Abstract
Short-term behavioral sensitization of the gill-withdrawal reflex after tail stimuli in Aplysia leads to an enhancement of the connections between sensory and motor neurons of this reflex. Both behavioral sensitization and enhancement of the connection between sensory and motor neurons are importantly mediated by serotonin. Serotonin activates two types of receptors in the sensory neurons, one of which is coupled to the cAMP/protein kinase A (PKA) pathway and the other to the inositol triphosphate/protein kinase C (PKC) pathway. Here we describe a genetic approach to assessing the isolated contribution of the PKA pathway to short-term facilitation. We have cloned from Aplysia an octopamine receptor gene, Ap oa1, that couples selectively to the cAMP/PKA pathway. We have ectopically expressed this receptor in Aplysia sensory neurons of the pleural ganglia, where it is not normally expressed. Activation of this receptor by octopamine stimulates all four presynaptic events involved in short-term synaptic facilitation that are normally produced by serotonin: (i) membrane depolarization; (ii) increased membrane excitability; (iii) increased spike duration; and (iv) presynaptic facilitation. These results indicate that the cAMP/PKA pathway alone is sufficient to produce all the features of presynaptic facilitation.
Sensitizing stimuli to the tail of Aplysia are capable of producing both short-term and long-term facilitation of the synaptic connections between the sensory and motor neuron as a function of a number of training trials (1–3). Tail stimuli activate several different types of modulatory neurons, of which the serotonergic neurons are particularly important for reflex enhancement to sensitizing stimuli (4). The short-term synaptic facilitation produced by serotonin or 5-hydroxytryptamine (5-HT) is accompanied by four changes in the sensory neurons: (i) membrane depolarization; (ii) increase in the duration of the action potential; (iii) enhanced membrane excitability; and (iv) enhanced neurotransmitter release (3, 5–10). Aplysia sensory neurons seem to have at least two types of 5-HT receptors, and these are coupled to different signal transduction pathways. One receptor is coupled to the adenylyl cyclase-cAMP-protein kinase A (PKA) system and the other to the inositol triphosphate (IP3)/protein kinase C (PKC) system (5, 10–12). The dissection of the relative contributions of PKA and PKC to presynaptic facilitation has been carried out mostly pharmacologically (for review, see ref. 3). We here attempt to address this issue genetically by overexpressing in the sensory neurons a heterologous receptor that activates only one signaling pathway by a naturally occurring ligand.
Toward this end, we have isolated from Aplysia an adenylyl cyclase-coupled octopamine receptor (Ap oa1) by using PCR based on homology screening (13). Pharmacological, biochemical, and physiological analyses indicate that this is a member of the G protein-linked receptor family that has as its natural ligand octopamine (OA), and that is positively coupled only to adenylyl cyclase and not to the IP3/PKC pathway. Ap oa1 is not endogenously expressed in Aplysia sensory neurons. We therefore expressed this receptor gene by microinjection in the sensory neurons (14, 15). Activation of this receptor by OA selectively engages the cAMP/PKA pathway. This selective activation of the cAMP/PKA pathway is sufficient to produce all four presynaptic events involved in short-term facilitation.
Materials and Methods
Molecular Cloning of Ap oa1.
PCR amplification was performed on 1 μg of genomic DNA with 1 μg of each of the two degenerate primer pools: 5′-AAG AAT TCT G(C,T)T GGT T(A,G)C CIT T(T,C)T TT-3′ and 5′-AAG CGG CCG CAG C(A,G)T A(A,G,T)A TIA (T,C)(A,C,G,T)G G(A,G)T T-3′, corresponding to the transmembrane domain sequences that are highly conserved among mammalian and Drosophila biogenic amine receptors. The PCR reaction yielded a DNA fragment of 210 bp, which was subsequently used as probe to obtain the entire ORF by screening the Aplysia californica genomic library and accessory radula closer muscle cDNA library (16). As a comparative study, we also cloned a cDNA sequence of Ap oa1 by a nested PCR strategy from Aplysia kurodai that is commonly found in Eastern Asian Pacific coastal lines. We amplified the full-length coding region of Ap oa1 from A. kurodai by PCR by using the primers 5′-CCCAAGCTTACAACCACCACGAAATG-3′ and 5′-GCTCTAGAGTACGACAGACGTGCGT-3′. The PCR product containing the DNA sequence encoding Ap oa1 was subcloned into the expression vector pNEXδ (14) by using HindIII and XbaI sites to create pNEXδ-Ap oa1.
Transfection and Second Messenger Analyses.
The entire amino acid coding region of Ap oa1 subcloned into pcDNAIII (Invitrogen) was introduced into HEK-293 cells by calcium phosphate-mediated transfection, and transformed cells were selected under 500 μM G418. Stable cell lines expressing the highest level of Ap oa1 mRNA were isolated by RNA Northern blot analysis.
For cAMP analysis, approximately 105 stable cells or 5 × 105 transient cells in each sample were treated with various concentrations of agonists in PBS in the presence of 500 μM 3-isobutyl-1-methylxanthine. The amount of cAMP of each sample was determined with the cAMP radioimmunoassay kit (New England Nuclear). Agonists used in our pharmacological and physiological assays were from Sigma.
For analysis of phosphoinositide (PI) metabolism, 5 × 105 cells of either HEK 293 or Chinese hamster ovary (CHO) cells were transfected with 2 μg of pcDNAIII-Ap oa1 or pcDNAIII-muscarinic acetylcholine receptor (mAChR) m3 (17) by using GenePORTER (Gene Therapy Systems, San Diego, CA). Transfection and PI assay for the two receptors were done in parallel at the same time, as described elsewhere (18, 19). cAMP assays were also done in these transfected cells. The data are representative of three independent experiments, each conducted in duplicate.
