Abstract
Programmed cell death is necessary for homeostasis in multicellular organisms and it is also widely recognized to occur in unicellular organisms. However, the mechanisms through which it occurs in unicells, and the enzymes involved within the final response is still the subject of heated debate. It is shown here that exposure of the unicellular microalga Dunaliella viridis to several environmental stresses, induced different cell death morphotypes, depending on the stimulus received. Senescent cells demonstrated classical and unambiguous apoptotic-like characteristics such as chromatin condensation, DNA fragmentation, intact organelles, and blebbing of the cell membrane. Acute heat shock caused general swelling and altered plasma membrane, but the presence of chromatin clusters and DNA strand breaks suggested a necrotic-like event. UV irradiated cells presented changes typical for necrosis, together with apoptotic characteristics resembling an intermediate cell-death phenotype termed aponecrosis-like. Cells subjected to hyperosmotic shock revealed chromatin spotting without DNA fragmentation, and extensive cytoplasmic swelling and vacuolization, comparable to a paraptotic-like cell death phenotype. Nitrogen-starved cells showed pyknosis, blebbing, and cytoplasmic consumption, indicating a similarity to autophagic/vacuolar-like cell death. The caspase-like activity DEVDase was measured by using the fluorescent substrate Ac-DEVD-AMC and antibodies against the human caspase-3 active enzyme cross-reacted with bands, the intensity of which paralleled the activity. All the environmental stresses tested produced a substantial increase in both DEVDase activity and protein levels. The irreversible caspase-3 inhibitor Z-DEVD-FMK completely inhibited the enzymatic activity whereas serine and aspartyl proteases inhibitors did not. These results show that cell death in D. viridis does not conform to a single pattern and that environmental stimuli may produce different types of cell death depending on the type and intensity of the stimulus, all of which help to understand the cell death-dependent and cell death-independent functions of caspase-like proteins. Hence, these data support the theory that alternative, non-apoptotic programmed cell death (PCDs), exist either in parallel or in an independent manner with apoptosis and were already present in single-celled organisms that evolved some 1.2-1.6 billion years ago.
Keywords: Aponecrosis-like, apoptosis-like, autophagic/vacuolar cell death, caspase-like, cell death, DEVDase activity, Dunaliella, environmental stress, microalgae, necrosis-like, paraptosis-like, phytoplankton, TUNEL
Introduction
Whether cell death is beneficial or detrimental for the organisms depends on the biological circumstances in which this happens and on the cell type or species. For both, the question arises concerning the significance of programmed cell death (PCD) in the biology of multi- and unicellular organisms. The origin of the capacity for self-destruction may be as ancient as the origin of the very first cell (Ameisen, 1998). Thus, if effectors of the cell survival machinery can also be effectors of the self-destruction of the cell in which they operate, then the requirement for coupling cell survival to the prevention of self-destruction may be as old as the origin of the first cell, and cell suicide is therefore an unavoidable consequence of self-organization. PCD is ubiquitous in multicellular systems and is essential for normal growth and development (Leist and Nicotera, 1997). The PCD type known as apoptosis, is characterized by very specific morphological and biochemical requirements such as chromatin condensation and margination, ordered DNA cleavage while the cytoplasm and organelles remain unchanged, and the participation of a family of cysteine proteases named caspases, as central regulators (Kerr et al., 1972). Apoptosis is clearly different from necrosis which is characterized by a loss of membrane integrity, cell swelling, and lyses. However, the classical necrotic nature described as passive, unprogrammed cell death, can be questioned. There are examples of cells presenting necrotic morphologies that are subjected to an active cell death programme called ‘necrotic-like’-PCD that can be either caspase-dependent or -independent (Edelstein et al., 1999; Kitanaka and Kuchino, 1999). Consistently, it is considered a type of PCD due to the presence of underlying regulatory mechanisms. However, other types of PCDs, which do not completely fulfil either the typical apoptotic features or the necrotic ones, have also been reported (Clarke, 1990; Sperandio et al., 2000; Leist and Jäätelä, 2001; Papucci et al., 2004; Elmore, 2007). In this regard, a cell death type named ‘paraptosis’, which is fundamentally different from apoptosis, was discovered. It involves cytoplasmic vacuolization, mitochondrial swelling, and the absence of caspase activation or typical nuclear changes, including pyknosis and DNA fragmentation, and is mediated by mitogen-activated protein kinases (MAPKs) (Sperandio et al., 2000, 2004). Other cells have been shown to exhibit features of both apoptosis and necrosis and the term ‘aponecrosis’ was coined (Formigli et al., 2000; Papucci et al., 2004). These authors suggested that apoptosis and necrosis represent two extremes of a wide range of aponecrotic responses that operate under caspase activation. One distinct model is ‘autophagic/vacuolar’-like cell death. The typical hallmark of this form of cell death is the consumption of the cytoplasm in the absence of leakage of the intracellular content and intact cell membranes. This type of cell death may display nuclear degradation and pyknosis, depending on the nature of the cell (Jones, 2000) and it has been shown to be a lysosomal degradation pathway, since final evidence for lysosome-like organelles in plants has been reported (Swanson et al., 1998).
Among all the cell death classes, apoptosis has been mainly studied in metazoans, but vascular plants, as well as some unicellular eukaryotic organisms, show characteristic apoptotic-like features. Apoptosis-like phenomena occur in vascular plants (Greenberg, 1996; Pennell and Lamb, 1997; Lam and del Pozo, 2000; Lam et al., 2001) and they have also been portrayed in unicellular organisms, including chlorophytes (Berges and Falkowski, 1998; Segovia et al., 2003; Segovia and Berges, 2005), dinoflagellates (Vardi et al., 1999; Dunn et al., 2004; Franklin and Berges, 2004; Segovia, 2007), diatoms (Casotti et al., 2005), yeast (Frohlich and Madeo, 2000), kinetoplastids and slime moulds (Cornillon et al., 1994), and bacteria (Lewis, 2000), including cyanobacteria (Berman-Frank et al., 2004). Berges and Falkowski (1998) described a form of autocatalysed cell death in the single cell algae D. tertiolecta. When this chlorophyte was deprived of light, it underwent apoptotic cell death (Segovia et al., 2003). During the process of cell dismantling in D. tertiolecta, the DNA suffered fragmentation and the nucleus disintegrated while the cytoplasm and organelles remained intact. In parallel, both caspase-like activity and expression increased. Despite the identification of metacaspases in vascular plants and protists their roles are not clear yet (Vercammen et al., 2007; Deponte, 2008) and neither is the nature of caspase-like activities in vascular plants (Bonneau et al., 2008). In the majority of cases, measurements of these activities in unicellular species have been carried out by using the classical aspartate-containing caspase substrates. Consequently, the activities measured must be ‘caspase-like’ activities. Although data are scarce, modes of cell death different from apoptosis are adopted by other phytoplanktonic species under stress conditions (Dunn et al., 2002, 2004; Franklin and Berges, 2004). The factors that cause cell death in unicells, their roles, and the details of the apoptotic process remain unclear and, nowadays, the existence and evolution of PCD in unicellular organisms is still controversial (Deponte, 2008). Hence evidence that these alternative, non-apoptotic, PCDs exist has important implications for understanding the fate of unicellular organisms.
Microalgae from the genus Dunaliella are among the most ubiquitous eukaryotic organisms in hypersaline environments, and often the major primary producers in salt lakes and in the evaporation ponds of salt works (Borowitzka, 1981). As an adaptation to the strong environmental seasonal changes operating in these systems, they show a remarkable degree of acclimation to salinity, temperature, nitrogen, and irradiance (Ginzburg, 1987). These features make this species a perfect candidate as a model organism.
Exposure of D. viridis to environmental stresses that impair cell division such as hyperosmotic shock, UV radiation, heat shock, and nutrient starvation, causes a marked decrease in the phosphorylated form of an extracellular signal-regulated kinase (ERK), known to be involved in cell proliferation and differentiation in mammalian cells, through protein kinase cascades (Jimenez et al., 2007). The authors had formerly demonstrated that ERK phosphorylation was critical for cell division in D. viridis (Jiménez et al., 2004). Cell numbers and viability of D. viridis cultures under these conditions were tested and suffered no changes when exposed to sub-lethal stress conditions.
In the present study, evidence is presented that D. viridis has the capacity to undergo different modes of cell death depending on the stress factor and on its intensity. The existence of several biochemical and morphological features typical of each cell death-like morphotype in this microalga is further demonstrated. In all the cases, cell death in D. viridis was linked to an increase in the caspase-like activity DEVDase that matched their accumulation.
