na+ activates anion current in EAAC1-WT without apparent cooperativity, a Hill coefficient of n = 1 was expected. This deviation from n = 1 indicates that substrate-free EAAC1 may have more than one Tl+ binding site. In any case, it is clear from comparison with the EAAC1-WT data (Fig. 1) that Tl+ induced anion current with a significantly lower affinity, which we estimated to be in the range between 25 and 50 mM (see solid line in Fig. 3A calculated with n = 1, Kcation = 50 ± 25 mM). Therefore, the affinity of the glutamate-free form of EAAC1-D367N for Tl+ is decreased at least 14 fold compared to wild type EAAC1, which is consistent with the 15-fold effect of the mutation on the Na+ affinity. To summarize, the D367N mutation has an equivalent inhibitory effect on both Na+ and Tl+ interaction with EAAC1.
Figure 3.
Differential effect of D367N and D454A mutations on affinities for Na+ and Tl+. (A) Tl+ concentration dependence of specific anion currents in EAAC1-D367N-expressing cells (filled circles). The specific component of the current was determined by subtracting the Tl+-induced current measured in control (non-transfected) cells (open squares) from that measured in EAAC1-transfected cells (open circles). The solid line represents a calculation according to the Michaelis-Menten equation with Kcation=50 mM. (B) Comparison of Kcation values obtained for Na+ (black bars) and Tl+ (light grey bars) activation of anion current in EAAC1-WT and the two mutant transporters. ** and * indicate statistical significance at the p<0.005 and 0.05 levels, respectively. All experiments were done under exchange conditions with 140 mM NaSCN and 10 mM glutamate in the pipette (V = 0 mV).
A second acidic amino acid residue, D454, is also highly conserved among all SLC1 members and is located in the central region of TM8. In contrast to D367N, neutralization of this aspartate by mutation to asparagine had only little effect on the sodium affinity of the glutamate-free form of the transporter (14). To further test this finding, we determined the Na+ affinity of a transporter with a less conservative mutation, D454A, which in contrast to D454N has no side chain oxygen that could potentially contribute a ligand to cation coordination. A Kcation of about 130 mM was found (Fig. 3B, black bars), confirming our previous results that showed minor effects of side chain neutralization in this position on cation affinity. Next, we studied the effect of the D454A mutation on Tl+ binding. In contrast to the results obtained for Na+, the Kcation for Tl+ was dramatically reduced by the D454A mutation (Kcation ~ 210 mM, Fig. 3B white bars), which is about 110 fold higher than that of wild-type EAAC1. All the mutagenesis results are summarized in Fig. 3B. They suggest that mutations in position D454 have an inhibitory effect on Tl+ interactions with the transporter, consistent with a recent study on GltPh (13), but only a minor inhibitory effect, if any, on Na+ interaction.
Amino acid substrate binds to EAAC1 in the presence of Tl+
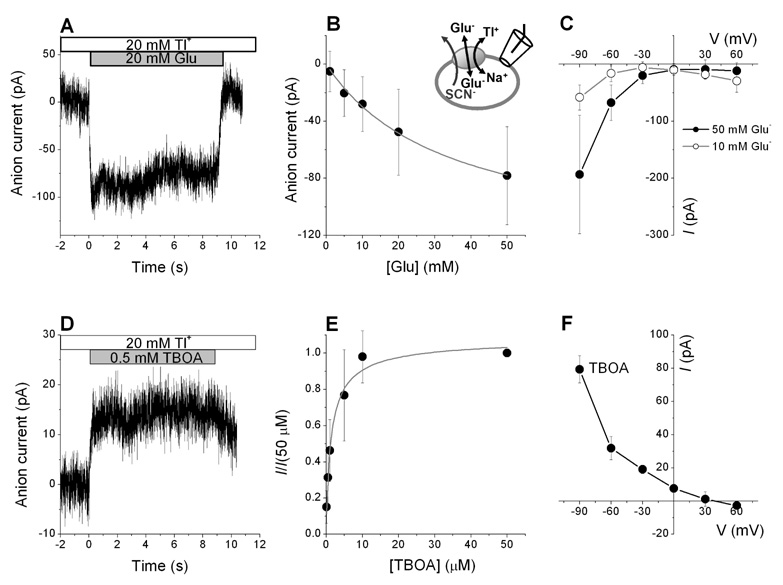
Under exchange conditions (with SCN− in the pipette), application of glutamate induced an anion current when Tl+ was the only extracellular cation present (Fig. 4A). As expected, this glutamate induced anion current was highly voltage dependent, showing the typical I-V-profile previously observed in the presence of Na+ for SCN− outflow, with higher electrical driving force resulting in larger inward currents (Fig. 4C). The currents were also glutamate concentration dependent (Fig. 4B). The Km for glutamate at 20 mM Tl+ was 33 ± 6 mM, which is about 2,000 times higher than that at 140 mM Na+. These results demonstrate that glutamate can bind to the Tl+-bound form of EAAC1, but only with low affinity.