Reverse Transcriptase—PCR (RT-PCR) Analysis.
RT-PCR analyses of Ap oa1 and 18S RNA were similarly done as described elsewhere (16). The gene-specific 32P-labeled probes of Ap oa1 and 18S RNA were 5′-ACC CGC TGC AGT ATG AGA GCA AGA TGA CGC GGC CG-3′ and 5′-GGG CAA GTC TGG TGC CAG CAG CCG CGG TAA TTC C-3′, respectively. Hybridization was done at 60°C for 12 hr. High stringent washings were sequentially performed at 60°C in 2× standard saline phosphate/EDTA (SSPE) (1×, 0.18 M NaCl/10 mM sodium phosphate, pH 7.6/1 mM EDTA)–0.1% SDS (15 min)/1× SSPE–0.1% SDS (15 min)/0.5× SSPE–0.1% SDS (15 min).
Heterologous Expression and Electrophysiology in Xenopus Oocyte.
Expression of Ap oa1, human β2-adrenergic receptor (20), the mouse 5-HT2c receptor (21), Lym oa1 (22), and human cystic fibrosis transmembrane regulator (CFTR) (23) in Xenopus oocytes and voltage clamp recordings were performed as described elsewhere (24).
Gene Transfer into Sensory Cells.
Animals (A. kurodai) weighing 150–250 g were purchased from a local supplier in Pusan, Korea. The sensory neurons in the ventrocaudal clusters of pleural ganglia were microinjected with the DNA construct of pNEXδ-green fluorescent protein (GFP) (25, 26) or with a mixture of pNEXδ-GFP and pNEXδ-Ap oa1 as described elsewhere (27). The GFP–DNA construct was used as the marker of DNA expression in the living cells.
Electrophysiological Recordings for Sensory Neurons: Membrane Potential, Spike Duration, and Membrane Excitability.
Standard intracellular recording techniques were used in a current clamp mode (28). The only cells used were those that had resting membrane potentials more negative than −40 mV. To determine the spike duration in the sensory cells, a 0.4-nA current pulse was delivered into the cells for 15 ms (short pulse). Spike duration was measured from the peak of the spike to repolarization of the spike to 25% of its amplitude. Depolarizing current pulse steps ranging from 0.1 to 0.3 nA with a duration of 500 ms (long pulse) were pretested in each cell to determine the threshold of current that produced only one spike before drug application. Immediately after these baseline recordings with the short pulse and long pulse, a drug was applied to the bath for 5–6 min before another drug application. The long pulse was usually delivered only once or twice within 1–2 min after each drug application. The number of spikes during the long pulse represented membrane excitability. Short (15-ms) pulses were delivered every 30 s and separated from long (500-ms) pulses by 30–60 s throughout the recordings. Only one sensory cell was recorded from each ganglion.
Rp-cAMPS Iontophoresis and Electrophysiological Recordings.
Electrophysiological recordings with OA or with 5-HT application were carried out as described above. The drug was completely washed out from the bath, and sensory neurons were rested for 2 hr before Rp-cAMPS (80 mM) (adenosine-3′,5′-cyclic monophosphorothioate, Rp-isomer, BIOLOG, Bremen, Germany) iontophoresis. Iontophoresis was done as described elsewhere (10) by injecting negative current (1.4 nA) for 20 min. After Rp-cAMPS iontophoresis, electrophysiological recordings with OA and 5-HT were repeated.
Excitatory Postsynaptic Potential (EPSP) Recording in Sensory Motor Connections.
EPSP was recorded from motor neurons in the pedal ganglion after eliciting a single spike by intracellular stimulation from sensory neurons in the pleural ganglion, as described elsewhere (28, 29). Sensory neurons were microinjected with pNEXδ-GFP and pNEXδ-Ap oa1 1 day before EPSP measurement. Two EPSP measurements, obtained before and after application of drugs for 5 min, were compared to calculate the change in amplitude.
Statistical Analysis.
Paired analyses for changes in membrane excitability and spike broadening were carried out by using the Wilcoxon signed rank test. The Mann–Whitney U test was used to measure the significance of EPSP changes by treatment with OA and 5-HT.
Results
Molecular Cloning of the OA Receptor and Its Expression in the Nervous System of Aplysia.
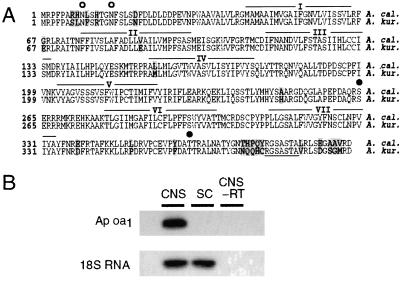
Using a PCR and homology screening of the Aplysia genomic and cDNA libraries, we have isolated an OA receptor, Ap oa1, from the genomes of A. californica and A. kurodai (Fig. 1A). Ap oa1 contains an ORF of 394 amino acids. The amino acid sequences of Ap oa1 from two Aplysia species are nearly identical (94% homologous). Sequence identity with other OA/tyramine receptors is 36–47% in transmembrane domains and 24–30% in the entire amino acid sequences. Furthermore, the lengths of second extracellular and third intracellular loops and C-terminal tail are very different from those of other OA/tyramine receptors. Specifically, Ap oa1 has a rather short third intracellular loop region. Interestingly, however, these hypervariable loops of Ap oa1 are comparable in length to those of mammalian β2-adrenergic receptors that are well known to couple to Gs protein. These features indicate that Ap oa1 may represent a new class of OA/tyramine receptors.