Materials and methods
Culture conditions
Dunaliella viridis Teodoresco was grown in Johnson et al. (1968) medium, at 2 M NaCl (Jiménez et al., 2004) and 25 °C under continuous photosynthetic active radiation (PAR) (400–700 nm) at 150 μmol quanta m−2 s−1, while maintaining gentle stirring and bubbling with filtered air. When cultures reached mid log-phase they were submitted to the following environmental stress treatments: (i) osmotic shock, (ii) UV radiation, (iii) heat shock, (iv) nitrogen starvation, and (v) cells were left to reach late stationary phase (senescence). All experiments were performed in triplicate and each sample was measured by triplicate.
Stress treatments
Osmotic shock:
Osmotic stress consisted of an increase in the osmotic pressure of the medium by the addition of NaCl. In acute hypertonic stress experiments, a final concentration of 4 M was considered non-lethal while 5.5 M NaCl was lethal. Cells were sampled at 0, 0.5, 1, and 2 h after the shock.
UV radiation (UVR):
Cultures were placed in 14 cm diameter Petri dishes transparent to UVR and exposed to either 40 (non-lethal) or 70 mJ cm−2 (lethal) of UVR in the range 200–400 nm using a GS Gene Linker UV chamber (Bio-Rad). The UVR spectral band is divided into three sub-bands corresponding to UV-C (<280 nm), UV-B (280–315 nm), and UV-A (315–400 nm). After UV exposure, cultures were maintained with continuous orbital shaking and 150 μmol m−2 s−1 PAR. Cultures were sampled at 0, 2, 4, and 6 h after UV radiation.
Heat shock:
For thermal stress, cultures were transferred to 50 ml conical tubes and submerged in a water bath at either 35 °C (non-lethal) or 40 °C (lethal). Continuous light was provided at the same irradiance as the PAR used during culture growth. Cultures were sampled at 0, 1, 2, 3, and 4 h after the heat shock.
Nitrogen starvation:
Cells were harvested by centrifuging the cultures at 1500 g for 10 min and resuspending them in nitrogen-free growth medium for 7 d. Control cultures were maintained with normal nitrogen-containing medium under the same conditions. Cultures were sampled at 0, 2, 5, and 7 d.
Senescence:
Cells were left to grow under continuous PAR, at the same irradiance as above, for 12 d until they reached late stationary phase. Sampling took place at 0, 2, 5, 7, 9, and 12 d after inoculation.
Transmission electron microscopy (TEM)
Cell pellets of the last point of the time-course for each treatment were used for morphological analysis. For that, such pellets were fixed in 2% glutaraldehyde for 1 h, washed and resuspended in 1 ml of 0.01 M phosphate buffer (pH 8). The pellets were post-fixed in 1% buffered osmium tetroxide followed by dehydration in a graded series of ethanol and embedded in epoxy resin. Ultra-thin sections were viewed and photographed on a Philips EM 201 electron microscope. TEM images of all the treatments were examined at ×3750, ×11 750, and ×25 000. Representative pictures (×25 000) under the different stress conditions are presented. Quantification of the cells under the TEM is always difficult, therefore counting of cells showing each different morphotype was carried out by analysing three fields of view (FOV) for each treatment under the smallest magnification.
Dead cells staining
Evans Blue is an acidic dye which has the inverse staining properties of a vital stain when determining the survival of plant and planktonic cells. Cells with intact semi-permeable membranes exclude the dye, whereas the dye penetrates and stains dead cells. Therefore, Evans Blue is referred as a mortal stain rather than a vital stain (Crippen and Perrier, 1974). Since this method does not give an indication of the mode of cell death, it should only be used in conjunction with other techniques. Accordingly, the method described by Crippen and Perrier (1974) was applied for marine plankton. Samples were measured in triplicate independent cultures for each treatment. Two aliquots of 1 ml of each culture were taken. One of them was preserved in Lugols iodine for counting the total number of cells. The other, was used for counting the number of Evans Blue-stained cells, by using a final concentration of 1:2000 (w/v) of Evans Blue stock 1% (w/v). Samples were counted under the microscope using a haemocytometer.
Confocal laser microscopy (CLM)
DAPI is a popular nuclear counterstain for use in multicolour fluorescent techniques. Its blue fluorescence stands out in vivid contrast to green, yellow, or red fluorescent probes of other structures and stains nuclei specifically, with little or no cytoplasmic labelling. DAPI (Invitrogen) was added to a concentration of 1–10 μM and incubated for 5 min at room temperature. A small droplet of algae suspension was placed on a pre-cleaned glass slide (VWR, Aurora, CO) and trapped under a coverslip. Algae were imaged on an inverted laser scanning confocal microscope (LSM 510, Carl Zeiss Inc., Thornwood, NY) through a ×40 1.2 N.A. water immersion objective. DAPI and endogenous chlorophyll fluorescence were simultaneously excited using two-photon excitation at 780 nm from a Ti: sapphire laser (Coherent Inc., Santa Clara, CA). A dichroic filter (HFT KP 700/543) was used to split the fluorescence emission into two channels. DAPI fluorescence was observed through a 435–485 nm band pass filter and chlorophyll fluorescence through a 650–710 nm bandpass filter. More than 100 cells from each condition were examined under a ×40 objective and representative pictures were taken.
TUNEL staining
Nuclear DNA fragmentation was identified in situ by TUNEL labelling (Gavrieli et al., 1992). Basically, cells were fixed with 0.1% glutaraldehyde, centrifuged for 5 min at 14 000 g at 4 °C. The pellet containing cells was permeabilized by the addition of 0.1% Triton-X100 for 15 min, washed with PBS, and labelled following the manufacturers’ instructions (Apoptag Direct Kit, Chemicon). Samples were then resuspended in PBS, and green fluorescence was observed using an epifluorescence microscope (Nikon, Eclipse E 800, Japan) (excitation 490 nm, emission of 525 nm). Samples were also analysed by using a DAKO cytomation flow cytometer (MoFlo, Beckman Coulter, Fullerton, CA, USA). Counts were triggered using forward scatter (FSC) signals. Positive controls consisted of cells pretreated with 10 μg ml−1 of DNAse I (nickase); for negative controls, distilled water was substituted for the terminal deoxynucleotidyl transferase.
DEVDase activity
For estimating the protein concentration of samples, 50 ml of culture were centrifuged at 1500 g for 10 min. The pellets were resuspended in 1 ml of lysis buffer and kept at 4 °C for 1 h. The lysis buffer contained 25 mM Na+ HEPES, 2 mM dithiothreitol (DTT), 1 mM EDTA, 0.1% 3-[(3-cholamidopropyl) dimethylammonio]-1-propanesulphonate (CHAPS), 10% sucrose, 1 mM phenylmethylsulphonyl fluoride (PMSF), and 1 μM pepstatin A, pH 7.2. Then the homogenate was sonicated and centrifuged at 4 °C at 100 000 g in a Beckman ultracentrifuge using a Ti70 rotor for 1 h. The resultant supernatants were immediately frozen in liquid N2 and stored at –80 °C until use. Lysate protein concentration was measured by the bicinchoninic acid method (Pierce, Rockford, IL), with bovine serum albumin as standard.
The DEVDase activity was determined by use of fluorescent substrates as previously described (Edelstein et al., 1999). The DEVDase assay was performed using 20–50 μl of the supernatant obtained as described above containing 50 μg total protein. A caspase assay buffer was added to the supernatant to achieve a total sample volume of 190 μl. The assay buffer consisted of 250 mM K+ HEPES, 50 mM KCl, 1 mM dithiothreitol, 1 mM EDTA, and 0.1% CHAPS, pH 7.4. The solution was pre-incubated for 10 min at 30 °C before the addition of the caspase substrate (Ac-Asp-Glu-Val-Asp-7-amido-4-methyl coumarin (Ac-DEVD-AMC) in 10% DMSO) (Thornberry et al., 1997). Ten μl of the substrate (25 μM final concentration) were added to make a final assay volume of 200 μl. Peptide cleavage was measured over 1 h at 25 °C using a Cytofluor 4000 series fluorescent plate reader (Perseptive Biosystems) at an excitation wavelength of 380 nm and an emission wavelength of 460 nm. An AMC standard curve was determined for each experiment. Caspase activity was expressed in nmol AMC released min−1 of incubation time mg−1 of lysate protein. To check whether or not these activities were real DEVDase, increasing concentrations of the irreversible caspase-3 inhibitor Z-DEVD-FMK (Calbiochem) were added to the homogenates according to Segovia et al. (2003) and samples were pre-incubated for 60 min before running reactions.
