Abstract
In adult rodents, neurons are continually generated in the subventricular zone of the forebrain, from where they migrate tangentially toward the olfactory bulb, the only known target for these neuronal precursors. Within the main olfactory bulb, they ascend radially into the granule and periglomerular cell layers, where they differentiate mainly into local interneurons. The functional consequences of this permanent generation and integration of new neurons into existing circuits are unknown. To address this question, we used neural cell adhesion molecule-deficient mice that have documented deficits in the migration of olfactory-bulb neuron precursors, leading to about 40% size reduction of this structure. Our anatomical study reveals that this reduction is restricted to the granule cell layer, a structure that contains exclusively γ-aminobutyric acid (GABA)ergic interneurons. Furthermore, mutant mice were subjected to experiments designed to examine the behavioral consequences of such anatomical alteration. We found that the specific reduction in the newly generated interneuron population resulted in an impairment of discrimination between odors. In contrast, both the detection thresholds for odors and short-term olfactory memory were unaltered, demonstrating that a critical number of bulbar granule cells is crucial only for odor discrimination but not for general olfactory functions.
Keywords: cell adhesion molecules, neurogenesis, interneurons, γ-aminobutyric acidGABA
The olfactory bulb is one of the few structures in the mammalian central nervous system in which there is a continued supplied of newly generated neurons (1–4). These progenitor cells originate from the subventricular zone of the lateral ventricle, which produces a proliferative population of stem cells throughout the life of the animal (5). Their progeny can undergo cell death (6) or give rise to neuronal progenitors that migrate through a tangential pathway, called the rostral migratory stream, into the core of the main olfactory bulb (7, 8). Here, the precursor cells turn radially to invade the granule and periglomerular layers, where they differentiate mainly into local interneurons (2, 9). Although this ongoing neurogenesis, as well as the migration process, has been extensively characterized, its functional consequences remain unknown.
The tangential migration of precursors from the cells in the subventricular zone into the olfactory bulb is associated with the expression of the neural cell adhesion molecule (NCAM) in its highly polysialylated form (10). Hence, NCAM-knockout mice, which also have been shown to be almost totally devoid of polysialic acid, show a defect in the rostral migration of subventricular zone precursors, resulting in an accumulation of precursors along the pathway and finally in a size reduction of the olfactory bulb (11, 12). A similar defect is observed in neonatal animals treated with an enzyme that specifically cleaves off the polysialic acid moiety from NCAM (13). From these findings, it was inferred that these alterations concern two classes of local inhibitory interneurons in the main olfactory bulb: (i) granule cells that impinge onto mitral or tufted cell secondary dendrites and that constitute, by far, the main target for the newly arriving neurons; and (ii) periglomerular neurons that make synapses onto the primary dendrites of mitral or tufted cells (see review in ref. 14).
Inhibitory synapses of such local interneurons from the olfactory bulb and the antennal lobe in insects (the analogue of the vertebrate olfactory bulb) shape the temporal patterns of output neurons, namely, the mitral cells in vertebrates and the projection neurons in insects (15–17). This temporal coding is thought to be responsible for the synchronization of output neurons, and it has been demonstrated that synchronization is crucial for odor processing (refs. 18 and 19; see review in ref. 20). Thus, if granule cells produced in adulthood are necessary for olfactory bulb function, then a defect in the rostral migration leading to a reduction of the recruitment of new bulbar interneurons should alter olfactory behavioral responses. We have tested this hypothesis, first, by quantifying the level of migration and the size of interneuron populations in NCAM-mutant mice and, second, by investigating their olfactory performance. We found that the dramatic reduction in granule cells in knockout mice was accompanied by impaired odor discrimination but not by impairments in olfactory detection level or memory, indicating a specific role for this cell population for downstream coding of odorant information.
Materials and Methods
Animals.
Male NCAM-mutant mice used in this study were generated by Cremer et al. (12). All experiments were performed “blind” to genotype on mice (C57BL/6J) between 10 and 14 months of age. Adult mice were kept under a 12-h light–dark cycle with lights on at 08:00 and with food and water ad libitum. A week before the start of behavioral experiments, mice were housed singly in 60 × 40 × 20 cm polycarbonate cages. All of the experiments were performed according to the principles of laboratory animal care published by the French Regulation (51).
Cytological and Immunohistological Analyses.
Control and mutant mice were deeply anesthetized with xylazine/ketamine and intracardially perfused with a solution of 4% paraformaldehyde in phosphate buffer (PB). The brain was dissected out and immersed overnight in the same fixative at 4°C. Coronal and sagittal sections were serially cut at 40 μm using a Vibratome 1000 (TPI, St. Louis, MO). Immunohistochemistry was performed on floating Vibratome sections as described previously (21, 22). Briefly, sections were first incubated overnight at 4°C with anti-glutamic acid decarboxylase (GAD)67 or anti-γ-aminobutyric acid (GABA) mAb (Sigma) before incubation with the corresponding fluorescent-labeled or biotinylated secondary antibody (Vector Laboratories). Sections were analyzed by using a standard Leitz confocal microscope and the complementary software package. To estimate the total number of cells in olfactory bulbs, the number of cell bodies in Nissl-stained (0.5% cresyl violet) or immuno-stained sections was determined within a defined grid area (100 × 100 μm).
BrdUrd Labeling and Detection.