Figure 1.
(A) Deduced amino acid sequences of Ap oa1 clones from A. californica (A. cal., Upper) and A. kurodai (A. kur., Lower). The coding region is determined as the longest ORF that begins with a methionine codon ATG. The different amino acids are shaded in gray. The putative transmembrane domains are overlined and numbered I–VII. Two potential N-linked glycosylation sites are indicated by open circles (○). Serine and threonine that are within the protein kinase C consensus sequences are indicated by closed circles (●). A potential N-myristoylation site is underlined. (B) Ap oa1 is expressed in the Aplysia CNS but not in the sensory cluster (SC). Neither Ap oa1 nor 18S RNA was amplified in CNS when reverse transcriptase was omitted in RT-PCR reaction mixture (CNS-RT).
We performed RT-PCR reactions to examine the expression of Ap oa1 mRNA in the central nervous system (CNS) of Aplysia. Total RNA was isolated either from total CNS or from only the pleural sensory cluster and reverse transcribed into cDNAs. As shown in Fig. 1B, we found that Ap oa1 is expressed in the Aplysia nervous system but not in the sensory neurons. In contrast, 18S RNA, an internal control, was detected in both CNS and sensory neurons at the same level. Neither Ap oa1 nor 18S RNA was amplified unless RT-PCR reactions contained reverse transcriptase, indicating the PCR products are originated from RNA, not from genomic DNA.
Ap oa1 Stimulates cAMP Production in Response to OA.
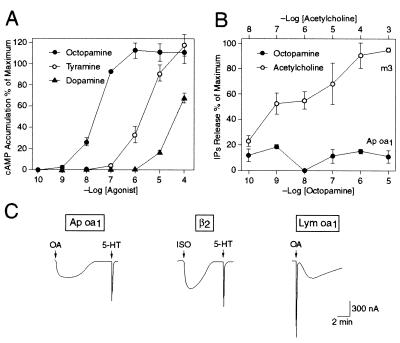
We stably expressed Ap oa1 in HEK 293 cells and tested the effects of a number of biogenic amine neurotransmitters on cAMP production to determine whether Ap oa1 encodes a functional receptor (Fig. 2A). Among all the biogenic amine neurotransmitters tested, OA stimulated cAMP production most effectively, and it did so in a dose-dependent saturable manner with an EC50 = 30 nM. Beside OA, tyramine and dopamine also stimulated cAMP production, but at a higher concentration. In control experiments, OA, tyramine, and dopamine had no effect on cAMP production in the untransfected HEK 293 cells at a concentration up to 10 μM (data not shown). Serotonin and histamine had no appreciable effect in a concentration up to 10 μM (data not shown). We also expressed Ap oa1 transiently in HEK 293 and CHO cells. We found that OA stimulated cAMP production in these cells in a dose-dependent manner (data not shown), indicating that this cAMP increase was not caused by the property of a particular cell line, but by expression of the receptor either stably or transiently.
Figure 2.
Coupling specificity of Ap oa1. (A) Dose-response curve of agonists of Ap oa1 on cAMP production in a HEK 293 cell line stably expressing Ap oa1. Neurotransmitters, OA, tyramine, and dopamine are tested at various concentrations in the presence of 500 μM 3-isobutyl-1-methylxanthine. Data are expressed as percentage of maximum response of cAMP production. (B) Dose-response curve of production of inositol phosphates (IPs) by Ap oa1 with OA (filled circle) and by cardiac m3 mAChR with acetylcholine (open circle) in HEK 293 cells heterologously expressing the receptors. Data are expressed as percentage of maximum response of IPs production. (C) The coupling specificity of Ap oa1 examined in Xenopus oocyte. Current tracings from three representative oocytes of voltage-clamp experiments. Either Ap oa1 or β2-adrenergic receptor cRNA (2.5 ng) were coinjected into oocytes with 5 ng CFTR cRNA and 0.1 ng mouse 5-HT2c cRNA. Lym oa1 (2.5 ng) cRNA was also coinjected with 5 ng CFTR cRNA. OA (100 nM), 100 nM 5-HT, and 1 μM isoproterenol (ISO) were used to induce currents from each oocyte. OA (1 μM) was used in stimulating Lym oa1 (EC50 = ≈5 μM). Drugs were applied to the bath solution (arrows) for 30 s and washed out. Holding potential was −60 mV.
We also performed the PI assay by using HEK 293 and CHO cells heterologously expressing Ap oa1 and examined whether activation of the receptor stimulates PI metabolism. When the HEK 293 cells expressing Ap oa1 were activated by OA in a dose-dependent manner, no significant [3H]inositol phosphate production was detected (Fig. 2B). Similarly, no significant increases of PI hydrolysis were detected in transfected CHO cells on application of OA (data not shown). In control experiments, we used the same cell lines but transfected with the porcine mAChR m3 that is known to be coupled to PI metabolism (17). In contrast to OA, application of acetylcholine to the HEK 293 (Fig. 2B) and CHO cells (data not shown) produced significant increases in [3H]inositol phosphates in a dose-dependent manner. These data indicate that Ap oa1 is coupled to adenylyl cyclase but not to phospholipase C activation.