Immunodetection
SDS-PAGE electrophoresis in 12% gels run in Tricine buffer, as well as immunedetection and band analysis were carried out according to Capasso et al. (2001). Antibodies against the full-length precursor form of caspase-3 of human origin were purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA). This antibody is highly specific and reacts with the active form and the full-length precursor of caspase-3. Jurkat (human T-cell leukemia) whole cell lysates were used (Santa Cruz Biotechnology Inc., Santa Cruz, CA) as positive controls.
Statistical analysis
Multiple group comparisons were performed using a one-way analysis of variance (ANOVA). Where significant differences were detected, post-hoc multiple comparisons were made by using the Tukey tests. A P value of <0.05 was considered statistically significant. To quantify the relationship between the variables, the Pearson product moment correlations were performed (considering P <0.05 as significant). Values are expressed as means ±standard deviation. The statistical analyses were carried out by using the STATISTICA 7 statistical package (StatSoft Inc. Tulsa, Oklahoma, USA).
Results
Cellular morphology
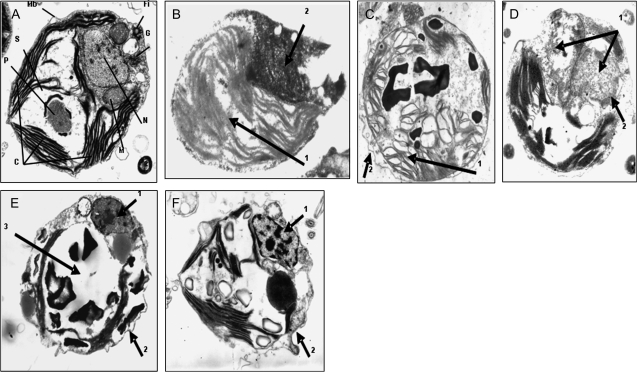
TEM micrographs of control cells actively growing in light (Fig. 1A) showed that D. viridis has one big cup-shaped chloroplast that occupies more than 50% of the cell volume with the pyrenoid in the centre containing starch granules. The nucleus is located in the apical part of the cell surrounded by a well-defined nuclear membrane. Mitochondria and Golgi apparatus are easily recognized. The flagellar insertion point can also be seen in the cellular apex. Ninety-eight per cent of the cells presented this morphology (Table 1). When cells were injured with sub-lethal doses of the environmental stressors, they did not die and were able to cope with the damage (essentially identical to that described by Jimenez et al., 2007). However, exposure of cells to sudden hyperosmotic shock (5.5 M NaCl) (Fig. 1B) revealed that 55% of the cells had swollen and showed chromatin condensation but also extensive cytoplasmic vacuolation (arrow 1) in the absence of nuclear fragmentation and cellular blebbing (arrow 2). This morphology seems to resemble the paraptotic cell death phenotype. Seventy-six per cent of cells exposed to lethal UV radiation (Fig. 1C) presented symptoms of morphological changes typical of necrosis such as cell swelling, disruption of organelle membranes, condensation of mitochondria, and the formation of cytoplasmic blebs (arrow 1). Nevertheless, other features clearly indicated some apoptotic characteristics such as an intact cell membrane and neat membrane blebbing (arrow 2). This appearance gives the impression of an intermediate cell-death phenotype combining both apoptotic and necrotic features, or aponecrosis. However, chromatin condensation was not clear. Fig. 1D corresponds to cells exposed to acute heat shock (40 °C). Under these conditions, 96% of the cells displayed swelling and nuclear oedema. Mitochondrial rupture and disrupted organelle membranes were also observed (arrow 1). Eventually, altered plasma membranes typical of necrosis were also apparent. However, since some chromatin clusters can be identified as spots within the nucleus, it is suggested that the morphology might be necrotic-like (arrow 2). In nutrient-starvation experiments, cells were deprived of nitrogen for 7 d (Fig. 1E) and 77% of the cells demonstrated margination of the nucleus in the cell, pyknosis, clumping and condensation in the nucleus (arrow 1), and intact cell membrane with blebbing (arrow 2) and the absence of leakage of the intracellular content. However, cytoplasmic consumption was observed as indicated by the disappearance of chloroplast as well as other organelles (arrow 3). This morphotype is very difficult to interpret as it seems to be coincident with autophagic/vacuolar cell death. When control cultures of Dunaliella are allowed to reach a late stationary phase of growth or senescence (Fig. 1F), cell death started to happen and cell density declined sharply. In this case, chromatin aggregation and a certain level of karyorrhexis can be seen (arrow 1) as well as membrane blebs (arrow 2). Cytoplasmic disassembling suggests secondary necrosis after apoptosis. Eighty-three per cent of the cells were in this state.
Fig. 1.
Representative transmission electron micrographs showing the morphological changes in Dunaliella viridis subjected to different lethal stress treatments. C, chloroplast; Fi, flagellar insertion; G, Golgi, M, mitochondria; Mb, plasmalemma; N, nucleus; P, pyrenoid; S, starch. Arrows indicate the alterations indicated in the text. (A) Normal vegetative cells grown in PAR. (B) Cells After 3 h of lethal hyperosmotic shock (5.5 M NaCl). Note chromatin condensation together with extensive cytoplasmic swelling and vacuolation (arrow 1) in the absence of nuclear fragmentation and cellular blebbing (arrow 2). (C) Cells after 4 h of UV radiation. Characteristic cell swelling, organelle membranes are disrupted, mitochondria are condensed and cytoplasmic blebs appear (arrow 1). Cell membrane is intact and shows blebbing (Arrow 2). (D) Cells after 2 h of heat shock. Cells experience nuclear edema and swelling, mitochondrial rupture, disrupted organelle membranes and eventually altered plasma membrane (arrow 1), some chromatin clusters are observed (arrow 2). (E) Cells after 7 d of nitrogen starvation. Note pyknosis (arrow 1) but not margination of chromatin, cell membrane with blebbing (arrow 2), and cytoplasmic consumption (arrow 3). (F) Senescent cells after 12 d. Nucleus suffers margination in the cell, chromatin is condensed (arrow 1), clumped and marginated in the nucleus, while cell membrane is intact with blebbing (arrow 2) and undamaged organelles. Picture augmentation was ×25 000.
Table 1.
Percentage of cells showing each different morphotype (Fig. 1) counted for each environmental stress under the TEM with the smallest magnification used (×3750)
| Cell morphotype | % of cells |
| (A) (normal) | 98 (8.76) |
| B (paraptotic-like) | 65 (11.23) |
| C (aponecrotic-like) | 76 (10.57) |
| D (necrotic-like) | 96 (2.23) |
| E (autophagic-like) | 77 (6.16) |
| F (apoptotic-like) | 83 (5.44) |
Standard deviation in brackets. (A) Control cells growing in PAR. (B) Cells exposed to sudden hyperosmotic shock (5.5 M NaCl). (C) Cells exposed to lethal UV radiation. (D) Cells submitted to acute heat shock. (E) Cells under nitrogen deprivation. (F) Cells left to reach the late stationary phase of growth, i.e. senescence. n=200–250 (cells treatment−1).
Dead cells
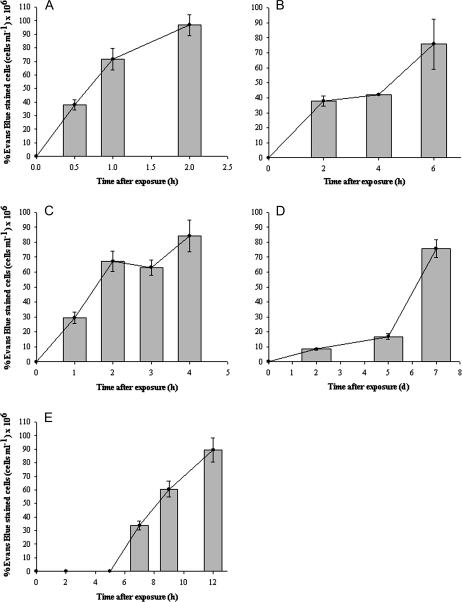
Counting of Evans Blue-stained cells revealed that under the non-lethal stress the cells did not incorporate the dye. After the first 30 min following osmotic shock around 35% of the cells were already dead. One and 2 h later, more than half of the population was dead (Fig. 2A). The response was different after UV radiation and thermal shock. After the UV stress, the cells also died during the first 4 h of treatment. However, the amount of dead cells increased 2-fold between 4 h and 6 h, reaching almost 80% of the total number of cells (Fig. 2B). The number of dead cells rose to about 3-fold, 2 h after the heat shock treatment and remained constant during the whole period of time (Fig. 2C). Sixty per cent of the cells died suddenly after day 7 under nitrogen deprivation whilst during the previous days only 5% or 10% (days 2 and 5, respectively) of the cells showed blue staining (Fig. 2D). Senescent cells started to die during day 7 and by the end of the sampling period (day 12), around 90% of the population was already dead (Fig. 2E).