To determine the quantity of adult-generated cells, 5-bromo-2′-deoxyuridine (BrdUrd, 10 mg/ml; Sigma), a marker of DNA synthesis, was administered intraperitoneally in mice with ages between postnatal days 60 and 120 (50 mg/kg of body weight in 0.07 M NaOH in 0.9% NaCl). Four injections were given over 2 days and animals were perfused 12 h after the last injection. Vibratome sections of the brains were processed for immunohistochemistry as described above. To denature DNA, sections were treated for 30 min at 37°C with 2 M HCl in PB containing 0.5% Triton X-100, then rinsed three times in sodium tetraborate buffer (0.1 M, pH 8.5) before incubation with anti-BrdUrd antibody (1:100; Dako). The anti-BrdUrd antibody was then revealed with a corresponding fluorescent-labeled secondary antibody, and only immuno-stained nuclei were numbered.
Olfactory Discrimination.
Odors were presented by placing a glass plate on the floor of the animal's home cage. Twenty-five microliters of a solution of paprika (or cinnamon) and sterilized water were placed onto a filter paper (Whatman no. 1) on the glass plate. Odorant solutions (10−4) were freshly prepared before each experiment. We used a habituation-dishabituation task in which each mouse was presented with the first odor (paprika or cinnamon) on one side of the plate and with water (as control) on the other side for five successive 5-min trials separated by 15-min intervals. On the sixth trial, the mouse was exposed for 5 min to a different odor (cinnamon or paprika, respectively) on one side of the plate and water on the other side (23). The mouse was considered to be sniffing whenever it had its nose 1 cm or less from the surface of the glass plate.
Olfactory Sensitivity.
Mice were exposed to a glass plate on which an odor (paprika) was put on one side and water on the other one. The amounts of time that mice spent investigating the odor or the water were recorded. Four different concentrations of paprika were used (10−4, 10−5, 10−6, and 10−8) in a descending order and in separate sessions. Lack of detection of the odorant stimulus was considered to be when mice spent as much time investigating the odor as the water control stimulus.
Olfactory Memory and Specificity.
To assess the time during which mice are able to recognize an odor, mice were exposed twice to the same odor with a 20-, 40-, 60-, 80-, or 100-min interval. These different intervals were tested in separate sessions spaced 2 days apart in a random order. Different odors were used in each test. In each 5-min session, we recorded the time spent by the animals investigating the odor to which they were exposed. A significant decrease in investigation time during the second presentation indicates that mice were able to recognize an odor that had been previously presented. Finally, to assess the specificity of odor recognition, mice were presented with odor, followed 40 min later by exposure to a different one.
Statistics.
Results are expressed as means ± SEM unless otherwise indicated. Comparisons of anatomical data between wild-type and transgenic mice were performed by using a Student's t test, whereas all other data were compared by ANOVA. Significant effects were further analyzed with the post hoc Newman–Keuls test. Levels of significance were set at 0.05.
Results
Laminar Width in Main Olfactory Bulbs.
All mice bulb slices showed the six well-described olfactory bulb layers: nerve, glomerular, external plexiform, mitral, internal plexiform, and granular. The most obvious difference in the overall size and structure of the central nervous system from adult NCAM-deficient animals was a decrease in the size of the olfactory bulb (Fig. 1 A and B) as reported previously for young mice (11, 12). From Nissl-stained sections, it appeared that this anatomical modification results predominantly from a marked decrease in the width of the granule cell layer, leading to a reduction of the bulbar diameter from transgenic animals as illustrated in Fig. 1A. This is shown strikingly both in photomicrographs of coronal (Fig. 1A) and sagittal (Fig. 1B) sections through the olfactory bulbs of homozygous and wild-type littermates and in measurements of the width of different layers: the glomerular (GL), external plexiform (EPL), internal plexiform (IPL), and granule cell (GCL) layers given in Table 1. Altogether, these data indicate that in transgenic mice, laminar width was significantly reduced for the GCL only (35% reduction; P < 10−5; n = 4 wild-type mice and n = 5 mutant mice).
Figure 1.
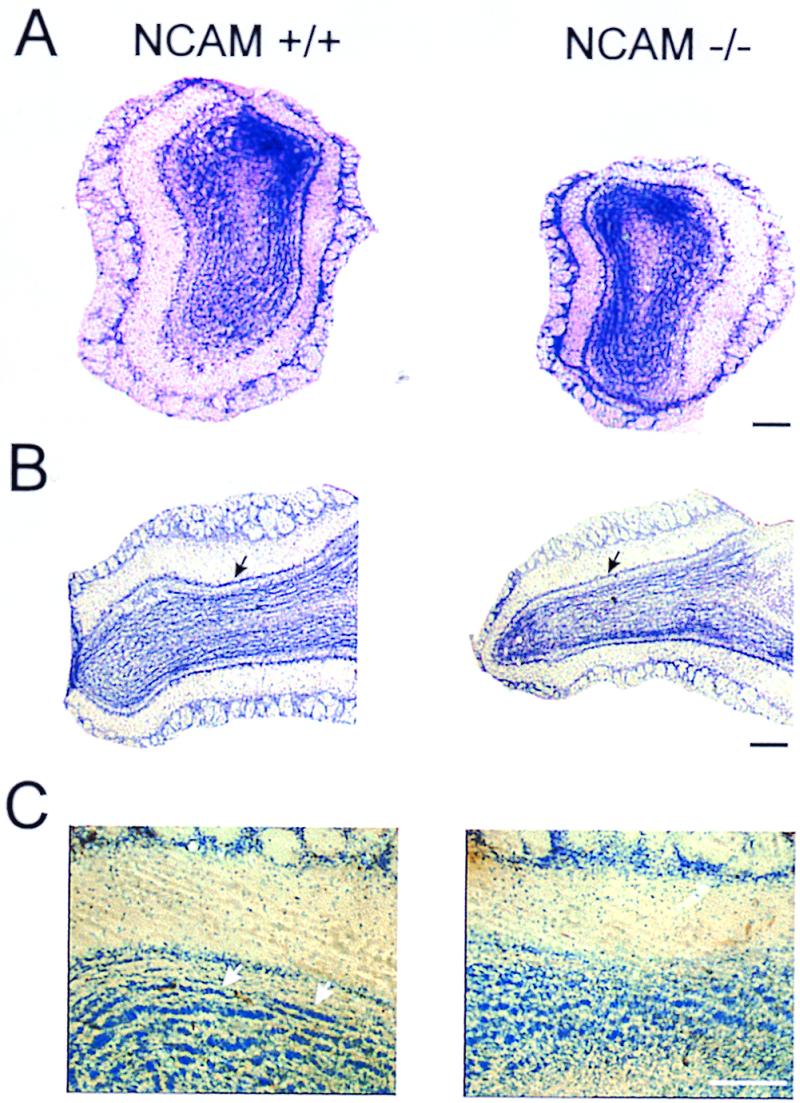
Histologic (Nissl) appearance of normal and mutant main olfactory bulbs in adult mice. Comparison of 40-μm thick Vibratome coronal (A) and sagittal (B) sections of olfactory bulbs from wild-type (NCAM+/+) and knockout (NCAM−/−) mice, showing the decreased size of the granule cell layer in the mutants. Arrows indicate the mitral cell layer. (C) Details of cresyl violet-stained granule cell layer showing the presence of striated aggregates of cells only in control animals (arrows). (Scale bars = 150 μm in A and B and 200 μm in C.)