Figure 4.
Amino acid substrates interact with the Tl+-bound transporter. (A) Typical anion current induced by 20 mM glutamate at 20 mM Tl+, V = −60 mV. (B) Determining the apparent dissociation constant (Km) of Tl+-bound EAAC1 for glutamate. Based on a fit of the Michaelis-Menten equation to the data (solid line), the Km of EAAC1 for glutamate is 34 ± 6 mM at 20 mM Tl+. (C) Voltage dependence of glutamate-induced anion currents at 20 mM Tl+ and 10 mM glutamate (open circles) and 50 mM glutamate (closed circles). (D) TBOA inhibits the leak anion current in the presence of 20 mM Tl+. (V = −60 mV). (E) Determining the apparent dissociation constant (Km) of EAAC1 for TBOA at 20 mM Tl+ (Km = 1.4 ± 0.4 µM, V = 0 mV). (F) Voltage dependence of 500 µM TBOA-induced anion current at 20 mM Tl+. All experiments were performed under exchange conditions with 140 mM NaSCN and 10 mM glutamate in the pipette.
Next, we tested the effect of TBOA in the presence of Tl+. Application of 0.5 mM TBOA to EAAC1-WT at 20 mM Tl+ induced an apparent outward current (Fig. 4D). This current was strongly voltage dependent with a larger current observed at negative membrane potentials (Fig. 4F). Consistent with previous results obtained in the presence of Na+ (15), these data indicate that the outward current is due to the inhibition of the leak outflow of SCN− induced by the sole presence of Tl+ (see also Fig. 1). By measuring the current at different TBOA concentrations (Fig. 4E), the apparent inhibition constant (Ki) of EAAC1-WT for TBOA was determined as 1.4 ± 0.4 µM at 20 mM Tl+ which is similar to that at 140 mM Na+ (1.4 ± 0.3 µM). Based on the data mentioned above, TBOA binding is almost unaffected by the replacement of Na+ with Tl+ in extracellular solution, while glutamate binding is strongly affected.
Tl+ inhibits Na+-dependent transport current
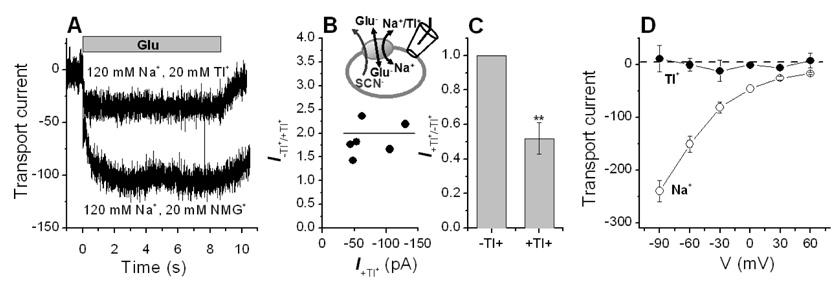
Next, we checked the effect of Tl+ on Na+-dependent transport current when it coexists with Na+ in the extracellular solution. Transport current induced by 1 mM glutamate was measured under forward transport conditions with KMes in the pipette and at 120 mM extracellular Na+ (Fig. 5A, 20 mM NMG+ was added to adjust the total cation concentration to 140 mM). In the absence of Tl+, the transport current induced by 1mM glutamate at 0 mV was 130 ± 30 pA. After replacing 20 mM NMG+ with 20 mM Tl+, the current was 60 ± 37 pA, which is less than half of that with NMG+ in solution (Fig. 5A). The relative inhibition of this current was independent of the absolute size of the transport current (Fig. 5B) and is quantified in Fig. 5C.