Fig. 2.
Cell death judged by Evans Blue mortal staining of cells under light microscopy with lethal stress treatments. The dashed horizontal line represents live control cells in PAR at t=0. (A) Cells after osmotic shock. (B) Cells after UV radiation. (C) Cells after heat shock. (D) Cells under nitrogen starvation. (E) Culture senescence.
DNA condensation
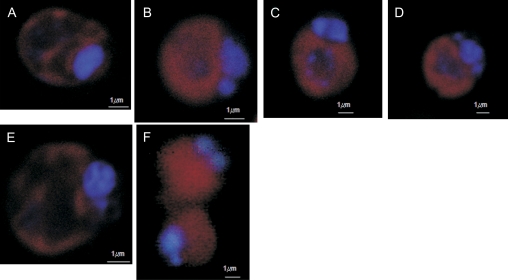
D. viridis cells were also analysed by means of CLM using DAPI nuclear staining. Normal cells growing in PAR (Fig. 3A) showed homogeneous DAPI staining well confined to the nuclear area. Two hours after the onset of a lethal osmotic shock (5.5 M NaCl) (Fig. 3B) cells presented irregular DAPI staining and a slight degree of chromatin clumping could be observed. Fig. 3C represents cells exposed for 2 h to lethal UV irradiation. In this case, chromatin aggregation was not only restricted to the nuclear area, blue-stained granules were also observed in the centre of the cell, therefore suggesting some degree of karyolysis. After 2 h of acute heat shock (Fig. 3D) cells demonstrated chromatin aggregation but again, not strictly restricted to the nuclear area. Figure 3E corresponds to cells subjected to 7 d of nitrogen starvation in which well-defined DNA condensation is observed, appearing only in the nuclear zone. Senescent cells also showed clear chromatin condensation in the nucleus (Fig. 3F).
Fig. 3.
DNA condensation in Dunaliella viridis revealed by DAPI staining and confocal laser microscopy. (A) Control cells in PAR. (B) Cells after osmotic shock (5.5 M NaCl). (C) Cells after 4 h of UV radiation. (D) Cells after 2 h of heat shock. (E) Cells after 7 d of nitrogen starvation. (F) Culture senescence. Horizontal bar is 1 μm. (This figure is available in colour at JXB online.)
DNA strand breaks
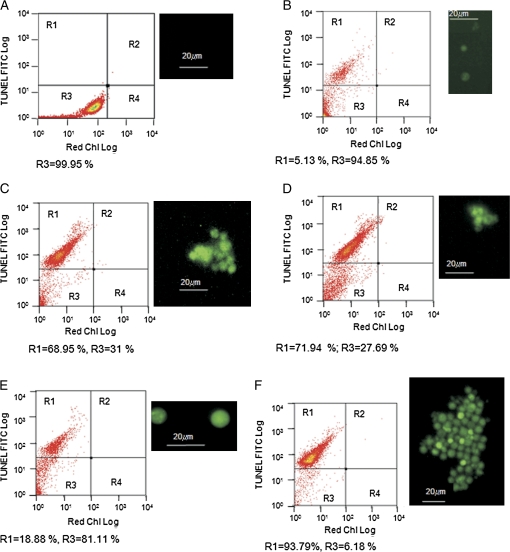
While the morphological changes mentioned above occurred, nuclear DNA was concurrently degraded in some of the stress treatments. Free 3′ OH ends of DNA, generated by activation of endonuclease activity in dying cells, were fluorescently labelled with a conventional TUNEL assay (Gavrieli et al., 1992). No labelling was observed either in cells growing in PAR (Fig. 4A) or in the the non-lethal conditions. In the FACS chart, quadrant R1 contain cells with positive TUNEL labelling, and quadrant R3 corresponds to the absence of TUNEL green fluorescence. Red chlorophyll fluorescence comprised both quadrants. After osmotic shock (Fig. 4B) only about 5% of the cells presented green fluorescence and such labelling was very faint. Around 68% of cells showed labelling after UV radiation (Fig. 4C) and a similar pattern, was observed after 3 h of heat shock (Fig. 4D). Results similar to those obtained under osmotic shock were also found under the nitrogen-starvation treatment (Fig. 4E), in which the number of green fluorescent cells was about 28%. A greater number of cells (93%) presented green fluorescence under the senescence treatment (Fig. 4F) after 12 d. Some of the counts in R1 correspond to cellular debris (for instance the dots at 100 both for FITC and red chlorophyll). Positive controls, consisting of cells treated with DNAse showed strong staining, indicative of DNA degradation. Negative controls were analysed by using 9 d senescent cells and by substituting MilliQ water for the TdT enzyme. These cells did not stain (controls data not shown).
Fig. 4.
DNA fragmentation in Dunaliella tertiolecta after different environmental stresses revealed by TUNEL staining. (A) Control cells in PAR. (B) Cells after 3 h of osmotic shock. (C) Cells after 4 h of UV radiation. (D) Cells after 3 h of heat shock. (E) Cells after 7 d of nitrogen starvation. (F) Senescence after 12 d. Horizontal bar is 20 μm. (This figure is available in colour at JXB online.)
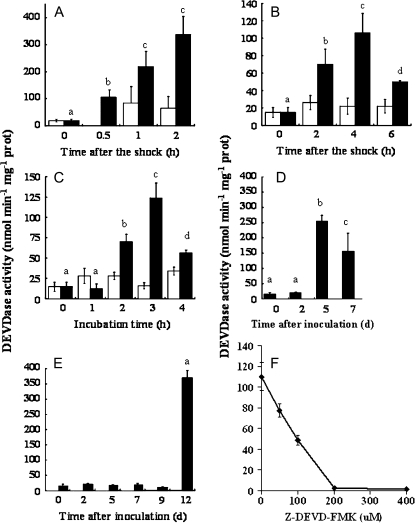
DEVDase activity
DEVDase activity was detected in D. viridis cell extracts, in response to the different stress conditions tested in this work (Fig. 5). Exposure of cultures of D. viridis to either lethal or sub-lethal hyperosmotic stress by the addition of NaCl (5.5 M and 4 M final concentrations, respectively) produced different results (Fig. 5A). While non-lethal hyperosmotic stress did not result in significant increases in activity, cells exposed to 5.5 M NaCl exhibited a rapid increase in DEVDase activity within 0.5 h after the shock, and resulted in values of 337±67 nmol min−1 mg−1 protein after 2 h (about a 17-fold increase). An increase in activity was also determined for cultures subjected to either lethal doses of UV radiation (70 mJ cm−2; Fig. 5B) or temperature (40 °C; Fig. 5C). In these cases a similar increase in DEVDase activity (100–120 nmol min−1 mg−1 protein) was noted under both treatments in the first few hours with a significant drop in activity at later time points most likely representing cell death. The activity of the enzymes was around half of that obtained under osmotic shock. Unlike hyperosmotic stress, sub-lethal UV and temperature shock did not induce a significant increase of DEVDase activity.
Fig. 5.
DEVDase activity in Dunaliella viridis following exposure to various stresses. Changes in enzymatic activity were measured as hydrolysis of 7-amino-4-fluoromethyl coumarin-labelled specific substrate DEVD, in all stress treatments. (A) Osmotic shock. (B) UV radiation. (C) Heat shock. Lethal conditions of stress factors are represented by black bars and sub-lethal conditions by white bars. (D) Nitrogen starvation. (E) Senescent cultures. (F) Inhibition of the enzymatic activity by using the irreversible caspase-3-inhibitor Z-DEVD-FMK after 4 h of UV lethal stress. The highest point of DEVDase activity for the rest of the treatments was inhibited with the highest concentration of the inhibitor (200 μM) and any of them showed activity. Statistical differences (P <0.05) during the time-course for the lethal stress are marked with letters. Same letter means no differences.