Table 1.
Laminar structures and cellular density in control and mutant olfactory bulbs
| Layer width, μm
|
Cell density, cells per 100 × 100 μm
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| GL* | EPL | IPL | GCL | Periglom. | Periglom. BrdUrd+ | Mitral | Granule | Granule BrdUrd+ | |
| WT | 155.2 ± 4.8 | 162.8 ± 6.4 | 45.3 ± 2.1 | 235.9 ± 12.5 | 104.7 ± 3.2 | 5.7 ± 0.4 | 10.7 ± 0.4 | 192.2 ± 11.3 | 4.2 ± 0.1 |
| KO | 147.0 ± 2.8 | 161.9 ± 2.4 | 43.4 ± 1.0 | 153.6 ± 4.9 | 110.9 ± 6.8 | 6.6 ± 0.4 | 11.5 ± 0.6 | 205.3 ± 7.8 | 4.8 ± 0.2 |
| P | 0.14 | 0.95 | 0.35 | <10−5 | 0.42 | 0.10 | 0.26 | 0.34 | 0.12 |
Values represent mean ± SEM and comparisons were analyzed by unpaired Student's t test from sagittal sections [n = 33 and 63 slices from 4 control (WT) and 5 mutant (KO) mice, respectively]. For counts of newly generated cells, three animals of each genotype were analyzed (45 sections per animal). EPL, external plexiform layer; GCL, granule cell layer; GL, glomerular layer; IPL, internal plexiform layer; Mitral, mitral cells; Granule, interneurons from the granule cell layer; Periglom., interneurons from the glomerular layer. These data were obtained from 12-month-old mice.
The mean diameter of glomeruli was not significantly different between both groups (77.1 ± 1.2 μm and 83.6 ± 2.7 μm for normal and mutant olfactory bulbs, respectively; P = 0.11).
Cytoarchitecture of Olfactory Bulbs.
To discriminate as closely as possible putative differences in cell density between the two groups, we focused our numeration on the main olfactory bulb functional levels, i.e., the glomerular, the mitral, and the granular cells. From Table 1, it is apparent that the reduction of the GCL was not accompanied by a change in the average cell density measured in all layers. Between mutants and normal mice, measurements of the glomerular diameter also revealed no significant difference in the mean diameter (P = 0.11; see Table 1). As shown in Fig. 1C, analysis of the GCL demonstrated that granule cells were irregularly organized in the mutant bulbs rather than being aligned in regular rows as they are in normal animals (arrows). This latter observation was also reported from GABA immuno-staining (data not shown).
Neurogenesis in the Olfactory Bulbs.
We used BrdUrd injections to investigate whether, in addition to the well described deficits in migration of olfactory bulb precursors in the rostral migratory stream, cellular addition to adult bulbs was also affected in the absence of polysialylated NCAM. After four injections of BrdUrd over 48 h, immuno-stained cells were found in the wild-type and in the mutant olfactory bulbs. BrdUrd immunostaining was characteristically associated with the nuclei of labeled cells. In all cases, this staining was closely linked to the expression of GAD67, another marker for GABAergic interneurons (Fig. 2). In mutants and controls, the majority of these newly generated neurons were located within the GCL (Fig. 2 A–D) and around glomeruli, suggesting that these neurons are periglomerular neurons (Fig. 2 E and F). Cell counts revealed no significant difference in the proportion of BrdUrd-positive cells relative to the total number of granule (wild type, 2.2%; mutant, 2.3%) or periglomular (wild type, 5.4%; mutant, 6.0%; see also Table 1) cells. Considering the smaller size of the mutant GCL in the absence of any change, either in the granule cell diameter (mean diameter of 6.55 ± 0.11 μm and 6.97 ± 0.13 μm for control and mutant mice, respectively; P > 0.05) or in the density of BrdUrd-positive cells, we inferred that the total number of newly generated cells in mutant olfactory bulbs should be reduced by a maximum of 35%.
Figure 2.