Figure 5.
Tl+ inhibits Na+-mediated glutamate transport, but does not support glutamate transport current. (A) Typical transport currents induced by 0.1 mM glutamate at 120 mM Na+ and 20 mM Tl+ (upper trace), or 120 mM Na+ and 20 mM NMG+ (lower trace). (B) Relative inhibition of the transport current by Tl+ is independent of the magnitude of the expression level (5 cells). (C) Quantification and statistical analysis of the transport current inhibition (** indicates significance at the p < 0.005 level). (D) Voltage-transport current relationships at 140 mM Tl+ (closed circles, 20 mM glutamate) and at 140 mM Na+ (open circles, 0.1 mM glutamate). All experiments were done in the forward transport mode with 140 mM KMes in the pipette.
When glutamate was applied to the cells in the presence of 20 mM Tl+, but in the absence of extracellular Na+, no transport currents were observed, while Na+ catalyzed large transport currents in the same cells (Fig. 5D). Together, these results suggest that Tl+ inhibits Na+-dependent transport current, but that it cannot support glutamate transport by itself.
Tl+ can replace intracellular potassium to mediate transport current
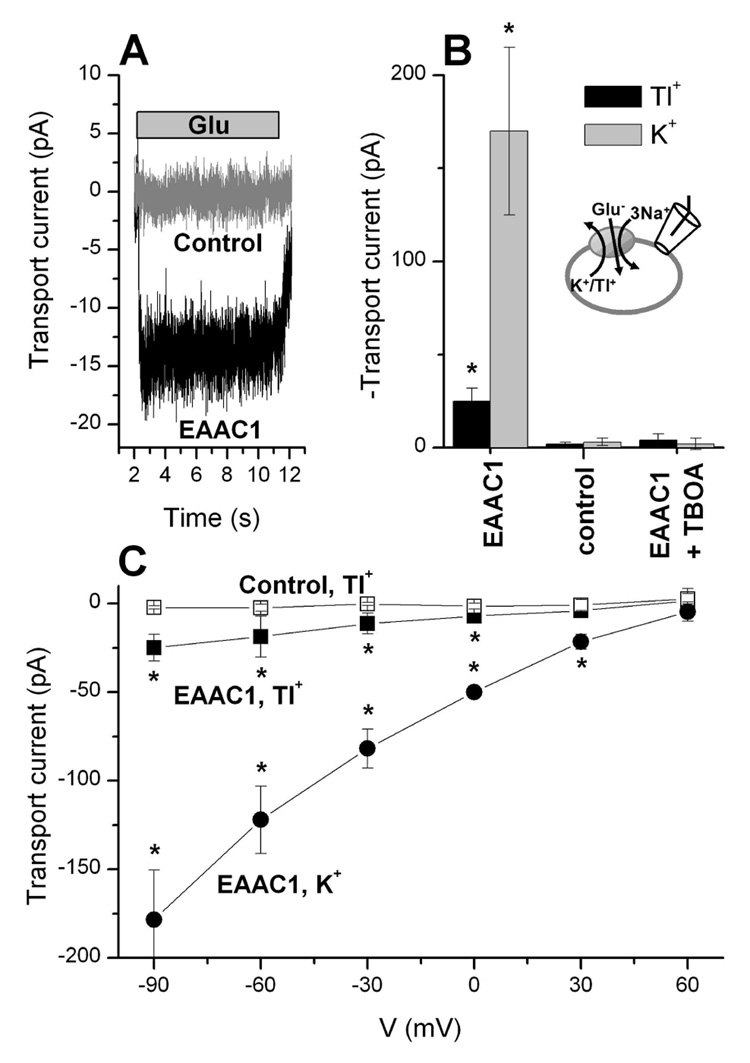
So far, our data indicate that Tl+ mediates effects similar to Na+ when present in the extracellular solution, but that it cannot replace sodium to mediate transport current. Because Tl+ is traditionally viewed as a K+ congener (22), due to its size, we wanted to know whether Tl+ can replace potassium in the intracellular solution in catalyzing the cation-driven transporter relocation and, ultimately, to mediate transport current. Whole cell transport current induced by 0.1 mM extracellular glutamate was recorded at 140 mM extracellular Na+ and 140 mM intracellular Tl+ (without K+). The extracellular anion was Mes− and the intracellular anion was F−, both of which do not permeate the anion conductance (16, 17). At 0 mV the transport current was small, but inward directed (Fig. 6A), as expected for the forward transport ionic conditions used in the experiment. No current was observed under the same conditions in non-transfected cells (Figs. 6A and 6B). With an increased electrical driving force at −60 mV membrane potential, the steady-state current in the presence of Tl+ was −25 ± 7 pA (n = 5), which is significant over background, but smaller than the K+-induced current of −170 ± 45 pA (Fig. 6B, n = 6). The transport current—voltage relationship is shown in Fig. 6C and shows exponential increase of the current with increasingly negative membrane potential. The Tl+-dependent transport current is specific for EAAC1, as it can be blocked by the co-application of extracellular TBOA (Fig. 6B).