We used a Xenopus oocyte system to examine electrophysiologically the coupling specificity of the receptor to second messenger pathways (Fig. 2C). We focused on two types of membrane current generated by activation of different GTP-binding proteins. We first examined the CFTR, a Cl− channel stimulated by PKA after activation of Gs (30). The β2-adrenergic receptor is known to activate this current (Fig. 2C). Xenopus oocytes also have an endogenous Cl− channel that is activated by IP3 after activation of Go or Gq (21). Activation of the 5-HT2c receptor is known to generate this current (Fig. 2C).
In vitro-transcribed cRNAs of Ap oa1 or β2-adrenergic receptor and 5-HT2c were injected into oocytes with CFTR cRNA to determine which type of current is activated by Ap oa1. Activation of Ap oa1 from either A. californica or A. kurodai by application of 100 nM OA led only to a slowly activating CFTR current (314 ± 44 nA: mean of peak amplitude ± SEM, n = 7) and not to an IP3-activated endogenous current (Fig. 2C). Similarly, activation of β2-adrenergic receptor with 1 μM isoproterenol led to generation of the CFTR current (425 ± 92 nA, n = 4), but did not activate IP3-stimulated endogenous current (Fig. 2C). Activation of coinjected 5-HT2c produced a fast endogenous current in both the absence (n = 6) and presence (n = 7) of CFTR expression (Fig. 2C). In contrast to the Ap oa1 and β2-adrenergic receptor, activation of Lym oa1, a receptor that is linked to both Gs and Go/Gq (22), gave rise to both IP3-activated Cl− current and CFTR current (n = 5) (Fig. 2C).
These electrophysiological analyses suggest that, like β2-adrenergic receptor, Ap oa1 (from either A. californica or A. kurodai) is positively linked only to Gs protein and not to Go or Gq proteins.
Aplysia Sensory Neurons Expressing Ap oa1 Show Characteristic Electrophysiological Responses.
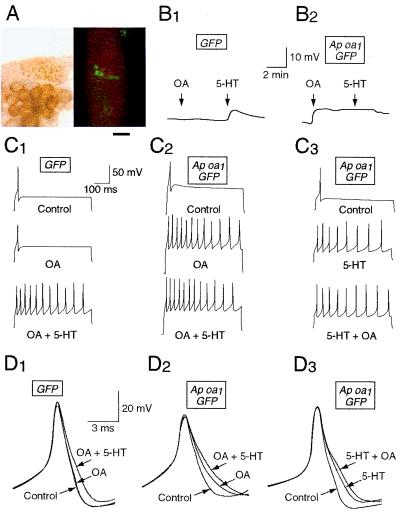
To study the role of cAMP/PKA in short-term facilitation, we expressed Ap oa1 in Aplysia sensory neurons by microinjecting pNEXδ-GFP and pNEXδ-Ap oa1. Fourteen to twenty-four hours after microinjection, the sensory neurons expressing the receptor were noninvasively selected on the basis of GFP fluorescence (Fig. 3A). We obtained intracellular recordings from GFP-positive neurons.
Figure 3.
Sample records of electrophysiological responses to OA and 5-HT in the sensory neurons expressing GFP (B1, C1, D1) or GFP plus Ap oa1 (B2, C2, C3, D2, D3). Resting potential (B1, B2), membrane excitability (C1–C3), and spike duration (D1–D3) were measured before (Control) and after drug applications. The cells B1, C1, and D1 were injected with pNEXδ-GFP, and the cells B2, C2, C3, D2, and D3 were injected with both pNEXδ-GFP and pNEXδ-Ap oa1. All these cells were GFP-positive. (A) Light (Left) and fluorescent (Right) photographs showing an example of GFP/Ap oa1-positive sensory neurons in a pleural ganglion. The cell bodies of these neurons are shown around the center of the fluorescent photograph. Bar = 250 μm. (B) Membrane depolarization by OA and 5-HT in a GFP-expressing cell (B1) and in a GFP/Ap oa1-expressing cell (B2). (C) Action potentials were recorded by injecting a depolarizing current (0.1–0.3 nA) for 500 ms. Membrane excitability was enhanced by 5-HT in a GFP-expressing cell (C1) and by OA and 5-HT in GFP/Ap oa1-expressing cells (C2, C3). (D) Superimposed action potentials demonstrating increases in spike duration after treatments with OA and 5-HT. Spike broadening by treatment with 5-HT in a GFP-expressing cell (D1) and with 5-HT and OA in GFP/Ap oa1-expressing cells (D2, D3). The shapes of single spikes were recorded by delivering a 0.4-nA current pulse for 15 ms. OA + 5-HT, 5-HT was applied later in the presence of OA (C1, C2, D1, D2). 5-HT + OA, OA was applied later in the presence of 5-HT (C3, D3).
In the control experiments, some neurons were injected only with GFP marker DNA construct. The expression of GFP protein did not affect the response of cells to 5-HT. 5-HT treatment of these GFP-expressing control cells produced membrane depolarization (Fig. 3B1), increase in membrane excitability (Fig. 3C1), and spike broadening (Fig. 3D1), as has previously been described in Aplysia sensory neurons (3). By contrast, OA treatment of the GFP-expressing cells produced none of these responses (Fig. 3 B1, C1, D1). This is consistent with RT-PCR data (Fig. 1B) demonstrating that Ap oa1 is not expressed endogenously in normal Aplysia sensory neurons.
Both OA and 5-HT Produced Membrane Depolarization.