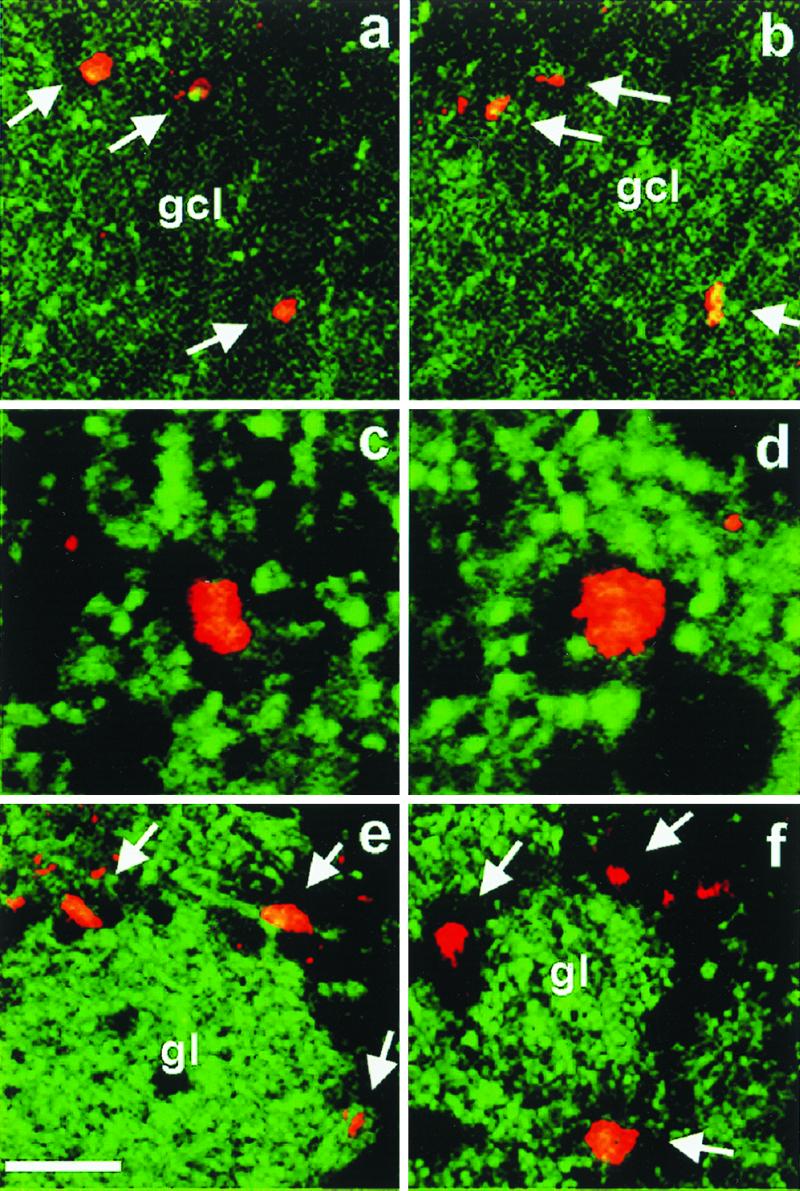
Proliferation in the olfactory bulb. BrdUrd immunohistochemistry on sagittal sections revealed the presence of dividing cells in the granule cell layer (gcl) (a–d) and the glomerular layer (e and f) of the olfactory bulb. Labeled cells in the granule cell layer (arrows) were more or less evenly distributed in mutant (a) and control (b). Double labeling and confocal microscopy demonstrated that BrdUrd-positive (red) cells were in all cases embedded in structures expressing GAD67 (green), a marker for GABAergic neurons. In the glomerular layer, which shows the most intense labeling for GAD67, newly generated cells (arrows) were located around glomeruli (gl), suggesting that they represent interneurons of the periglomerular type. (Scale bars = 25 μm in a and b, 10 μm in c and d, and 20 μm in e and f.)
Behavioral Olfactory Responses.
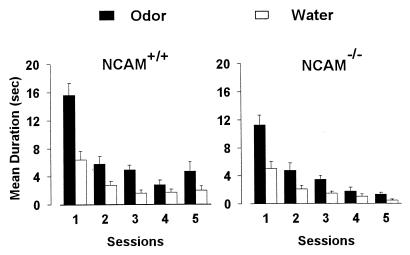
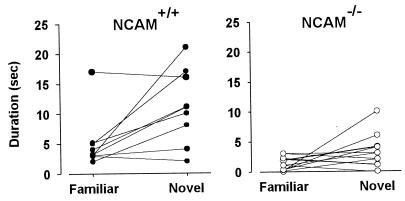
In the first behavioral experiment, both animal groups (10 wild-type and 13 transgenic mice) showed a significant decline in investigation time between the first and subsequent presentations (Newman–Keuls, P < 0.001; Fig. 3). This change in sniffing behavior indicates that both animal types habituated to the repeated presentation of the first odor. However, during the course of this habituation, the animals spent a greater time investigating odors than water [ANOVA, F(1, 21) = 102.14; P < 0.001], indicating that the familiar odor was still detected and elicited more interest than control water (Fig. 3). Interestingly, knockout mice spent less time in odor investigation than wild-type animals [ANOVA, genotype × odors, F(1, 21) = 5.61; P < 0.05]. Most importantly, during the discrimination task, the time spent investigating the familiar odor (fifth trial) and the novel odor (sixth trial) differed between wild-type and transgenic mice [ANOVA, genotype × presentations, F(1, 21) = 4.48; P < 0.05; Fig. 4]. In contrast to wild-type mice (P < 0.001), knockout mice did not significantly increase their level of investigation when a novel, unreinforced odor was presented on the sixth trial (P > 0.05; Fig. 4). In both groups, there was no significant increase in the investigation time for water (P > 0.05).