Figure 6.
Intracellular Tl+ supports glutamate transport. (A) Typical transport current generated by application of 1 mM glutamate (bar) in the presence of 140 mM intracellular Tl+ in EAAC1-expressing and non-transfected control cells (V = 0 mV). The counteranion was fluoride, which does not generate anion current. (B) Quantification of transport currents in the presence of intracellular K+ (grey bars) and Tl+ (black bars) and their inhibition by 100 µM TBOA. (C) Voltage dependence of glutamate-induced transport currents in the presence of intracellular K+ (circles) and Tl+ in EAAC1-expressing (filled squares) and control cells (open squares). The stars indicate statistical significance with respect to control at the p < 0.05 level, as determined by two-way ANOVA.
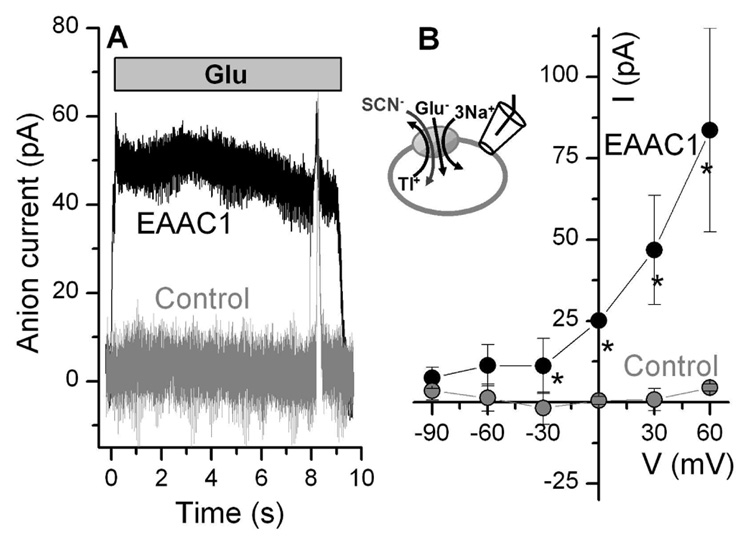
To further test Tl+’s ability to induce the cation-induced relocation reaction, we determined whether anion current is induced by extracellular glutamate application in the presence of intracellular Tl+ (forward transport conditions). It was shown previously that steady state anion current is only observed when an intracellular cation is present that supports relocation, such as K+, whereas intracellular choline+ did not support anion current (15). Consistent with this result, anion current was induced by extracellular glutamate in EAAC1-expressing cells with Tl+ as the sole intracellular cation (25 ± 7 pA, Fig. 7A), whereas no such currents were seen in non-transfected cells. This anion current was outward, as expected, as it is caused by SCN− inflow. The voltage dependence of the current is shown in Fig 7B and is consistent with an anion current, as no reversal of the current was observed, even at very negative potentials (infinite inward-directed SCN− concentration gradient). Together with the results on Tl+-induced transport current, these data suggest that Tl+ can replace K+ to catalyze the cation-dependent relocation reaction of glutamate transporters, consistent with Tl+’s predicted role as a K+ congener.
Figure 7.
