Abstract
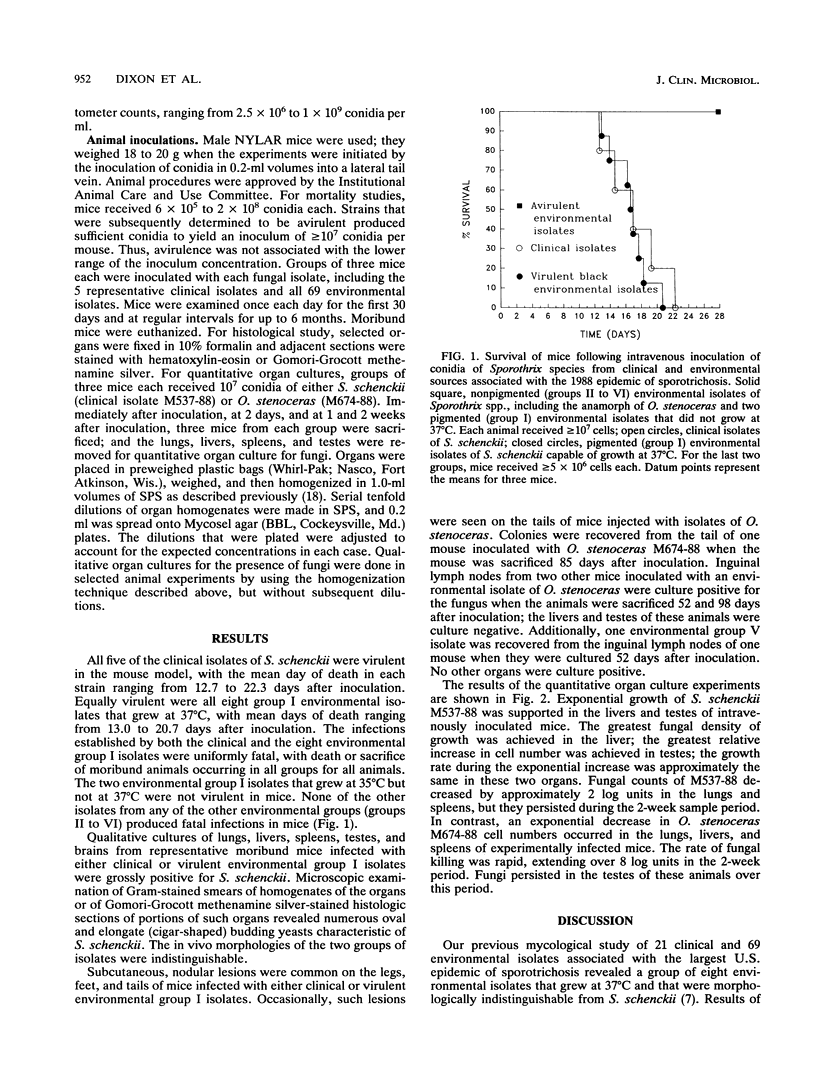
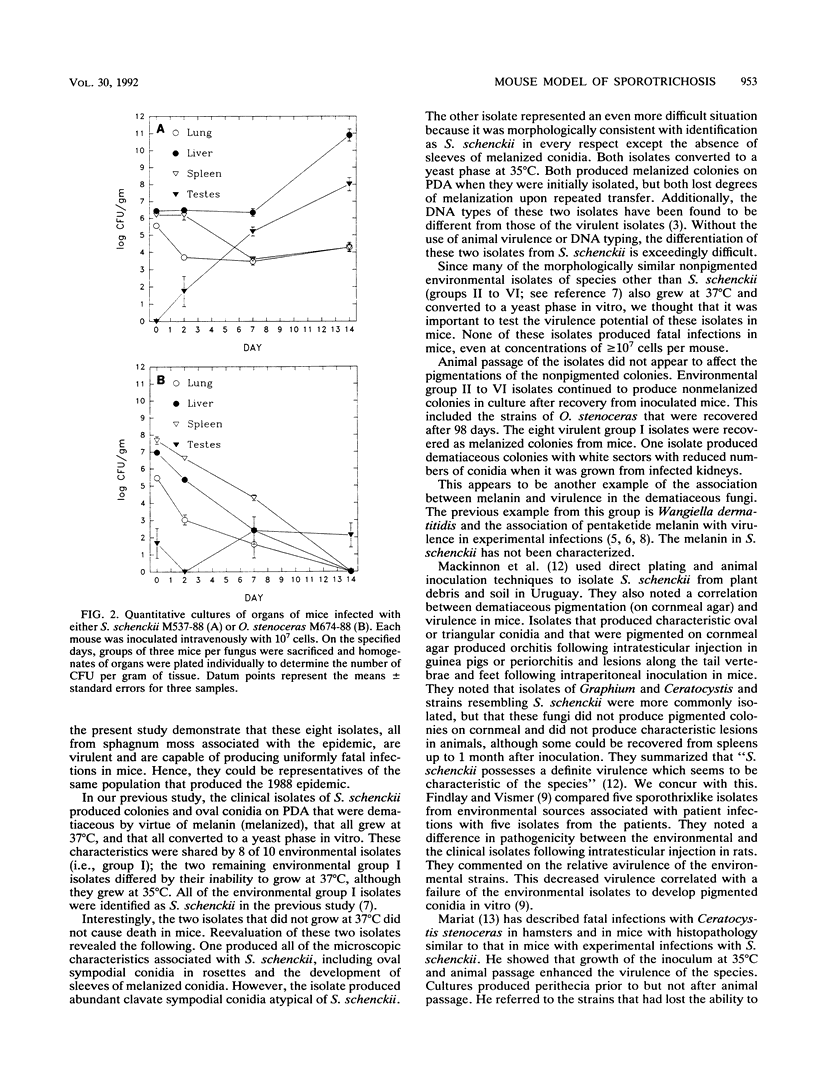
Five clinical and 69 environmental isolates from the largest U.S. epidemic of sporotrichosis were evaluated in NYLAR male mice following intravenous injection of 5 x 10(6) to 2 x 10(8) conidia per mouse. The clinical isolates and eight environmental isolates produced 100% mortality in groups of three mice each between 12 and 24 days after injection. These virulent isolates grew at 37 degrees C, were dematiaceous by virtue of melanin (melanized) on permissive media (e.g., potato dextrose agar), produced ovoid conidia borne sympodially on lateral conidiophores and pleurogenously about the main hyphal axis, and were identified as Sporothrix schenckii. Two melanized environmental isolates that grew at 35 degrees C but not at 37 degrees C were not virulent and had subtle morphological differences from S. schenckii. The remaining environmental isolates were not melanized, were not virulent, and were not S. schenckii; five were identified as Ophiostoma stenoceras and the remainder were identified as Sporothrix spp. Quantitative organ cultures revealed that clinical isolates grew exponentially in livers and testes, in contrast to an isolate of O. stenoceras that was eliminated from liver, lung, and spleen but that persisted in the testes throughout the 14-day sample period. This model helped to confirm the identification of S. schenckii isolates obtained from the environment.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Block E. R., Jennings A. E., Bennett J. E. Experimental therapy of cladosporiosis and sporotrichosis with 5-fluorocytosine. Antimicrob Agents Chemother. 1973 Jan;3(1):95–98. doi: 10.1128/aac.3.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson C. L., Taylor R. L., Drutz D. J. Susceptibility of congenitally athymic (nude) mice to sporotrichosis. Infect Immun. 1983 Apr;40(1):417–420. doi: 10.1128/iai.40.1.417-420.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon D. M., Polak A., Conner G. W. Mel- mutants of Wangiella dermatitidis in mice: evaluation of multiple mouse and fungal strains. J Med Vet Mycol. 1989;27(5):335–341. [PubMed] [Google Scholar]
- Dixon D. M., Polak A., Szaniszlo P. J. Pathogenicity and virulence of wild-type and melanin-deficient Wangiella dermatitidis. J Med Vet Mycol. 1987 Apr;25(2):97–106. doi: 10.1080/02681218780000141. [DOI] [PubMed] [Google Scholar]