Figure 3.
Performance in a habituation task in wild-type and NCAM-deficient mice. Values represent the mean time (±SEM) that wild-type (NCAM+/+, n = 10) and transgenic (NCAM−/−, n = 13) mice spent investigating the side of a glass plate scented with an odor (black columns) diluted at 10−4 (wt/wt) and the other side containing sterilized water (white columns).
Figure 4.
Performance in a discrimination task in wild-type and NCAM-deficient mice. Time (in sec) spent investigating a familiar odor (fifth trial) before the novel one (sixth trial) by individual animals in wild-type (filled circles, n = 10) and NCAM-mutant (open circles, n = 13) mice.
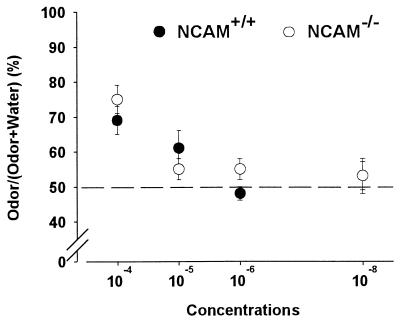
These results suggest that NCAM-deficient mice were impaired in discriminating between familiar and novel, unreinforced odorants. However, it could be argued that wild-type and knockout mice differ only by their olfactory detection thresholds. Therefore, we examined the performance of both groups (n = 13 mice in each group) in a spontaneous olfactory detection task by submitting the animals to a four-step, descending-concentration series (10−4, 10−5, 10−6, and 10−8) of a given odor (paprika) versus water (Fig. 5). At the highest concentration of paprika (10−4), mice of both groups investigated the scented side of the plate more than the water side (Newman–Keuls; wild type, P < 0.001; knockout, P < 0.01), indicating detection of the odorant solution. However, as illustrated in Fig. 5, when the concentration of paprika was set at 10−5, 10−6, or 10−8, no difference was observed between groups in the time spent sniffing the scented side and the clean side of the plate (Newman–Keuls; wild type and knockout, P > 0.05 for the three concentrations used). Thus, wild-type and knockout mice do not differ in their olfactory behavioral thresholds. Therefore, the lack of change in sniffing behavior when NCAM-deficient mice were exposed to a novel, unreinforced odor was not caused by different levels of olfactory sensitivity.
Figure 5.
Detection threshold in wild-type and NCAM-deficient mice. Normalized values are expressed as the mean ratio (±SEM) between the time spent investigating the odor and the total sniffing time (i.e., odor + water). NCAM-deficient (n = 13) and wild-type (n = 13) mice submitted to a four-step, descending-concentration series (10−4, 10−5, 10−6, and 10−8) of a given odor (paprika) versus water. Note that only odor at 10−4 is discriminated from water for both groups.
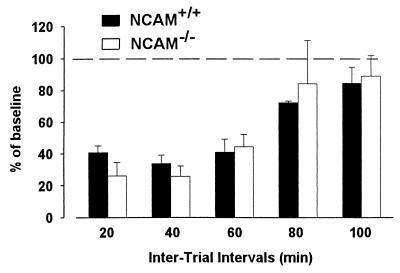
To assess the time interval in which wild-type and knockout mice are still able to recognize a previously encountered odor, animals were exposed twice to the same odor with 20-, 40-, 60-, 80-, or 100-min intertrial intervals (Fig. 6). A three-way ANOVA (two groups × five intervals × two exposures) for the investigation time of the odor revealed a significant effect of all factors [F(1, 18) = 70.54, P < 0.001; F(4, 72) = 8.64, P < 0.001; and F(1, 18) = 101.56, P < 0.001, respectively; n = 10], interaction between groups and exposures [F(1, 18) = 15.32, P < 0.01], and between intervals and exposures [F(4, 72) = 6.14, P < 0.001]. As in the first experiment, wild-type mice spent more time investigating the odor stimulus than knockout mice. The mean overall investigation time in wild-type mice was significantly lower when the second exposure to the odor took place after 20, 40, 60, or 80 min (Newman–Keuls, P < 0.001 for delays ranging from 20 to 60 min, and P < 0.05 for 80 min), but not 100 min after the first exposure (P > 0.05). In NCAM-deficient mice, odor recognition seems to hold to a lesser extent because investigation times significantly decreased on the second exposure, only 20, 40, and 60 min after the first presentation (Newman–Keuls, P < 0.01, P < 0.01, and P < 0.05, respectively), but not after 80 and 100 min (P > 0.05). To determine in both groups whether the decrease in investigation time is specific to the previously presented odor, mice were exposed to a different odor 40 min after the initial exposure. No significant decrease in investigation time was observed in either group (Newman–Keuls, P > 0.05; n = 12 wild-type mice and n = 10 knockout mice), demonstrating that data from Fig. 6 were related to short-term memory rather than to a nonspecific process (data not shown).
Figure 6.
Performance in a short-term memory task in wild-type and NCAM-deficient mice. Effect of different intertrial intervals on odor recognition in NCAM-deficient (n = 10) and wild-type (n = 10) mice. Each bar represents the mean percentage (±SEM) of time investigating a given odor on the second exposure compared with the time spent investigating the same odor during the first presentation.
Discussion
The persistence of a high level of neuron production within the olfactory bulb throughout adulthood and its conservation throughout evolution (24) suggest that this process is of fundamental biological significance. Our study has directly addressed the functional consequence of constant interneuron recruitment within the main olfactory bulb of adult animals. In this region, and in contrast to the selective elimination of some neurons that characterizes late stages of development in other systems, the adult olfactory bulb shows, indeed, both cell addition and cell elimination events. We propose that the combination of these two phenomena may create conditions under which the odor discrimination level may be regulated.