Extracellular glutamate induces anion current in the presence of intracellular Tl+. (A) Typical anion current recordings (SCN− influx) from EAAC1-transfected and control (non-transfected) cells in the presence of 1 mM glutamate (application time indicated by bar) and 140 mM intracellular Tl+ (V = 0 mV). (B) Voltage dependence of glutamate-induced anion currents. The experiments were done under forward transport conditions, as illustrated in the inset. The stars indicate statistical significance with respect to control at the p < 0.05 level, as determined by two-way ANOVA.
Discussion
In this paper, we studied the effects of thallium ions on the functional properties of the mammalian glutamate transporter EAAC1. The major conclusion is that Tl+, which is typically used as an analogue of potassium with higher atomic weight (22), can replace both K+ and Na+ in EAAC1 and mediate partial function (by replacing Na+) or full function (by replacing potassium) of this protein.
Tl+ can replace intracellular potassium to mediate transport current
When Tl+ was used to replace potassium at the intracellular side of the membrane, a small, but significant voltage dependent inward current was observed, which was most likely mediated by electrogenic inward glutamate transport, coupled to the countertransport of Tl+. Although this transport current, which was inhibited by the competitive inhibitor TBOA, was about 7-fold less than that when potassium was used to drive countertransport, the data indicate that Tl+ can replace intracellular K+ to support the cation-dependent relocation process, and, thus, transport current. This result is in line with previous reports on the ability of Tl+ to act as a K+ congener in transport proteins, most notably in the Na/K-ATPase (22, 23). Moreover, the ionic radii of Tl+ and K+ are very similar to each other (r = 1.40 Å for Tl+, r = 1.33Å for K+), supporting the hypothesis of a specific interaction of Tl+ with the EAAC1 K+ binding site. However, Tl+ is less efficient than potassium in driving relocation, resulting in a significantly reduced turnover rate in the presence of Tl+. In previous work by the Jahr group (24), Cs+ was found to be able to replace intracellular K+ and support glutamate transport, but with much lower rate. Together with our data on Tl+ interaction with the glutamate-free transporter, these results indicate that the cation specificity of the K+ binding site of glutamate transporters is low. This is not unusual for transport proteins, as the serotonin transporter, which belongs to the SLC6 family, is also potassium dependent, but the function of potassium can be replaced by a proton (25).
Tl+ interaction with the glutamate-free form of EAAC1 resembles that of Na+, but is of higher affinity
When applied to the extracellular side of the membrane Tl+ induces functional behavior in EAAC1 that resembles that of the Na+-bound transporter. However, despite some similarities in function, there are also differences in the properties. First, Tl+ binds with about 50-fold higher affinity to EAAC1 as Na+. Second, the amplitude of the anion current induced by nearly saturating concentration of Tl+ (20 mM) is much smaller than that induced by half saturating concentrations of Na+ (140 mM). The exact reason is unknown, but three possibilities (separate from each other or in combination) can be considered. 1) The conformations of EAAC1 induced by Tl+ binding or Na+ binding are different, raising the possibility that the pathways for the anion in these two cases are different; 2) the bigger bound Tl+ ion obstructs the anion-conducting pathway; 3) Tl+ is in the anion pathway and is involved in binding of the anion, but due to its different ionic properties anion binding is altered. Although our data do not allow the distinction of these possibilities, it has to be considered that, compared to Na+, Tl+ has a has a bigger ionic radius (r = 0.95 Å for Na+) and is a highly polarizable ion. Thus, differential function of the Tl+ and Na+-bound transporters is highly plausible.
The involvement of the D367 and D454 residues in cation binding
Neutralization of the highly conserved acidic amino acid residue D367 by mutation to asparagine (D367N) has been shown to decrease the affinity of Na+ to the glutamate-free form of EAAC1 (14). Therefore, it was hypothesized that D367 contributes, directly or indirectly, to a cation binding site, which is deeply buried in the transmembrane domain of the transporter. Here, we show that the affinity of Tl+ to the glutamate-free form of transporter is also decreased by D to N mutation in position D367, by about the same factor as Na+ affinity is decreased. This result indicates that D367 in EAAC1 is involved in both Na+ and Tl+ binding. In the crystal structure of GltPh (13), no electron density assignable to a bound Tl+ ion was observed near D312 (D367 in EAAC1). This could mean that the effect of the D367 mutation on cation binding is indirect. Two other possibilities have to be considered: 1) Based on the proposed stoichiometry of GltPh, two sodium ions (instead of three in EAAC1) bind to the transporter. Therefore, one cation binding site is not conserved between the two proteins. While D367 may contribute to this non-conserved binding site in EAAC1, this site is not present in GltPh. 2) D312 of GltPh may contribute to a third sodium binding in GltPh, but the affinity of this binding site for thallium ions is so low that no Tl+ can be observed in the crystal structure. While further studies will be necessary to resolve this issue, the results presented here are compatible with the hypothesis of an additional cation binding site near D367 that is not observed in GltPh.