Nitrogen starvation (Fig. 5D) is a much slower process leading to cell death, and a peak of maximal DEVDase activity (17-fold increase compared with control cells) was found 5 d after nitrogen removal from the medium. The maximum DEVDase activity in nitrogen-starved cells at 5 d was more than double compared to treatment with UV or temperature and similar to that caused by osmotic stress (Fig. 5B, C, respectively), reaching average values of 250 nmol min−1 mg−1 protein.
Having demonstrated that DEVDase activity was induced in cells exposed to different forms of lethal stress, the influence of natural cell deterioration due to culture ageing was studied. Fig. 5E depicts a dramatic increase in DEVDase activity in senescent cultures of D. viridis at 12 d following initial inoculation (late stationary phase of growth). The activity reached values of more than 350 nmol min−1 mg−1 protein, indicating that activation of this particular caspase-like activity naturally occurs in ageing cultures of D. viridis. DEVDase activity dropped 50% after the addition of 100 μM of the irreversible caspase 3 inhibitor Z-DEVD-FMK (Fig. 5F), and was not inhibited at all by 1 mM PMSF or 1 μM pepstatin A, well-known inhibitors of serine proteases and acid proteases (aspartyl peptidases), respectively.
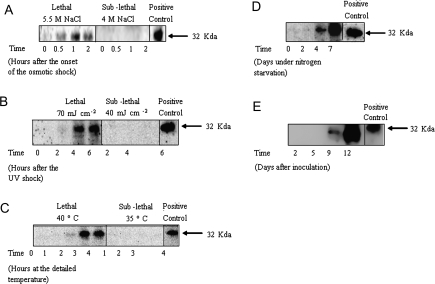
Immunoblot analysis
A mammalian anti-caspase 3 antibody was used to detect the caspase-like protein in D. viridis in response to lethal environmental stress (Fig. 6). Such protein was essentially absent in normal steady-state cells; however, a rapid increase in the intensity of the active 32 kDa caspase-like band was detected after the shock treatments. According to the apparent molecular weight and to the migration of the caspase-3 positive control, the band is similar to the caspase-3 of human origin. The presence of the caspase-like band was only slightly detected after the lethal hyperosmotic shock (Fig. 6A) and no bands appeared under the sub-lethal condition. Under UV radiation (Fig. 6B), maximal band intensity occurred 4–6 h after the treatment, while 3–4 h was needed following heat shock (Fig. 6C). It started to accumulate 4 d after the transfer of cells to nitrogen-free medium, reaching maximal band intensity after 7 d (Fig. 6D). Senescence of cultures of D. viridis also induced the appearance of the caspase-like enzyme (Fig. 6E) 9 d after inoculation, showing maximal band intensity after 12 d (late stationary phase of growth). Thus, there was a clear induction of caspase-like enzyme synthesis in response to lethal stress, irrespective of the cell death morphology presented.
Fig. 6.
Western blot showing cross-reactions of protein extracts from Dunaliella viridis with antibodies raised against human caspase 3. Lanes correspond to the hours or days exposed to each particular stress treatment. (A) Osmotic shock. (B) UV radiation. (C) Heat shock. (D) Nitrogen starvation. (E) Culture senescence.
Discussion
Apoptosis is an active form of cell death by which individual cells commit suicide. It is a highly controlled and organized process characterized by well-defined morphological changes. The possible role of programmed cell death (PCD) in unicellular organisms has received much attention recently (Cornillon et al., 1994; Ameisen, 1996; Vardi et al., 1999; Frolich and Madeo, 2000; Lewis, 2000; Ning et al., 2002; Segovia et al., 2003). The presence of key components of cell death pathways in some of the earliest-evolved organisms (Berman-Frank et al., 2004) suggests that their origins are truly ancient, and it has been speculated that they may be the result of viral-eukaryote genomic mixing during ancient evolutionary history (Berges and Falkowski, 1998; Segovia et al., 2003; Bidle and Falkowski, 2004). However, the existence and evolution of PCD in unicellular organisms is controversial (Deponte, 2008) and obviously confusing because unlike multicellular organisms, it results in complete loss of the organism. However, apoptosis is not the only way by which cells may die. Evidence shows that other alternative forms of non-apoptotic PCD exist in parallel or in an independent manner with apoptosis (Golstein and Kroemer, 2005; Bredesen, 2007), with important implications for understanding the type of PCD that occurs in unicellular microalgae and how they operate. It is now commonly thought that subtle or dramatic changes in the cell-death phenotype are a direct result of the relative degree of the injury to which the cells are exposed. Supporting this contention, there are data that environmental stimuli can produce different types of cell death depending on the intensity of the stimulus and on ATP availability within the cell, and that classic apoptosis and necrosis may represent only two extremes of a continuum of intermediate forms of cell death (Papucci et al., 2004).
In the present work, both morphological and biochemical approaches have been used to study the forms of cell death in D. viridis. Only senescent cultures showed unambiguous features of apoptosis, followed by secondary necrosis; in turn, the other stress factors provoked different cell-death phenotypes under lethal conditions. In the case of heat shock, necrosis-like was the single cell-death process, whilst UV radiation produced an intermediate cell-death morphotype combining both apoptotic and necrotic features, with aponecrotic-like characteristics. A different pattern was observed under hyperosmotic shock, where cells highly resembled the paraptotic-like phenomenon. Finally, algae exposed to nitrogen starvation showed a different death morphotype, that, acording to the results obtained, suggest that it is a similar mechanism to that of autophagic/vacuolar-like cell death. The ends of the death chain would then correspond to necrosis and apoptosis (Aigner, 2002; Papucci et al., 2004), fitting within the cell-death phenotypes found in D. viridis under heat shock and senescence, respectively. The necrotic-like morphology observed after heat stress implies active cell death (Kitanaka and Kuchino, 1999). Chromatin condensation was not observed, but the cells presented chromatin clusters as well as disorganized spots accompanied by cytoplasmic swelling, also described by Leist and Jäätelä (2001), and TUNEL labelling was positive. This is opposed to the necrotic event per se, which is passive and unprogrammed. Cell disintegration under heat stress seems to be programmed and organized in Chlorella pyrenoidosa (Leu and Hsu, 2004) and shows apoptotic morphology as well as intermediate morphotypes in Chlorella saccharopila (Zuppini et al., 2007). Interestingly, the temperature used in both species was higher than the temperature used for D. viridis, which showed a necrotic-like pattern. In the same context, Symbiodinium sp., the symbiotic dinoflagellate of the sea anemone Aiptasia sp., presented PCD characteristics under experimental bleaching, that shifted between both necrosis and apoptosis, depending on the extent of the stress (Dunn et al., 2002, 2004). When D. viridis cells were left to reach late stationary phase, they died apoptotically-like. The cell-death phenotype shown by these cells was totally coincident with that described for D. tertiolecta under light deprivation (Segovia et al., 2003). This is not surprising as ageing has been widely reported to be one of the conditions by which apoptosis takes place in vascular plants (Fukuda, 1994; Buchanan-Wollaston et al., 2003) and by which phytoplankton blooms disappear (Berges and Falkowski, 1998; Vardi et al., 1999; Ross and Sharples, 2007). In fact, dead cells in natural phytoplankton populations closely resemble senescent cultured cells (Veldhuis et al., 2001).
Intermediate features of both apoptosis and necrosis morphotypes or aponecrosis (Formigli et al., 2000) were exhibited when D. viridis was irradiated with UV, including a high degree of TUNEL labelling. UV has been reported to cause apoptotic-like cell death in the unicellular alga Chlamydomonas reinhardtii subjected to high doses of UV (100 J m−2) (Moharikar et al., 2006). However, the difference between D. viridis showing an aponecrotic-like appearance and C. reinhardtii with apoptotic-like morphology might reside in the UV doses received, i.e. in the level of the injury caused to the cells. So, depending on the damage infringed, the cells seem to ‘choose’ how to die.
Completely distinct responses took place under nitrogen starvation and hyperosmotic shock. Under nitrogen starvation, cells may die by means of a mechanism similar to autophagic/vacuolar-like cell death as indicated by the disappearance of clear cytoplasm. Nevertheless, the nucleus was not degraded and chromatin was slightly clumped and condensed. Strikingly, a small percentage of cells showed TUNEL positive labelling. Several reports support that autophagic cell death goes through without the generation of DNA strand breaks (Kissova et al., 2006; Bassham, 2007). Nuclear degradation and pyknosis seem not to be universal in all cells undergoing autophagic death (Jones 2000), and this would rather depend on the cell type. Although the positive TUNEL assay was not statistically significant, the reason lying beyond the chromatin cluttering observed with DAPI and TEM, deserves further studies. Yet, the most important fact is that autophagic/vacuolar cell death occurs in nutrient-starved cells, serving as a cellular protective mechanism up-regulated by nutrient starvation (Yue et al., 2003; Shimizu et al., 2004; Levine and Yuan, 2005). In vascular plants, autophagy has been known for some time to be important for nutrient remobilization during sugar and nitrogen starvation and leaf senescence, and recent reports focus on its role in housekeeping functions related to oxidative stress (Bassham, 2007).