Behavioral Odor Responses.
The present set of behavioral studies shows that NCAM-deficient mice deviate substantially from wild-type mice in their persistence in investigating odors and their ability to spontaneously discriminate between odors. The lack of spontaneous discrimination between odors in transgenic mice cannot be attributed to a differential sensitivity to odors, because both mutant and wild-type mice present the same detection threshold. The major difference found in the cytoarchitecture of the olfactory bulb between NCAM-deficient and wild-type mice suggests that granule cells play a key role in olfactory discrimination. This local interneuron population constitutes the main target of centrifugal fibers, which originate from multiple sources and play important roles in regulating neural excitability in the bulb (see reviews in refs. 25–27). The substantial reduction in granule cell number in NCAM-deficient mice may reduce the sensitivity of the olfactory bulb to these neuromodulators, which in turn could contribute to the reduced odor-discrimination capabilities seen in transgenic mice. Furthermore, our results also indicate that deletion of the NCAM gene does not interfere with odor recognition. Mutant mice, like wild-type mice, recognize odors to which they had been previously exposed for 5 min, although the duration of that form of memory seems slightly shorter in knockout than in wild-type mice. This finding is in agreement with previous studies showing that mice can form a short-term memory of a specific odor and that this memory can last at least 60 min (28).
Regulation of Neuron Production and Migration in Adulthood.
Neurogenesis in the adult brain was first described in the high vocal center of the adult canary neostriatum (29), and has since remained best characterized among songbirds. This neurogenesis, however, differs somewhat from that reported in mammalian vertebrates. First, in contrast to the widespread neurogenesis in birds, the mammalian forebrain uses its resident progenitors to generate new neurons destined for only a few regions: the neocortex, the hippocampal dentate gyrus, and the olfactory bulb. Second, neurogenesis in the avian brain is usually tied to reproductive cycles, whereas in the mammal olfactory system, it is continuous throughout life. Finally, neurogenesis in birds concerns relay neurons, whereas in mammals it is only related to interneurons.
The signals that regulate the differentiation and survival of the young neurons constantly arriving in the olfactory bulb are not known. Mice living in so-called enriched environments show an increase in the number of surviving granule cells in the hippocampus (30). Recently, it was also found that training in hippocampal-dependent learning tasks increases the number of new granule cells (31). In the olfactory bulb, it remains undetermined whether the migration of neuroblasts requires bulbar activity. In fact, conflicting results exist as to whether the proliferation of neuroblasts destined for the bulb can be influenced by exogenous factors such as afferent activity levels. Permanent naris closure does not reduce proliferation levels in early postnatal rats (32), however, it decreases proliferation in adult mice (33). Perhaps the regulation of subventricular-zone cell proliferation changes from a preprogrammed state during bulb growth to an activity-dependent state during bulb maintenance. Regardless of whether proliferation can be regulated, substantial evidence from many brain regions indicates that activity is important for cell survival (see reviews in refs. 34 and 35).
A Role for Adult-Generated Neurons in the Olfactory Bulb?
The consequences of ongoing neurogenesis have long been the subject of speculation. New neurons in the dentate gyrus of the hippocampus seem to be added throughout juvenile and adult life, suggesting that they do not replace neurons that die (36). Alternatively, work in the song-control system of birds has shown that neuronal replacement occurs in some nuclei, perhaps to play a role in song learning (37). Concerning olfaction, one possibility is that new interneurons are simply added to the bulbs, as they are in the hippocampus. Yet, although increases in the number of interneurons have been reported in the adult, substantial granule cell death has also been observed, suggesting that newly generated neurons may replace dying ones (38).
This ongoing recruitment of interneurons may also open new opportunities to investigate the cellular basis for olfactory processing and its functional plasticity. The presence of a pool of new neurons accompanied by the emergence of new synapses could play a role in synchronizing temporal patterns. Indeed, odors are represented by temporally distributed ensembles of coherently firing mitral cells whose firing activity is under the control of local interneurons (see reviews in refs. 14, 20, and 39). In the antennal lobe of the honeybee, pharmacological blockade of GABAergic inhibition, although sparing the general odor selectivity, impairs discrimination between close odorants (19). We thus propose not only that granule cell activity enhances, through lateral inhibition, the refinement of odor-molecule tuning (40), but also that it is crucial for olfactory discrimination, through reciprocal inhibition, in facilitating the synchronization of individual members of mitral cell subpopulations that may be widely distributed in the bulb.
The existence of adult neurogenesis suggests that remodeling occurs continually within the local synaptic circuits of the bulb. Thus, the adult olfactory bulb should comprise neurons with a wide range of maturity levels, given the continual arrival of new cells. Interestingly, granule cells have been reported to be heterogeneous in terms of location and morphology (41–43) as well as physiological characteristics (A. Carleton, P. E. Castillo, and P.-M.L., unpublished data). A classification of these interneurons can thus be proposed that distinguishes mature from immature granule cells; the latter could be particularly important in olfactory discrimination. Adult-generated, immature granule cells might have unusual structural characteristics that induce a proportionately larger effect on olfactory bulb physiology than mature interneurons. Future studies are needed to determine the extent to which neurons produced in adulthood differ from those produced during development.