In the GltPh crystal structure (13), a Tl+ ion was observed with the side chain of D405 as one of the ligands (D454 in EAAC1). Electron density from this bound Tl+, as well as Na+ binding to one of the two cation binding sites, was reduced when D405 was mutated to asparagine, indicating a destabilization of the bound cation by the mutation to the Na1 site of GltPh. In our previous work (14), the analogous D454N in EAAC1 mutation was shown to have little effect on sodium binding affinity of the glutamate-free form of the transporter, making a direct contribution to Na+ binding unlikely. Here, we studied another, less conservative mutant in the same position, D454A. While EAAC1-D454N had unchanged affinity for Na+ compared to the wild-type transporter, the D454A mutation had a moderate effect on Na+ affinity (less than 3-fold decrease in affinity). However, a dramatic effect on Tl+ affinity was observed for the D454A mutation (about 100-fold reduction in affinity). This data strongly indicates that, like D405 in GltPh, D454 in EAAC1 contributes a ligand to a Tl+ binding site. However, the relatively small effect of the mutations on Na+ binding is more ambiguous and argues against an important contribution of the EAAC1 cation binding site analogous to the GltPh Na1 site to Na+ binding. Therefore, the possibility has to be considered that the GltPh Na1 site is predominantly a potassium binding site in the mammalian transporters.
The Tl+-bound transporter binds TBOA and glutamate, but does not support glutamate transport
Our data show that, like Na+, Tl+ supports binding of the competitive inhibitor TBOA. The affinity of EAAC1 for TBOA is similar in the Tl+-bound as in the Na+-bound transporter. In contrast, glutamate binding to the transporter, while still possible in the presence of Tl+, occurs with dramatically reduced affinity in comparison to Na+ binding. This result is consistent with data on aspartate affinity of GltPh, which is about 100-fold higher in the presence of Na+ than in the presence of Tl+ (13). Therefore, it appears that the nature of the bound cation has a strong impact on the affinity and, most likely, the geometry of the substrate binding site. In a previous study it was shown that the nature of the amino acid substrate affects the affinity of the transporter for Na+ (21). Moreover, it was demonstrated that the affinity for the amino acid substrate is different for Na+ and Li+-bound transporters (26). Our data on reduced glutamate interaction with the Tl+-bound transporter add to the results from these reports, indicating that the bound amino acid substrate and the cation (presumably in the Na2 site) interact with each other.
In contrast to extracellular Na+, extracellular Tl+ cannot support glutamate transport, even when Tl+ is present at saturating concentrations. This result is consistent with data published on aspartate transport by GltPh, which is also inhibited in the presence of Tl+ (13). This suggests that the conformational changes most likely associated with substrate translocation and transport are dramatically slower in the Tl+-bound form, or that they do not occur at all.
In conclusion, our data demonstrate that Tl+ can interact with the mammalian glutamate transporter EAAC1 with high affinity. When applied intracellularly, it can replace K+ to mediate substrate transport. In contrast Tl+ is a less efficient congener for Na+, which it can replace to mediate cation-dependent and glutamate-dependent anion currents, but which it cannot replace to mediate glutamate transport. Two mutations to conserved amino acid residues potentially contributing to cation sites (D367N and D454A) mediate differential effects on Tl+ and Na+ affinity. The mutagenesis results indicate that while D367 is important for general cation affinity, D454 may contribute to coordinating Tl+ (and possibly K+), but not Na+. Overall, our data highlight the conserved nature of the Tl+ effect on bacterial and mammalian glutamate transporters, but also show differences due to the ability of the mammalian transporters to transport potassium, which can be efficiently replaced by Tl+.