Finally, when cells were subjected to hyperosmotic shock they showed an analogous appearance to paraptotic-like morphology, i.e. chromatin condensation and also cytoplasmic swelling and vacuolation. Like apoptosis, it seems to be an active process requiring transcription and de novo protein synthesis (Aigner, 2002). Paraptosis has been reported to occur in the unicellular dinoflagellate A. carterae when cultured in darkness and during culture senescence (Franklin and Berges, 2004) concurring with rapid vacuolization, loss of internal structure, intact membranes, and lack of DNA fragmentation. Paraptosis is mediated by mitogen-activated protein kinases (MAPKs) in human cells (Sperandio et al., 2004). Coincidently, authors have demonstrated the presence of mitogen-activated protein (MAP) kinase signalling pathways in D. viridis, and that operation of the p38 and the c-Jun N-terminal kinase (JNK) cascades are crucial for adaptation and survival of this microalga upon hyperosmotic stress (Jiménez et al., 2004), the very stress treatment described in this work under which the cells seem to undertake a paraptotic-like demise. Moreover, hyperosmotic shock, nitrogen starvation, and UV irradiation, impaired cell division and caused a marked decrease in the phospho-ERK levels in D. viridis (Jiménez et al., 2007). These data suggest that, indeed, D. viridis might undergo a paraptotic-like event.
Hence, it is now generally accepted that multiple forms of programmed cell death exist and that some of them do not require the activation of caspases (Leist and Jäätelä, 2001; Clarke, 2002). Thus, the term apoptosis in most cases, but not always, is exclusively used for caspase-dependent cell death (Blagosklonny, 2000; Leist and Jäätelä, 2001). On the other hand, while necrosis does not require caspase activation, necrosis-like, being active cell death might or might not require caspase activation (Kitanaka and Kuchino, 1999), and the same is true for aponecrosis (Papucci et al., 2004). However, paraptosis and autophagic/vacuolar cell death traditionally do not call for the participation of caspases (Sperandio, 2000; Jones, 2000; Wyllie and Golstein, 2001; Leist and Jäätelä, 2001). A highly significant increase of DEVDase activity and activation in cell extracts was found after the induction of cell death by means of the environmentally relevant stresses. Caspase-like enzymatic activities and immunodetection were reported for the first time in the unicellular cholorophyte D. tertiolecta (Segovia et al., 2003), other reports regarding metacaspase activity in several microalgal species have also been described (Berman-Frank et al., 2004; Moharikar et al., 2006; Zuppini et al., 2007) and analyses of completed genome sequences of prokaryotic and eukaryotic phytoplankton have revealed the widespread presence of metacaspases in some cyanobacteria, and in unicellular eukaryotic microalgae (Bidle and Falkowski, 2004). However, despite the fact that metacaspases have been reported to operate in an analogous manner to caspases, they are distinct in terms of target site specificity from caspases, at least in vascular plants [their target substrate contains either lysine or arginine at the P1 position, whilst caspase-like enzymes seem to have specificity for aspartate (Vercammen et al., 2004., 2007; Watanabe and Lam, 2005)], and their roles in protists are not obvious yet (Deponte, 2008). DEVDase was not apparent in steady-state D. viridis cells; however, a rapid increase in the intensity of the 32 kDa band was detected after shock treatments. Our data suggest that these activities must be DEVDases because PMSF (an inhibitor of serine proteases) and pepstatin A (an inhibitor of aspartyl peptidases) did not inhibit the activity, whilst the irreversible caspase-3 inhibitor Z-DEVD-FMK inhibited them completely. In this context, it seems clear that environmental treatments induced the activation and the increase in DEVDase activity in unicellular organisms. Yet, the nature of caspase-like proteins in plants is diverse (Bonneau et al., 2008) and a more thorough inhibitor analyses is necessary. Similar results were obtained in other unicellular chlorophytes exposed to UV (Moharikar et al., 2006) and to heat shock (Zuppini et al., 2007). In both cases, antibodies against a mammalian caspase-3 from human origin cross-reacted with a protein of 28–32 kDa and its pattern of expression correlated with the onset of cell death. DEVDase activity and accumulation of the 32 kDa band was observed in cells undergoing all the different cell-death types described in this work, despite the fact that some of those cell-death events, as for example autophagic-like or paraptotic-like deaths, do not necessarily concur with caspase activation. A non-apoptotic role for proapoptotic caspases was previously proposed (Zeuner et al., 1999), indicating that high caspase-3 activity is not exclusive of apoptotic cell death, and in phytoplankton they have been reported to be constitutive and having housekeeping functions (Segovia and Berges, 2005). Thus, growing evidence suggests the participation of caspases in other cellular processes such as development, cell cycle, cell proliferation, and receptor internalization (Algeciras-Schimnich et al., 2002), in addition to their well-characterized role in apoptosis, helping to understand apoptosis-dependent and apoptosis-independent functions of caspases.
PCD is difficult to explain as it is a mechanism that offers negative selective pressure. In spite of this, cells have managed to use PCD for several ecologically relevant purposes. One theory suggests that PCD in unicellular organisms would provide for evolutionary advantage for their genome to survive harsh conditions, optimize adaptation of cell numbers to environmental conditions (i.e. nutrient availability), maintain tight regulation of the cell cycle and differentiation (i.e. formation of resistant forms: cysts), enhance defence against pathogens (i.e. viral infection), and to promote and maintain clonality within the population (Welburn et al., 1997; Ameisen, 2000). In this sense, PCD in unicellular organisms may be considered as a safety mechanism for the population. The evidence that alternative, non-apoptotic PCD exists in unicellular organisms has important implications for understanding cell dynamics. That environmental stimuli can produce different types of cell death depending on the intensity of the stimulus, and that classic apoptosis and necrosis may represent only two extremes of a continuum of intermediate forms of cell death, is also applicable to unicellular organisms. If PCD in phytoplankton is truly the result of a programme activated by environmental factors, then it is important to understand how activation happens and what occurs within the cell in response. The existence of genetically driven cell-death phenomena in phytoplankton indicates that the regulation mechanisms responsible for the dichotomy cell survival/cell death were already present in single-celled organisms 1.2–1.6 billion years ago and preceeded multicellularity.
It has been demonstrated in this work, by using morphological and biochemical approaches, that the presence of different cell-death programmes, mediated by DEVDase activity, can occur in a single-celled organism such as the unicellular chlorophyte D. viridis, and that cell death may vary from apoptosis to necrosis, going through different intermediate morphotypes such as aponecrosis, or adopt autophagic/vacuolar or paraptotic-like appearance, depending on the stimulus received. New advances in the elucidation of the molecular pathways leading to cell death in phytoplankton will decipher the genes involved in the responses to different stress factors.
Acknowledgments
This research was supported by grants from the Ministry of Science and Innovation (MICINN) (Spain) to C Jimenez (CGL05-01071 partially funded by FEDER) and to M Segovia (CTM06-09710), and grants from the National Institutes of Health (NIH) to CL Edelstein (DK-56851). We thank the two anonymous reviewers for their exhaustive criticisms.