The olfactory bulb has been implicated in certain types of olfactory learning and memory (see review in ref. 44). One theory proposes that the olfactory bulb plays a transient role in memory storage (see reviews in refs. 45 and 46). Support for the idea of temporary storage comes from electrophysiological studies (47, 48). The naturally occurring replacement of bulbar interneurons provides a rationale for the transferal of memories out of the olfactory bulb (see review in ref. 46). The loss of interneurons may be programmed to occur after the transferal of the memories held by these neurons to other parts of the brain. Alternatively, if the olfactory bulb is necessary for temporary processing of the information that is sent elsewhere for storage, then a rejuvenating population of neurons capable of rapidly forming synaptic connections may be well suited to participate in such a function. Further evidence for a possible role of adult-generated cells in learning comes from studies of mice demonstrating that early environmental complexity enhances the number of newly generated neurons (49). It will be interesting to determine how learning, which leads to changes in the meaning of an odor, correlates with variation in the adult-generated granule cell population. Finally, although NCAM-deficient mice display a strong impairment in olfactory discrimination as tested in the present conditions, they could nevertheless be operantly conditioned to distinguish odors that they cannot normally discriminate without intensive training (50).
Acknowledgments
We thank A. Carleton, A. Hsia, and D. Desmaisons for their comments on the manuscript and C. Rochefort and N. Senant for expert technical help. This work was supported by the Centre National de la Recherche Scientifique, the Institut Universitaire de France, Fondation pour la Recherche Médicale, and by a grant from the European Community Biomed program to H.C. (BIO4-CT96–0730).
Abbreviations
- GCL
granule cell layer
- NCAM
neural cell adhesion molecules
- GABA
γ-aminobutyric acid
References
- 1.Alvarez-Buylla A. Semin Cell Dev Biol. 1997;8:207–213. doi: 10.1006/scdb.1996.0134. [DOI] [PubMed] [Google Scholar]
- 2.Luskin M B. J Neurobiol. 1998;36:221–233. [PubMed] [Google Scholar]
- 3.Gould E, Tanapat P, Hastings N B, Shors T J. Trends Cognit Sci. 1999;3:186–192. doi: 10.1016/s1364-6613(99)01310-8. [DOI] [PubMed] [Google Scholar]
- 4.Gould E, Reeves A J, Graziano M S A, Gross C G. Science. 1999;286:548–552. doi: 10.1126/science.286.5439.548. [DOI] [PubMed] [Google Scholar]
- 5.Temple S, Alvarez-Buylla A. Curr Opin Neurobiol. 1999;9:135–141. doi: 10.1016/s0959-4388(99)80017-8. [DOI] [PubMed] [Google Scholar]
- 6.Morshead C, van der Kooy D. J Neurosci. 1992;12:249–256. doi: 10.1523/JNEUROSCI.12-01-00249.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luskin M B. Neuron. 1993;11:173–189. doi: 10.1016/0896-6273(93)90281-u. [DOI] [PubMed] [Google Scholar]
- 8.Lois C, Alvarez-Buylla A. Science. 1994;264:1145–1148. doi: 10.1126/science.8178174. [DOI] [PubMed] [Google Scholar]
- 9.O'Rourke N A. Neuron. 1996;16:1061–1064. doi: 10.1016/s0896-6273(00)80130-0. [DOI] [PubMed] [Google Scholar]
- 10.Rousselot P, Lois C, Alvarez-Buylla A. J Comp Neurol. 1995;351:51–61. doi: 10.1002/cne.903510106. [DOI] [PubMed] [Google Scholar]
- 11.Tomasiewicz H, Ono K, Yee D, Thompson C, Goridis C, Rutishauser U, Magnuson T. Neuron. 1993;11:1163–1174. doi: 10.1016/0896-6273(93)90228-j. [DOI] [PubMed] [Google Scholar]
- 12.Cremer H, Lange R, Cristoph C, Plomann M, Vopper G, Roes J, Brown R, Baldwin S, Kraemer P, Scheff S, et al. Nature (London) 1994;367:455–459. doi: 10.1038/367455a0. [DOI] [PubMed] [Google Scholar]
- 13.Ono K, Tomasiewicz H, Magnuson T, Rutishauser U. Neuron. 1994;13:595–609. doi: 10.1016/0896-6273(94)90028-0. [DOI] [PubMed] [Google Scholar]
- 14.Shepherd G M, Greer C A. In: The Synaptic Organization of the Brain. 4th Ed. Shepherd G M, editor. New York: Oxford Univ. Press; 1998. pp. 159–203. [Google Scholar]
- 15.Laurent G, Davidowitz H. Science. 1994;265:1872–1875. doi: 10.1126/science.265.5180.1872. [DOI] [PubMed] [Google Scholar]
- 16.MacLeod K, Laurent G. Science. 1996;274:976–979. doi: 10.1126/science.274.5289.976. [DOI] [PubMed] [Google Scholar]