In neurons expressing Ap oa1, exposure to OA in the bath led to depolarization of membrane potential by 4.75 ± 0.7 mV (n = 16) (Fig. 3B2). Subsequent application of 5-HT to the bath (n = 6) did not depolarize the cells further. Application of 5-HT alone either to the cells expressing GFP only (5.7 ± 0.6 mV; n = 13) or to the uninjected cells (4.4 ± 0.6 mV; n = 12) produced a depolarization to a similar extent that was achieved by OA in neurons expressing Ap Oa1. (Fig. 3B1). In the control experiments, application of OA either to the cells expressing GFP only (0.5 ± 0.3 mV; n = 10) or to the uninjected cells (0.2 ± 0.2 mV; n = 5) did not change the resting potential significantly.
OA and 5-HT Produce Similar Increases in Membrane Excitability.
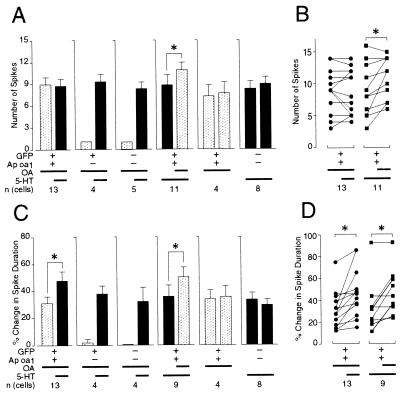
Exposure to OA produced an increase in membrane excitability in sensory cells that expressed Ap oa1 (Fig. 3C2). During the 500-ms depolarization step, the number of spikes in GFP/Ap oa1-expressing cells changed from 1 to 8.9 ± 1.0 (n = 13) after OA application and to 8.7 ± 1.0 (n = 13) after 5-HT application in addition to OA (Fig. 4A). The paired analysis shown in Fig. 4B Left for 13 cells revealed that the additional change in the number of spikes by 5-HT was not statistically significant (P > 0.7, n = 13).
Figure 4.
Group data showing that treatment with 1 μM OA causes increase in membrane excitability (A and B) as well as spike broadening (C and D) in sensory cells that expressed Ap oa1. Changes in membrane excitability are described as number of action potentials under fixed current step command during 500 ms (see Materials and Methods). Data represent the average response to drugs ± SEM. Striped bars indicate the response of the neurons when OA was applied alone or added later on top of 5-HT in the bath. Black bars indicate the response of the neurons when 5-HT was applied alone or added later on top of OA in the bath. +, expression of specific DNA constructs; −, no injection of DNA molecules. Thick lines illustrate the existence of applied drugs (OA or 5-HT) in bath solutions for 10 min (long lines) and 5 min (short lines), respectively. n (cells) is the number of cells examined. (B and D) The paired analyses of percentage change in the number of spikes (B) and spike duration (D) of individual cells. Data were individually compared by using the two-tailed Wilcoxon signed rank test from each cell before and after 5-HT or OA application to the bath that already contained OA or 5-HT, respectively: *, P < 0.02 (n = 11); P < 0.001 (n = 13), P < 0.04 (n = 9).
Next we reversed the order of drug treatment by applying 5-HT to the sensory cells expressing Ap oa1 before OA treatment (Fig. 3C3). The number of spikes per cell produced by 5-HT was 8.9 ± 1.3 (n = 11), and later application of OA to the bath containing 5-HT generated 10.9 ± 1.0 spikes (n = 11) during 500 ms (Fig. 4A). The paired analysis shown in Fig. 4B Right revealed that the additional change in the number of spikes by OA was statistically significant (P < 0.02, n = 11). By contrast, the number of spikes in GFP-expressing cells (n = 4) or in uninjected cells (n = 5) remained 1.0 after application of OA but changed to 9.3 ± 1.0 (n = 4) or to 8.2 ± 0.9 (n = 5), respectively, with 5-HT application to the bath containing OA (Fig. 3C1) (Fig. 4A).
We also tested the effect of prolonged application (≈10 min instead of 5 min) of either OA or 5-HT on the increase in membrane excitability. Prolonged application produced no further significant increase in membrane excitability either by OA (P > 0.3, n = 4) or by 5-HT (P > 0.3, n = 8) (Fig. 4A). Therefore, these data indicate that OA and 5-HT produce very similar increases in membrane excitability, and that the increase in membrane excitability achieved by 5-HT application could be still further enhanced slightly by exposure to OA.
Both OA and 5-HT Produce Spike Broadening.
Spike duration was increased by 31.4% ± 5.0% (n = 13) with exposure to OA for 5 min (Fig. 3D2). In GFP/Ap oa1-expressing cells, the broadening was further increased to 48.2% ± 6.2% in these cells (n = 13) by applying 5-HT for another 5 min on top of OA (Fig. 4C). The paired analysis shown in Fig. 4D Left revealed that the additional 5-HT-induced increase of spike duration (16.8% ± 4.7%, P < 0.001, n = 13) was statistically significant.
Next, we reversed the order of drug applications by treating 5-HT to the sensory cells (n = 9) expressing Ap oa1 before OA treatment (Fig. 3D3). Application of 5-HT for 5 min increased spike duration by 36.5% ± 8.1%, and application of OA for additional 5 min to the bath containing 5-HT further increased spike duration to 50.7% ± 7.4% (Fig. 4C). The paired analysis shown in Fig. 4D Right revealed that the additional increase of spike duration by OA was statistically significant (14.2% ± 4.9%, P < 0.04, n = 9).
In contrast, spike duration was not significantly changed by application of OA either in GFP-expressing cells (Fig. 3D1) (2.1% ± 2.1%, n = 4) or in uninjected sensory cells (0.0% ± 0.0%, n = 4). However, spike broadening was achieved by later application of 5-HT on top of OA either to the control cells expressing the GFP protein alone (38.3% ± 6.2%, n = 4) or to the noninjected sensory cells (32.7% ± 10.7%, n = 4) (Fig. 4C).