- Dixon D. M., Salkin I. F., Duncan R. A., Hurd N. J., Haines J. H., Kemna M. E., Coles F. B. Isolation and characterization of Sporothrix schenckii from clinical and environmental sources associated with the largest U.S. epidemic of sporotrichosis. J Clin Microbiol. 1991 Jun;29(6):1106–1113. doi: 10.1128/jcm.29.6.1106-1113.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findlay G. H., Vismer H. F. Studies in sporotrichosis: fungal morphogenesis and pathogenicity in differing environments. Mycopathologia. 1986 Nov;96(2):115–122. doi: 10.1007/BF00436670. [DOI] [PubMed] [Google Scholar]
- Kan V. L., Bennett J. E. Efficacies of four antifungal agents in experimental murine sporotrichosis. Antimicrob Agents Chemother. 1988 Nov;32(11):1619–1623. doi: 10.1128/aac.32.11.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon-Chung K. J. Comparison of isolates of Sporothrix schenckii obtained from fixed cutaneous lesions with isolates from other types of lesions. J Infect Dis. 1979 Apr;139(4):424–431. doi: 10.1093/infdis/139.4.424. [DOI] [PubMed] [Google Scholar]
- Mackinnon J. E., Conti-Díaz I. A., Gezuele E., Civila E., da Luz S. Isolation of Sporothrix schenckii from nature and considerations on its pathogenicity and ecology. Sabouraudia. 1969 Feb;7(1):38–45. [PubMed] [Google Scholar]
- Mariat F. Adaptation de Ceratocystis à la vie parasitaire chez l'animal--étude de l'aquisition d'un pouvoir pathogene comparable à celui de Sporothrix schenckii. Sabouraudia. 1971 Nov;9(3):191–205. [PubMed] [Google Scholar]
- Miyaji M., Nishimura K. Defensive role of granuloma against Sporothrix schenckii infection. Mycopathologia. 1982 Nov 19;80(2):117–124. [PubMed] [Google Scholar]
- Miyaji M., Nishimura K. Increased resistance of splenectomized mice to Sporothrix schenckii infection. Mycopathologia. 1986 Dec;96(3):143–151. doi: 10.1007/BF00437380. [DOI] [PubMed] [Google Scholar]
- TSUBURA E., SCHWARZ J. Treatment of experimental sporotrichosis in mice with griseofulvin and amphotericin B. Antibiot Chemother (Northfield) 1960 Dec;10:753–757. [PubMed] [Google Scholar]
- Taylor J. J. A comparison of some Ceratocystis species with Sporothrix schenckii. Mycopathol Mycol Appl. 1970 Dec 29;42(3):233–240. doi: 10.1007/BF02051951. [DOI] [PubMed] [Google Scholar]
- Walsh T. J., McEntee C., Dixon D. M. Tissue homogenization with sterile reinforced polyethylene bags for quantitative culture of Candida albicans. J Clin Microbiol. 1987 May;25(5):931–932. doi: 10.1128/jcm.25.5.931-932.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]