References
- Aigner T. Apoptosis, necrosis, or whatever: how to find out what really happens? Journal of Pathology. 2002;198:1–4. doi: 10.1002/path.1172. [DOI] [PubMed] [Google Scholar]
- Algeciras-Schimnich A, Barnhart BC, Peter ME. Apoptosis: independent functions of killer caspases. Current Opinion in Cell Biology. 2002;14:721–726. doi: 10.1016/s0955-0674(02)00384-8. [DOI] [PubMed] [Google Scholar]
- Ameisen JC. The origin of programmed cell death. Science. 1996;272:1278–1279. doi: 10.1126/science.272.5266.1278. [DOI] [PubMed] [Google Scholar]
- Ameisen JC. The evolutionary origin and role of programmed cell death in single celled organisms: a new view of executioners, mitochondria, host–pathogen interactions, and the the role of death in the process of natural selection. In: Lockshin R, Zakeri Z, Tilly J, editors. When cells die. New York: Wiley-Liss; 1998. pp. 3–56. [Google Scholar]
- Ameisen JC. On the origin, evolution, and nature of programmed cell death: a timeline of four billion years. Cell Death and Differentiation. 2002;9:367–393. doi: 10.1038/sj.cdd.4400950. [DOI] [PubMed] [Google Scholar]
- Bassham DC. Plant autophagy: more than a starvation response. Current Opinion in Plant Biology. 2007;10:587–593. doi: 10.1016/j.pbi.2007.06.006. [DOI] [PubMed] [Google Scholar]
- Berges JA, Falkowski PG. Physiological stress and cell death in marine phytoplankton: induction of proteases in response to nitrogen or light limitation. Limnology Oceanography. 1998;43:129–135. [Google Scholar]
- Berman-Frank I, Bidle KD, Haramaty L, Falkowski PG. The demise of the marine cyanobacterium, Trichodesmium spp., via an autocatalyzed cell death pathway. Limnology and Oceanography. 2004;49:997–1005. [Google Scholar]
- Bidle KD, Falkowski PG. Cell death in planktonic, photosynthetic microorganisms. Nature Reviews in Microbiology. 2004;2:643–655. doi: 10.1038/nrmicro956. [DOI] [PubMed] [Google Scholar]
- Blagosklonnyn MV. Cell death beyond apoptosis. Leukemia. 2000;14:1502–1508. doi: 10.1038/sj.leu.2401864. [DOI] [PubMed] [Google Scholar]
- Bonneau L, Ge Y, Drury GE, Gallois P. What happened to plant caspases? Journal of Experimental Botany. 2008;59:491–499. doi: 10.1093/jxb/erm352. [DOI] [PubMed] [Google Scholar]
- Borowitzka LJ. The microflora: adaptations to life in extremely saline lakes. Hydrobiologia. 1981;81:33–46. [Google Scholar]
- Bredesen DE. Toward a mechanistic taxonomy for cell death programs. Stroke. 2007;38:652–660. doi: 10.1161/01.STR.0000257802.82826.a7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan-Wollaston V, Earl S, Harrison E, Mathas E, Navabpour S, Page T, Pink D. The molecular analysis of leaf senescence: a genomics approach. Plant Biotechnology Journal. 2003;1:3–22. doi: 10.1046/j.1467-7652.2003.00004.x. [DOI] [PubMed] [Google Scholar]
- Capasso JM, Rivard C, Berl T. The expression of the gamma subunit of Na-K-ATPase is regulated by osmolality via C-terminal Jun kinase and phosphatidylinositol 3-kinase-dependent mechanisms. Proceedings of The National Academy of Sciences, USA. 2001;98:13414–13419. doi: 10.1073/pnas.231309198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casotti R, Mazza S, Brunet C, Vantrepotte V, Ianova A, Miralto A. Growth inhibition and toxicity of the diatom aldehyde 2-trans, 4-trans-decadienal on Thalassiosira weissflogii (Bacillariophyceae) Journal of Phycology. 2005;41:7–20. [Google Scholar]
- Clarke PGH. Developmental cell death: morphological diversity and multiple mechanisms. Anatomy and Embryoology. 1990;181:195–213. doi: 10.1007/BF00174615. [DOI] [PubMed] [Google Scholar]
- Clarke PGH. Apoptosis: from morphological types of cell death to interacting pathways: comment from Clarke. Trends in Pharmacological Science. 2002;23:308–309. doi: 10.1016/s0165-6147(02)02041-2. [DOI] [PubMed] [Google Scholar]
- Cornillon S, Foa C, Davoust J, Buonavista N, Gross JD, Golstein P. Programmed cell-death in Dictyostelium. Journal of Cell Sciences. 1994;107:2691–2704. doi: 10.1242/jcs.107.10.2691. [DOI] [PubMed] [Google Scholar]
- Crippen RW, Perrier JL. The use of neutral red and Evans blue for live–dead determinations of marine plankton. Staining Technology. 1974;49:97–104. doi: 10.3109/10520297409116949. [DOI] [PubMed] [Google Scholar]
- Deponte M. Programmed cell death in protists. Biochemica et Biophysica Acta. 2008;1783:1396–1405. doi: 10.1016/j.bbamcr.2008.01.018. [DOI] [PubMed] [Google Scholar]
- Dunn SR, Bythell JC, Le Tissier MDA, Burnett WJ, Thomason JC. Programmed cell death and cell necrosis activity during hyperthermic stress-induced bleaching of the symbiotic sea anemone Aiptasia sp. Journal of Experimental Marine Biology and Ecology. 2002;272:29–53. [Google Scholar]
- Dunn SR, Thomason JC, Thissler ML, Bythell JC. Heat stress induces different forms of cell death in sea anemones and their endosymbiotic algae depending on temperature and duration. Cell Death and Differentiation. 2004;1:1213–1222. doi: 10.1038/sj.cdd.4401484. [DOI] [PubMed] [Google Scholar]
- Edelstein CL, Shi YX, Schrier RW. Role of caspases in hypoxia-induced necrosis of rat renal proximal tubules. Journal of the American Society of Nephrology. 1999;10:1940–1949. doi: 10.1681/ASN.V1091940. [DOI] [PubMed] [Google Scholar]
- Elmore S. Apoptosis: a review of programmed cell death. Toxicologic Pathology. 2007;35:495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Formigli L, Papucci L, Tani A, Schiavone N, Tempestini A, Orlandini GE, Capaccioli S, Orlandini SZ. Aponecrosis: morphological and biochemical exploration of a syncretic process of cell death sharing apoptosis and necrosis. Journal of Cellular Physiology. 2000;182:41–49. doi: 10.1002/(SICI)1097-4652(200001)182:1<41::AID-JCP5>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Franklin DJ, Berges JA. Mortality in cultures of the dinoflagellate Amphidinium carterae during culture senescence and darkness. Proceedings of the Royal Society London, Biology. 2004;271:2099–2107. doi: 10.1098/rspb.2004.2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frohlich KU, Madeo F. Apoptosis in yeast: a monocellular organism exhibits altruistic behaviour. FEBS Letters. 2000;473:6–9. doi: 10.1016/s0014-5793(00)01474-5. [DOI] [PubMed] [Google Scholar]
- Fukuda H. Programmed cell death of tracheary elements as a paradigm in plants. Plant Molecular Biology. 1994;44:245–253. doi: 10.1023/a:1026532223173. [DOI] [PubMed] [Google Scholar]
- Gavrieli Y, Sherman Y, Ben-Sasson SA. Identification of programmed cell-death in situ via specific labelling of nuclear-DNA fragmentation. Journal of Cell Biology. 1992;119:493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginzburg M. Dunaliella: a green alga adapted to salt. Advances in Botanical Research. 1987;14:93–183. [Google Scholar]
- Golstein P, Kroemer G. Redundant cell death mechanisms as relics and backups. Cell Death and Differentiation. 2005;12:1490–1496. doi: 10.1038/sj.cdd.4401607. [DOI] [PubMed] [Google Scholar]
- Greenberg JT. Programmed cell death: a way of life for plants. Proceedings of the Natural Academy of Sciences, USA. 1996;93:12094–12097. doi: 10.1073/pnas.93.22.12094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez C, Cossio BR, Rivard CJ, Berl T, Capasso JM. Cell division in the unicellular microalga Dunaliella viridis depends on phosphorylation of extracellular signal-regulated kinases (ERKs) Journal Experimental Botany. 2007;58:1001–1011. doi: 10.1093/jxb/erl260. [DOI] [PubMed] [Google Scholar]
- Jimenez C, Berl T, Rivard CJ, Edelstein C, Capasso JM. Phosphorylation of MAP kinase-like proteins mediates the response of the halotolerant alga Dunaliella viridis to hypertonic shock. Biochimica et Biophysica Acta. 2004;1644:61–69. doi: 10.1016/j.bbamcr.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Johnson MK, Johnson EJ, MacElroy RD, Speer Hl Bruff BS. Effects of salts on halophilic alga Dunaliella viridis. Journal of Bacteriology. 1968;95:1461–1468. doi: 10.1128/jb.95.4.1461-1468.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones A. Does the plant mitochondrion integrate cellular stress and regulate programmed cell death? Trends in Plant Science. 2000;5:225–230. doi: 10.1016/s1360-1385(00)01605-8. [DOI] [PubMed] [Google Scholar]