We also tested the effects of prolonged application (≈10 min instead of 5 min) of either OA or 5-HT on spike broadening. This application produced no additional broadening of action potential either by OA (P > 0.3, n = 4) or by 5-HT (P > 0.1, n = 8) (Fig. 4C). These data suggest that the additional increase in spike duration by different drugs is specific to the drugs that were applied.
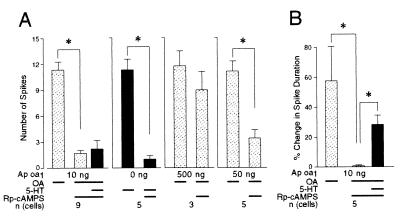
Rp-cAMPS Blocked the Increase in Membrane Excitability and Spike Broadening Produced by Ap oa1.
To determine whether the increase of membrane excitability and spike broadening are caused solely by PKA activity, we iontophoresed Rp-cAMPS (80 mM), a specific PKA inhibitor, into sensory neurons expressing Ap oa1. Rp-cAMPS blocked completely the increase in membrane excitability produced by OA of the sensory neurons that expressed Ap oa1 (1.7 ± 0.3, n = 9) (Fig. 5A). Before Rp-cAMPS iontophoresis, the same neurons showed a normal increase in membrane excitability when treated with OA (11.4 ± 0.9, n = 9) (Fig. 5A). This shows that PKA activity is necessary for the increase in membrane excitability.
Figure 5.
Iontophoresis of Rp-cAMPS into the sensory neurons expressing GFP/Ap oa1. (A) Rp-cAMPS blocked the increase in membrane excitability produced by either Ap oa1 or endogenous 5-HT receptor. (B) Rp-cAMPS blocked spike broadening produced by Ap oa1. However, later application of 5-HT produced spike broadening in the presence of Rp-cAMPS. *, P < 0.04 (n = 5), P < 0.002 (n = 9), one-tailed Wilcoxon signed rank test. Expression level of Ap oa1 was controlled by using different concentrations (10, 50, and 500 ng/μl) of pNEXδ-Ap oa1 in the microinjection solution. Thick lines under histograms indicate the presence of drugs in the bath, as described in the legend of Fig. 4.
Later application of 5-HT on top of OA did not increase membrane excitability significantly in the presence of Rp-cAMPS (2.2 ± 1.0, n = 9) (Fig. 5A). This suggests that Rp-cAMPS still blocked effectively PKA activity produced by 5-HT on top of OA. Consistent with this finding, 5-HT treatment of the uninjected control cells failed to increase membrane excitability in the presence of Rp-cAMPS (1.0 ± 0.4, n = 5) (Fig. 5A). Taken together, these data indicate that the increase of membrane excitability to both OA and 5-HT is generated predominantly by PKA activity.
The increase in membrane excitability induced by OA became resistant to Rp-cAMPS as the sensory neurons were microinjected with 50 ng/μl or higher concentration of pNEXδ-Ap oa1 (Fig. 5A). The expression level of the protein is known to correlate with the copy number of the microinjected DNA construct (27). Therefore, Rp-cAMPS may fail to effectively block PKA when the Ap oa1 is highly overexpressed and generates a greater amount of cAMP by OA.
Rp-cAMPS also completely abolished spike broadening produced by OA (0.5% ± 0.5%, n = 5) (Fig. 5B). Without Rp-cAMPS injection, the same neurons showed spike broadening by the treatment of OA (57.3% ± 22.8%, n = 5) (Fig. 5B). However, unlike the case of membrane excitability, application of 5-HT on top of OA still produced a significant increase in spike duration even in the presence of Rp-cAMPS (27.9% ± 6.3%, n = 5) (Fig. 5B). Our data cannot distinguish whether spike broadening is being further enhanced by an additional increase in cAMP produced by 5-HT or whether a second messenger system other then cAMP/PKA can contribute to additional spike broadening.
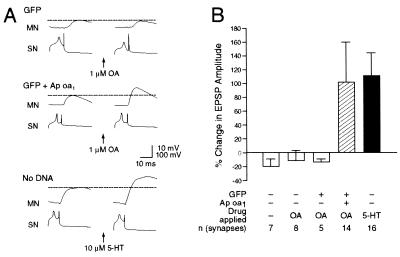
Sensory Neurons Expressing Ap oa1 Showed Short-Term Synaptic Facilitation by Treatment with OA.
Activation of Ap oa1 also generated synaptic facilitation between genetically engineered sensory cells and motor neurons. Application of OA produced a 102.1% ± 58.7% increase in amplitude of EPSP (n = 14), (Fig. 6B). This synaptic facilitation by OA was statistically significant (P < 0.002) and comparable to that observed by application of 10 μM 5-HT in positive control experiments (119.5% ± 33.8%, P < 0.002, n = 16) (Fig. 6B). Indeed, the extent of synaptic facilitation by OA is not significantly different from that by 5-HT (P > 0.5). In control experiments, when no drugs were applied to uninjected cells, repeated stimulation yielded a small decrease in EPSP amplitude by 18.6% ± 11.3% (P > 0.1, n = 7). When OA was applied to uninjected cells or to cells expressing GFP alone, we observed similarly a small decrease of amplitude of EPSP by 8.5% ± 10.3% (P > 0.3, n = 8) or by 12.4% ± 5.5% (P < 0.05, n = 5), respectively.