Acknowledgments
This work was supported by grants of the National Institutes of Health (R01-NS049335 and R56 NS049335-06) to CG, and by the American Heart Association 0525485B to ZT.
Abbreviations
- EAAC1
Excitatory amino acid carrier 1
- CNS
Central nervous system
- ALS
amyotrophic lateral sclerosis
- GltPh
Glutamate/Aspartate transporter from pyrococcus horikoshii
- TBOA
dl-threo-β–benzyloxyaspartate
- EGTA
Ethylene glycol-bis(2-aminoethylether)-N,N,N',N'-tetraacetic acid
- HEK
Human embryonic kidney
- HEPES
4-(2-Hydroxyethyl)piperazine-1-ethanesulfonic acid
- Mes
Methanesulfonate
- NMG+
N-methylglucamine+
- SLC
Solute carrier
References
- 1.Storck T, S S, Hofmann K, Stoffel W. Structure, expression, and functional analysis of a Na+-dependent glutamate/aspartate transporter from rat brain. Proc. Natl. Acad. Sci. USA. 1992;89:10955–10959. doi: 10.1073/pnas.89.22.10955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Danbolt NC, Storm Mathisen J, Kanner BI. A Sodium and Potassium Coupled L Glutamate Transporter Purified From Rat Brain Is Located in Glial Cell Processes. Neuroscience. 1992;51:295–310. doi: 10.1016/0306-4522(92)90316-t. [DOI] [PubMed] [Google Scholar]
- 3.Pines G, Danbolt NC, Bjoras M, Zhang Y, Bendahan A, Eide L, Koepsell H, Storm Mathisen J, Seeberg E, Kanner BI. Cloning and Expression of a Rat Brain L Glutamate Transporter. Nature. 1992;360:464–467. doi: 10.1038/360464a0. [DOI] [PubMed] [Google Scholar]
- 4.Kanai Y, Hediger MA. Primary Structure and Functional Characterization of a High-Affinity Glutamate Transporter. Nature. 1992;360:467–471. doi: 10.1038/360467a0. [DOI] [PubMed] [Google Scholar]
- 5.Fairman WA, Vandenberg RJ, Arriza JL, Kavanaugh MP, Amara SG. An excitatory amino-acid transporter with properties of a ligand-gated chloride channel. Nature. 1995;375:599–603. doi: 10.1038/375599a0. [DOI] [PubMed] [Google Scholar]
- 6.Arriza JL, Eliasof S, Kavanaugh MP, Amara SG. Excitatory amino acid transporter 5, a retinal glutamate transporter coupled to a chloride conductance. Proc Natl. Acad. Sci. USA. 1997;94:4155–4160. doi: 10.1073/pnas.94.8.4155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zerangue N, Kavanaugh MP. Flux coupling in a neuronal glutamate transporter. Nature. 1996;383:634–637. doi: 10.1038/383634a0. [DOI] [PubMed] [Google Scholar]
- 8.Tanaka K, Watase K, Manabe T, Yamada K, Watanabe M, Takahashi K, Iwama H, Nishikawa T, Ichihara N, Kikuchi T, Okuyama S, Kawashima N, Hori S, Takimoto M, Wada K. Epilepsy and Exacerbation of Brain Injury in Mice Lacking the Glutamate Transporter GLT-1. Science. 1997;276:1699–1702. doi: 10.1126/science.276.5319.1699. [DOI] [PubMed] [Google Scholar]
- 9.Kanner BI, Bendahan A. Binding order of substrates to the sodium and potassium ion coupled L-glutamic acid transporter from rat brain. Biochemistry. 1982;21:6327–6330. doi: 10.1021/bi00267a044. [DOI] [PubMed] [Google Scholar]
- 10.Billups B, Rossi D, Attwell D. Anion conductance behavior of the glutamate uptake carrier in salamander retinal glial cells. J. Neurosci. 1996;16:6722–6731. doi: 10.1523/JNEUROSCI.16-21-06722.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levy LM, Warr O, Attwell D. Stoichiometry of the glial glutamate transporter GLT-1 expressed inducibly in a Chinese hamster ovary cell line selected for low endogenous Na+-dependent glutamate uptake. J. Neurosci. 1998;18:9620–9628. doi: 10.1523/JNEUROSCI.18-23-09620.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Watzke N, Rauen T, Bamberg E, Grewer C. On the mechanism of proton transport by the neuronal excitatory amino acid carrier 1. J. Gen. Physiol. 2000;116:609–622. doi: 10.1085/jgp.116.5.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boudker O, Ryan RM, Yernool D, Shimamoto K, Gouaux E. Coupling substrate and ion binding to extracellular gate of a sodium-dependent aspartate transporter. Nature. 2007;445:387–393. doi: 10.1038/nature05455. [DOI] [PubMed] [Google Scholar]