- Kerr JFR, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. British Journal of Cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kissova I, Plamondon LT, Brisson L, Priault M, Renouf V, Schaeffer J, Camougrand N, Manon S. Evaluation of the roles of apoptosis, autophagy, and mitophagy in the loss of plating efficiency induced by bax expression in yeast. Journal of Biological Chemistry. 2006;281:36187–36197. doi: 10.1074/jbc.M607444200. [DOI] [PubMed] [Google Scholar]
- Kitanaka C, Kuchino Y. Caspase-independent programmed cell death with necrotic morphology. Cell Death and Differentiation. 1999;6:508–515. doi: 10.1038/sj.cdd.4400526. [DOI] [PubMed] [Google Scholar]
- Lam E, Del Pozo O. Caspase-like protease involvement in the control of plant cell death. Plant Molecular Biology. 2000;44:417–428. doi: 10.1023/a:1026509012695. [DOI] [PubMed] [Google Scholar]
- Lam E, Kato N, Lawton M. Programmed cell death, mitochondria and the plant hypersensitive response. Nature. 2001;411:848–853. doi: 10.1038/35081184. [DOI] [PubMed] [Google Scholar]
- Leist M, Nicotera P. The shape of cell death. Biochemical and Biophysical Research Communications. 1997;236:1–9. doi: 10.1006/bbrc.1997.6890. [DOI] [PubMed] [Google Scholar]
- Leist M, Jäättelä M. Four deaths and a funeral: from caspases to alternative mechanisms. Nature Reviews in Molecular and Cell Biology. 2001;2:589–598. doi: 10.1038/35085008. [DOI] [PubMed] [Google Scholar]
- Leu KL, Hsu BD. A programmed cell disintegration of Chlorella after heat stress. Plant Science. 2005;168:145–152. [Google Scholar]
- Levine B, Yuan J. Autophagy in cell death: an innocent convict? Journal of Clinical Investestigation. 2005;115:2679–2688. doi: 10.1172/JCI26390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis K. Programmed cell death in bacteria. Microbiology and Molecular Biology Reviews. 2000;64:503–514. doi: 10.1128/mmbr.64.3.503-514.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moharikar S, D'Souza JS, Kulkarni AB, Rao BJ. Apoptotic-like cell death pathway is induced in unicellular chlorophyte Chlamydomonas reinhardtii (Chlorophyceae) cells following UV irradiation: detection and functional analyses. Journal of Phycology. 2006;42:423–433. [Google Scholar]
- Ning SB, Guo HL, Wang L, Song YC. Salt stress induces programmed cell death in prokaryotic organism Anabaena. Journal of Applied Microbiology. 2002;93:15–28. doi: 10.1046/j.1365-2672.2002.01651.x. [DOI] [PubMed] [Google Scholar]
- Papucci L, Formigli L, Schiavone N, et al. Apoptosis shifts to necrosis via intermediate types of cell death by a mechanism depending on c-myc and bcl-2 expression. Cell and Tissue Research. 2004;316:197–209. doi: 10.1007/s00441-004-0872-z. [DOI] [PubMed] [Google Scholar]
- Pennell RI, Lamb C. Programmed plant cell in plants. The Plant Cell. 1997;9:1157–1168. doi: 10.1105/tpc.9.7.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross ON, Sharples J. Phytoplankton motility and the competition for nutrients in the thermocline. Marine Ecology-Progress Series. 2007;347:21–38. [Google Scholar]
- Segovia M. Programmed cell death in dinoflagellates. In: Perez Martin JM, editor. Programmed cell death in Protozoa. Church Street Georgetown USA: Landes Bioscience-Springer Wiley; 2007. pp. 126–142. [Google Scholar]
- Segovia M, Berges JA. Effect of inhibitors of protein synthesis and DNA replication on the induction of proteolytic activities, caspase-like activities and cell death in the unicellular chlorophyte Dunaliella tertiolecta. European Journal of Phycology. 2005;40:21–30. [Google Scholar]
- Segovia M, Haramaty L, Berges JA, Falkowski PG. Cell death in the unicellular chlorophyte Dunaliella tertiolecta: an hypothesis on the evolution of apoptosis in higher plants and metazoans. Plant Physiology. 2003;132:99–105. doi: 10.1104/pp.102.017129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu S, Kanaseki T, Mizushima N, Mizuta T, Arakawa-Kobayashi S, Thompson Cb, Tsujimoto Y. Role of bcl-2 family proteins in a non-apoptotic programmed cell death dependent on autophagy genes. Nature Cell Biology. 2004;6:1221–1228. doi: 10.1038/ncb1192. [DOI] [PubMed] [Google Scholar]
- Sperandio S, de Belle I, Bredesen DE. An alternative, nonapoptotic form of programmed cell death. Proceedings of the National Academy of Sciences, USA. 2000;97:14376–14381. doi: 10.1073/pnas.97.26.14376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperandio S, Poksay K, de Belle I, Lafuente MJ, Liu B, Nasir J, Bredesen DE. Paraptosis: mediation by MAP kinases and inhibition by AIP-1/Alix. Cell Death and Differentiation. 2004;11:1066–1075. doi: 10.1038/sj.cdd.4401465. [DOI] [PubMed] [Google Scholar]
- Swanson SJ, Bethke PC, Jones RL. Barley aleurone cells contain two types of vacuoles: characterization of lytic organelles by use of fluorescent probes. The Plant Cell. 1998;10:685–698. doi: 10.1105/tpc.10.5.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornberry NA, Ranon TA, Pieterson EP, et al. A combinatorial approach defines specificities of members of the caspase family and granzyme B: functional, relationships established for key mediators of apoptosis. Journal of Biological Chemistry. 1997;272:17907–17911. doi: 10.1074/jbc.272.29.17907. [DOI] [PubMed] [Google Scholar]
- Vardi A, Berman-Frank I, Rozenberg T, Hadas O, Kaplan A, Levine A. Programmed cell death of the dinoflagellate Peridinium gatunense is mediated by CO2 limitation and oxidative stress. Current Biology. 1999;9:1061–1064. doi: 10.1016/s0960-9822(99)80459-x. [DOI] [PubMed] [Google Scholar]
- Veldhuis MJW, Kraay GW, Timmermans KR. Cell death in phytoplankton: correlation between changes in membrane permeability, photosynthetic activity, pigmentation and growth. European Journal of Phycology. 2001;36:167–177. [Google Scholar]
- Vercammen D, Declercq W, Vandenabeele P, Van Breusegem F. Are metacaspases caspases? Journal of Cell Biology. 2007;179:375–380. doi: 10.1083/jcb.200705193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vercammen D, Van de Cotte B, De Jaeger G, Eeckhout D, Casteels P, Vandepoele K, Vandenberghe I, Van Beeumen J, Inzé D, Van Breusegem F. Type II metacaspases Atmc4 and Atmc9 of Arabidopsis thaliana cleave substrates after arginine and lysine. Journal of Biological Chemistry. 2004;44:45329–45336. doi: 10.1074/jbc.M406329200. [DOI] [PubMed] [Google Scholar]
- Watanabe N, Lam E. Two Arabidopsis metacaspases AtMCP1b and AtMCP2b are 18 arginine/lysine-specific cysteine proteases and activate apoptosis-like cell death in 19 yeast. Journal of Biological Chemistry. 2005;280:14691–14699. doi: 10.1074/jbc.M413527200. [DOI] [PubMed] [Google Scholar]
- Welburn SC, Barcinski MA, Williams GT. Programmed cell death in trypanosomatids. Parasitotology Today. 1997;13:22–26. doi: 10.1016/s0169-4758(96)10076-4. [DOI] [PubMed] [Google Scholar]
- Wyllie AH, Golstein P. More than oneway to go. Proceedings of the National Academy of Sciences, USA. 2001;98:11–13. doi: 10.1073/pnas.98.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue Z, Jin S, Yang C, Levine AJ, Heintz N. Beclin 1, an autophagy gene essential for early embryonic development, is a haploin sufficient tumor suppressor. Proceedings of the National Academy of Sciences, USA. 2003;100:15077–15082. doi: 10.1073/pnas.2436255100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeuner A, Eramo A, Peschle C, De Maria R. Caspase activation without death. Cell Death and Differerentiation. 1999;6:1075–1080. doi: 10.1038/sj.cdd.4400596. [DOI] [PubMed] [Google Scholar]
- Zuppini A, Andreoli C, Baldan B. Heat stress: an inducer of programmed cell death in Chlorella saccharophila. Plant and Cell Physiology. 2007;48:1000–1009. doi: 10.1093/pcp/pcm070. [DOI] [PubMed] [Google Scholar]