Figure 6.
Treatment with OA produced short-term facilitation in EPSP. (A) Representative monosynaptic EPSPs evoked by stimulating the sensory cells (SN) expressing GFP alone (Top) or expressing both GFP and Ap oa1 (Middle) or an uninjected sensory cell (SN) (Bottom). EPSPs were recorded at the motor neurons (MN) before (Left) and 5 min after (Right) the application of 1 μM OA (Top and Middle) or 10 μM 5-HT (Bottom) to the Aplysia pleural-pedal connections. (B) These group data indicate that OA enhanced amplitudes of EPSPs of motoneurons connected to sensory cells expressing Ap oa1. 5-HT also facilitated synaptic efficacy between uninjected sensory cell and motoneuron. Changes in EPSP amplitude are represented by blank bars (control for no activation of receptors), striped bar (activation of ectopic Ap oa1), and black bar (activation of endogenous 5-HT receptors). The height of each bar shows the mean ± SEM.
Taken together, these data show that intracellular cAMP synthesized by application of OA in the Ap oa1-expressing sensory neurons facilitates their synaptic connections to motor neurons, not only in the nondepressed case but even when that connection is slightly depressed (3, 31).
Discussion
We have taken a genetic approach to dissecting the relative role of cAMP/PKA and PKC signaling pathways in synaptic facilitation of connections between sensory and motor neurons of the gill-withdrawal reflex. We cloned an Aplysia OA receptor (Ap oa1) and have shown, pharmacologically and electrophysiologically, that this receptor couples exclusively to adenylyl cyclase to stimulate cAMP production. The deduced amino acid sequences suggest that Ap oa1 represents a new member of the OA receptor family. Our RT-PCR result shows that this receptor is not expressed in Aplysia sensory neurons, which therefore allowed us to express this receptor ectopically in these cells. Thus, by simply applying OA instead of 5-HT, we can selectively activate only cAMP/PKA pathways in the Ap oa1-expressing sensory neurons and ask to what degree cAMP is responsible for each process involved in short-term facilitation.
We find that in Ap oa1-expressing neurons, 1 μM OA produced all four changes characteristics of 5-HT: (i) membrane depolarization; (ii) spike broadening; (iii) increase in membrane excitability; and (iv) increase in transmitter release. Thus, the data suggest that cAMP plays a major role in these four features of synaptic facilitation.
It is not clear from our data, however, to what degree the increase in synaptic efficacy by OA results from spike broadening or from other processes. We would also emphasize that, as in other studies of plasticity, the results are likely to depend on the particular protocol that we used. Specifically, we have worked primarily with nondepressed or only slightly depressed synapses. There is good evidence that when transmitter release is depressed, PKC becomes importantly involved in facilitation. Also, our data do not exclude the possibility that higher doses of 5-HT or more prolonged exposure to 5-HT would recruit second messenger pathways other than cAMP.
Because cAMP is a key second messenger in the late phase of long-term potentiation in the hippocampus (32) and in associative learning in Drosophila (33, 34), Ap oa1 may provide a useful tool to study the role of cAMP in learning and memory by gene transfer of this exogenous receptor to these experimental systems.
Acknowledgments
We thank Drs. Norman Davidson (Caltech) (5-HT2c), Lap-Chee Tsui (The Hospital for Sick Children, Toronto) (CFTR), Robert Lefkowitz (Duke University) (β2-adrenergic receptor), Ham van Heerikhuizen (Vrije Universiteit, Amsterdam) (Lym oa1), and Douglas Melton (Harvard University) (pSP64T) for their generous gifts of the plasmids used in this study. This research was supported by the Korean Ministry of Science and Technology under the Brain Science Research Program and by the Korea Research Foundation in the program year of 1998 to B.-K.K. E.R.K. is supported by the Howard Hughes Medical Institute laboratories and K.R.W. by the National Institute of Mental Health.
Abbreviations
- 5-HT
5-hydroxytryptamine
- PKA
protein kinase A
- PKC
protein kinase C
- CHO
Chinese hamster ovary
- CFTR
cystic fibrosis transmembrane regulator
- GFP
green fluorescent protein
- EPSP
excitatory postsynaptic potential
- IP3
inositol triphosphate
- OA
octopamine
- CNS
central nervous system
- PI
phosphoinositide IPs, inositol phosphates
- RT-PCR
reverse transcriptase–PCR
- mAChR
muscarinic acetylcholine receptor
Footnotes
Data deposition: The sequences reported in this paper have been deposited in the GenBank database (accession nos. AF117654 and AF222978).