- 17.Desmaisons D, Vincent J-D, Lledo P-M. J Neurosci. 1999;19:10727–10737. doi: 10.1523/JNEUROSCI.19-24-10727.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laurent G, Wehr M, Davidowitz H. J Neurosci. 1996;16:3837–3847. doi: 10.1523/JNEUROSCI.16-12-03837.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stopfer M, Bhagavan S, Smith B, Laurent G. Nature (London) 1997;390:70–74. doi: 10.1038/36335. [DOI] [PubMed] [Google Scholar]
- 20.Laurent G. Trends Neurosci. 1996;19:489–496. doi: 10.1016/S0166-2236(96)10054-0. [DOI] [PubMed] [Google Scholar]
- 21.Calaora V, Chazal G, Nielsen P J, Rougon G, Moreau H. Neuroscience. 1996;73:581–594. doi: 10.1016/0306-4522(96)00042-5. [DOI] [PubMed] [Google Scholar]
- 22.Cremer H, Chazal G, Carleton A, Goridis C, Vincent J-D, Lledo P-M. Proc Natl Acad Sci USA. 1998;95:13242–13247. doi: 10.1073/pnas.95.22.13242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnston R E, Derzie A, Chiang G, Jernigan P, Lee H C. Anim Behav. 1993;45:1061–1070. [Google Scholar]
- 24.Nottebohm F, Alvarez-Buylla A. Restor Neurol. 1993;6:227–236. [Google Scholar]
- 25.Macrides F, Davis B J. In: Chemical Neuronanatomy. Emson P C, editor. New York: Raven; 1983. pp. 391–426. [Google Scholar]
- 26.Halász N, Shepherd G M. Neuroscience. 1983;10:579–619. doi: 10.1016/0306-4522(83)90206-3. [DOI] [PubMed] [Google Scholar]
- 27.Shipley M T, Ennis M. J Neurobiol. 1996;30:123–176. doi: 10.1002/(SICI)1097-4695(199605)30:1<123::AID-NEU11>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 28.Bluthé R M, Gheusi G, Dantzer R. Psychoneuroendocrinology. 1993;18:323–335. doi: 10.1016/0306-4530(93)90028-j. [DOI] [PubMed] [Google Scholar]
- 29.Goldman S A, Nottebohm F. Proc Natl Acad Sci USA. 1983;80:2390–2394. doi: 10.1073/pnas.80.8.2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kempermann G, Kuhn H G, Gage F H. Nature (London) 1997;386:493–495. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- 31.Gould E, Beylin A, Tanapat P, Reeves A, Shors T. Nat Neurosci. 1999;2:260–265. doi: 10.1038/6365. [DOI] [PubMed] [Google Scholar]
- 32.Frazier-Cierpial L, Brunjes P C. J Comp Neurol. 1989;289:355–370. doi: 10.1002/cne.902890312. [DOI] [PubMed] [Google Scholar]
- 33.Corotto F S, Henegar J R, Maruniak J A. Neuroscience. 1994;61:739–744. doi: 10.1016/0306-4522(94)90397-2. [DOI] [PubMed] [Google Scholar]
- 34.Cameron H A, McKay R. Curr Opin Neurobiol. 1998;8:677–680. doi: 10.1016/s0959-4388(98)80099-8. [DOI] [PubMed] [Google Scholar]
- 35.Kempermann G, Brandon E P, Cage F H. Curr Biol. 1998;8:939–942. doi: 10.1016/s0960-9822(07)00377-6. [DOI] [PubMed] [Google Scholar]
- 36.Bayer S A, Yackel J W, Puri P S. Science. 1982;216:890–892. doi: 10.1126/science.7079742. [DOI] [PubMed] [Google Scholar]
- 37.Alvarez-Buylla A, Ling C-Y, Nottebohm F. J Neurobiol. 1992;23:396–406. doi: 10.1002/neu.480230406. [DOI] [PubMed] [Google Scholar]
- 38.Kaplan M, McNelly N A, Hinds J W. J Comp Neurol. 1985;239:117–125. doi: 10.1002/cne.902390110. [DOI] [PubMed] [Google Scholar]
- 39.Mori K. Prog Neurobiol. 1987;29:275–320. doi: 10.1016/0301-0082(87)90024-4. [DOI] [PubMed] [Google Scholar]
- 40.Yokoi M, Mori K, Nakanishi S. Proc Natl Acad Sci USA. 1995;92:3371–3375. doi: 10.1073/pnas.92.8.3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Struble R G, Walters C P. Brain Res. 1982;236:237–251. doi: 10.1016/0006-8993(82)90711-9. [DOI] [PubMed] [Google Scholar]
- 42.Mori K, Kishi K, Ojima H. J Comp Neurol. 1983;219:339–355. doi: 10.1002/cne.902190308. [DOI] [PubMed] [Google Scholar]
- 43.Orona E, Scott J W, Rainer E C. J Comp Neurol. 1983;217:227–237. doi: 10.1002/cne.902170209. [DOI] [PubMed] [Google Scholar]
- 44.Brennan P A, Keverne E B. Prog Neurobiol. 1997;51:457–481. doi: 10.1016/s0301-0082(96)00069-x. [DOI] [PubMed] [Google Scholar]
- 45.Freeman W J, Skarda C A. Brain Res Rev. 1985;10:147–175. doi: 10.1016/0165-0173(85)90022-0. [DOI] [PubMed] [Google Scholar]
- 46.Lynch G, Granger R. In: Olfaction. Davis J L, Eichenbaum H, editors. Cambridge, MA: MIT Press; 1991. pp. 141–165. [Google Scholar]
- 47.Gray C, Freeman W J, Skinner J E. Behav Neurosci. 1986;100:585–596. doi: 10.1037//0735-7044.100.4.585. [DOI] [PubMed] [Google Scholar]
- 48.Gray C, Skinner J E. Exp Brain Res. 1988;73:189–197. doi: 10.1007/BF00279672. [DOI] [PubMed] [Google Scholar]
- 49.Rosselli-Austin L, Williams J. Dev Brain Res. 1990;51:135–137. doi: 10.1016/0165-3806(90)90267-3. [DOI] [PubMed] [Google Scholar]
- 50.Schellinck H M, Brown R E, Slotnick B M. Anim Learn Behav. 1991;19:223–233. [Google Scholar]
- 51.French Ministry of Health. Principles of Laboratory Animal Care. France: French Ministry of Health; 1987. , Décret no. 87-848. [Google Scholar]