- 14.Tao Z, Zhang Z, Grewer C. Neutralization of the Aspartic Acid Residue Asp-367, but Not Asp-454, Inhibits Binding of Na+ to the Glutamate-free Form and Cycling of the Glutamate Transporter EAAC1. J. Biol. Chem. 2006;281:10263–10272. doi: 10.1074/jbc.M510739200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grewer C, Watzke N, Wiessner M, Rauen T. Glutamate translocation of the neuronal glutamate transporter EAAC1 occurs within milliseconds. Proc. Natl. Acad. Sci. USA. 2000;97:9706–9711. doi: 10.1073/pnas.160170397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Watzke N, Bamberg E, Grewer C. Early intermediates in the transport cycle of the neuronal excitatory amino acid carrier EAAC1. J. Gen. Physiol. 2001;117:547–562. doi: 10.1085/jgp.117.6.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wadiche JI, Amara SG, Kavanaugh MP. Ion fluxes associated with excitatory amino acid transport. Neuron. 1995;15:721–728. doi: 10.1016/0896-6273(95)90159-0. [DOI] [PubMed] [Google Scholar]
- 18.Otis TS, Jahr CE. Anion currents and predicted glutamate flux through a neuronal glutamate transporter. J. Neurosci. 1998;18:7099–7110. doi: 10.1523/JNEUROSCI.18-18-07099.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Watzke N, Grewer C. The anion conductance of the glutamate transporter EAAC1 depends on the direction of glutamate transport. FEBS Lett. 2001;503:121–125. doi: 10.1016/s0014-5793(01)02715-6. [DOI] [PubMed] [Google Scholar]
- 20.Wadiche JI, Arriza JL, Amara SG, Kavanaugh MP. Kinetics of a human glutamate transporter. Neuron. 1995;14:1019–1027. doi: 10.1016/0896-6273(95)90340-2. [DOI] [PubMed] [Google Scholar]
- 21.Tao Z, Grewer C. Cooperation of the Conserved Aspartate 439 and Bound Amino Acid Substrate Is Important for High-Affinity Na+ Binding to the Glutamate Transporter EAAC1. J. Gen. Physiol. 2007;129:331–344. doi: 10.1085/jgp.200609678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rossi RC, Norby JG. Kinetics of K(+)-stimulated dephosphorylation and simultaneous K+ occlusion by Na, K-ATPase, studied with the K+ congener Tl+. The possibility of differences between the first turnover and steady state. J. Biol. Chem. 1993;268:12579–12590. [PubMed] [Google Scholar]
- 23.Jensen J, Nørby J. Thallium binding to native and radiation-inactivated Na+/K+-ATPase. Biochim. Biophys. Acta. 1989;985:248–254. doi: 10.1016/0005-2736(89)90409-4. [DOI] [PubMed] [Google Scholar]
- 24.Bergles DE, Tzingounis AV, Jahr CE. Comparison of Coupled and Uncoupled Currents during Glutamate Uptake by GLT-1 Transporters. J. Neurosci. 2002;22:10153–10162. doi: 10.1523/JNEUROSCI.22-23-10153.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Keyes SR, Rudnick G. Coupling of transmembrane proton gradients to platelet serotonin transport. J. Biol. Chem. 1982;257:1172–1176. [PubMed] [Google Scholar]
- 26.Menaker D, Bendahan A, Kanner BI. The substrate specificity of a neuronal glutamate transporter is determined by the nature of the coupling ion. J. Neurochem. 2006;99:20–28. doi: 10.1111/j.1471-4159.2006.04003.x. [DOI] [PubMed] [Google Scholar]