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.030524297.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.030524297
References
- 1.Kandel E R, Schwartz J H. Science. 1982;218:433–443. doi: 10.1126/science.6289442. [DOI] [PubMed] [Google Scholar]
- 2.Frost W N, Clark G A, Kandel E R. J Neurobiol. 1988;19:297–334. doi: 10.1002/neu.480190402. [DOI] [PubMed] [Google Scholar]
- 3.Byrne J H, Kandel E R. J Neurosci. 1996;16:425–435. doi: 10.1523/JNEUROSCI.16-02-00425.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mackey S L, Kandel E R, Hawkins R D. J Neurosci. 1989;9:4227–4235. doi: 10.1523/JNEUROSCI.09-12-04227.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brunelli M, Castellucci V, Kandel E R. Science. 1976;194:1178–1181. doi: 10.1126/science.186870. [DOI] [PubMed] [Google Scholar]
- 6.Klein M, Kandel E R. Proc Natl Acad Sci USA. 1978;75:3512–3516. doi: 10.1073/pnas.75.7.3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klein M, Camardo J, Kandel E R. Proc Natl Acad Sci USA. 1982;79:5713–5717. doi: 10.1073/pnas.79.18.5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walters E T, Byrne D A, Carew T J, Kandel E R. J Neurophysiol. 1983;50:1543–1559. doi: 10.1152/jn.1983.50.6.1543. [DOI] [PubMed] [Google Scholar]
- 9.Pollock J D, Bernier L, Carmardo J S. J Neurosci. 1985;5:1862–1871. doi: 10.1523/JNEUROSCI.05-07-01862.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hochner B, Kandel E R. Proc Natl Acad Sci USA. 1992;89:11476–11480. doi: 10.1073/pnas.89.23.11476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mercer A R, Emptage N J, Carew T J. Science. 1991;254:1811–1813. doi: 10.1126/science.1662413. [DOI] [PubMed] [Google Scholar]
- 12.Stark L L, Mercer A R, Emptage N J, Carew T J. J Neurophysiol. 1996;75:855–866. doi: 10.1152/jn.1996.75.2.855. [DOI] [PubMed] [Google Scholar]
- 13.Vanhoutte P M, Humphrey P P A, Spedding M. Pharmacol Rev. 1996;48:1–2. [PubMed] [Google Scholar]
- 14.Kaang B K, Kandel E R, Grant S G N. Neuron. 1993;10:427–435. doi: 10.1016/0896-6273(93)90331-k. [DOI] [PubMed] [Google Scholar]
- 15.Kaang B K, Pfaffinger P J, Grant S G N, Kandel E R, Furukawa Y. Proc Natl Acad Sci USA. 1992;89:1133–1137. doi: 10.1073/pnas.89.3.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li X-C, Giot J-F, Kuhl D, Hen R, Kandel E R. J Neurosci. 1995;15:7585–7591. doi: 10.1523/JNEUROSCI.15-11-07585.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Akiba I, Kubo T, Maeda A, Bujo H, Nakai J, Mishina M, Numa S. FEBS Lett. 1988;235:257–261. doi: 10.1016/0014-5793(88)81274-2. [DOI] [PubMed] [Google Scholar]
- 18.Berridge M J, Dawson R M, Downes C P, Heslop J P, Irvine R F. Biochem J. 1983;212:473–482. doi: 10.1042/bj2120473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hwang J M, Chang D J, Kim U S, Lee Y S, Park Y S, Kaang B K, Cho N J. Receptors Channels. 2000;6:415–424. [PubMed] [Google Scholar]
- 20.Kobilka B K, MacGregor C, Daniel K, Kobila T S, Caron M G, Lefkowitz R J. J Biol Chem. 1987;262:15796–15802. [PubMed] [Google Scholar]
- 21.Lubbert H, Hoffman B J, Snutch T P, Dyke T V, Levine A J, Henry P R, Lester H A, Davidson N. Proc Natl Acad Sci USA. 1987;94:4322–4336. doi: 10.1073/pnas.84.12.4332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gerhardt C C, Bakker R A, Piek G J, Planta R J, Vreugdenhil E, Leysen J E, van Heerikhuizen H. Mol Pharmacol. 1997;51:293–300. doi: 10.1124/mol.51.2.293. [DOI] [PubMed] [Google Scholar]
- 23.Bear C E, Duguay F, Naismith A L, Hanrahan J W, Riordan J R. J Biol Chem. 1991;266:19142–19145. [PubMed] [Google Scholar]
- 24.Lee Y S, Park Y S, Chang D J, Hwang J M, Min C K, Kaang B K, Cho N J. J Neurochem. 1999;72:58–65. doi: 10.1046/j.1471-4159.1999.0720058.x. [DOI] [PubMed] [Google Scholar]
- 25.Kaang B K. Mol Cell. 1996;6:285–295. [Google Scholar]
- 26.Kim H K, Kaang B K. Brain Res Bull. 1998;47:35–41. doi: 10.1016/s0361-9230(98)00020-3. [DOI] [PubMed] [Google Scholar]
- 27.Kaang B K. Neurosci Lett. 1996;221:29–32. doi: 10.1016/s0304-3940(96)13279-1. [DOI] [PubMed] [Google Scholar]
- 28.Lim C S, Chung D Y, Kaang B K. Mol Cell. 1997;7:399–407. [PubMed] [Google Scholar]
- 29.Emptage N J, Carew T J. Science. 1993;262:253–256. doi: 10.1126/science.8211146. [DOI] [PubMed] [Google Scholar]
- 30.Uezono Y, Bradley J, Min C, McCarty N A, Quick M, Riordan J R, Chavkin C, Zinn K, Lester H A, Davidson N. Receptors Channels. 1993;1:233–241. [PubMed] [Google Scholar]
- 31.Ghirardi M, Montarolo P G, Kandel E R. Neuron. 1995;14:413–420. doi: 10.1016/0896-6273(95)90297-x. [DOI] [PubMed] [Google Scholar]
- 32.Bailey C H, Bartsch D, Kandel E R. Proc Natl Acad Sci USA. 1996;93:13445–13452. doi: 10.1073/pnas.93.24.13445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Davis R L. Physiol Rev. 1996;76:299–317. doi: 10.1152/physrev.1996.76.2.299. [DOI] [PubMed] [Google Scholar]
- 34.Tully T. Proc Natl Acad Sci USA. 1996;93:13460–13467. doi: 10.1073/pnas.93.24.13460. [DOI] [PMC free article] [PubMed] [Google Scholar]