Abstract
The acquisition of terminal cell fate and onset of differentiation are instructed by cell type–specific master control genes. Loss of differentiation is frequently observed during cancer progression, but the underlying causes and mechanisms remain poorly understood. We tested the hypothesis that master regulators of differentiation may be key regulators of tumor formation. Using loss- and gain-of-function analyses in Drosophila, we describe a critical anti-oncogenic function for the atonal transcription factor in the fly retina, where atonal instructs tissue differentiation. In the tumor context, atonal acts by regulating cell proliferation and death via the JNK stress response pathway. Combined with evidence that atonal's mammalian homolog, ATOH1, is a tumor suppressor gene, our data support a critical, evolutionarily conserved, function for ato in oncogenesis.
Author Summary
During embryonic development, cells become more and more specialized, and this process is referred to as differentiation. In contrast to normal adult cells, cancer cells—like embryonic cells—display fewer differentiated properties. It has been postulated that the acquisition of terminal differentiation helps inhibit tumor formation; however, no direct evidence for this hypothesis was available. The development of the eye in the fruit fly, Drosophila melanogaster, has long been used as a model for studying genetic factors controlling differentiation. More recently, eye development has also been used to study how tumors can form and progress. In this study, we used this model to show that genes, such as atonal, that instruct the differentiation of specific tissues can act as tumor suppressers and inhibit the formation and progression of tumors in those tissues. Losing such genes can generate tumors, whereas activating them can strongly inhibit these tumors.
We establish a direct genetic link between cancer and the initiation of differentiation in theDrosophila eye.
Introduction
Cell fate commitment in neural and neuroendocrine lineages of the peripheral nervous system (PNS) as well as secretory epithelia is controlled by genes of the basic helix-loop-helix (bHLH) superfamily of transcription factors. One of the most structurally and functionally conserved groups within this family is the Atonal (Ato) group proteins [1,2]. Drosophila ato (CG7508) and mammalian ATOH1 (Ensembl accession number: ENSG00000172238) are required for cell fate specification and the induction of differentiation in the PNS and the secretory lineages in all animal species. In Drosophila, ato is necessary for cell fate specification and differentiation of mechano- and photoreceptors [3–5].
The acquisition of differentiated cell fate endows cells with two key features. First, it allows them to become distinct from one another and, accordingly, functionally specialized. Second, it inhibits further cell division under physiological conditions, thus controlling tissue size. When the regulation of cell division fails, cancer may develop. Cancer, however, is the result of a selective process in which cells accumulate several genetic and epigenetic mutations giving them a growth advantage over surrounding cells by, for example, the inhibition of apoptosis, induction of angiogenesis, and growth factor–independent survival [6]. More than one mutation is needed for cancer to arise, and it is therefore thought that mutations occur in undifferentiated cells that are proliferative. As such, oncogenesis might select for cells that have lost their capacity to induce differentiation. In this context, it has been a long-standing postulate that cancer is a disease of loss of differentiation [7,8]. Work in the seventies and eighties by Harris and colleagues shows that hybrids of malignant and diploid cells only become malignant again after losing chromosomal loci required for differentiation (e.g., [9]). More recently, the interplay between differentiation and cancer has gained renewed attention through the study of a pool of undifferentiated cells in tumors, the so-called cancer stem or tumor-initiating cells [10]. A major theme emerging from these studies is the importance of the maintenance of an undifferentiated state in this niche for tumor growth to occur. Furthermore, the fact that signals implicated in regulating differentiation across various lineages, such as the WNT and Notch pathways, also have been implicated both in the promotion and suppression of cancer [11,12] suggests a mechanistic link between the regulation of differentiation and tumor progression. Importantly, however, these pathways are also implicated in stem cell or progenitor cell maintenance and do not act in a lineage-restricted fashion. As such, the definition of their role in tumor progression vis-a-vis differentiation is unclear. For this hypothesis to be correct, at least one key prediction should hold true: master control genes that instruct cell fate commitment in specific lineages should act as brakes on the oncogenic process, either by preventing uncontrolled proliferation or by inducing cell death when a differentiated state can no longer be maintained. Thus, we hypothesize that such master control genes suppress both tumor formation and progression.
To test this prediction in lineages in which ato is the key regulator of cell fate commitment, we asked two experimental questions. First, does ato loss of function contribute to tumor initiation or progression in tissues where ato instructs differentiation, such as the Drosophila retina? Second, can ato gain of function inhibit the formation or progression of these tumors? Finally, we examined the genetic pathway by which ato suppresses tumor formation.
We find that loss of ato strongly enhances the formation and progression of tumors in flies. Conversely, gain of ato function strongly inhibits tumor formation and metastasis. Finally, we describe a highly conserved anti-oncogenic genetic pathway that links ato activity to the stress sensor Jun N-terminal kinase (JNK) pathway. Combined with genetic and molecular evidence from mouse and human cancer models [13], these data support a key role for ato in a very early step of the oncogenic process and suggest that mutations in master control genes of cell fate commitment may be pivotal switches during tumorigenesis.
Results
Gain and Loss of ato Function Suppresses and Enhances Tumor Formation, Respectively, in a Drosophila Cancer Paradigm
We took advantage of the genetic power of Drosophila melanogaster to investigate whether the gain and loss of function of Drosophila ato suppresses and enhances tumor formation, respectively. ato instructs differentiation in the Drosophila eye [4]. We therefore turned to a well-established in vivo eye cancer model, namely “eyeful” flies, which has been used to study the mechanisms of Rb and the PTEN-AKT pathway in cancer [14,15]. The eyeful flies have activated Notch signaling in the developing eye due to overexpression of the Notch ligand Delta (Dl, CG3619), combined with overexpression of lola (CG12052) and psq (CG2368). Flies overexpressing only Dl, leading to an increase in eye size but no tumors, will henceforth be called “sensitized” flies.
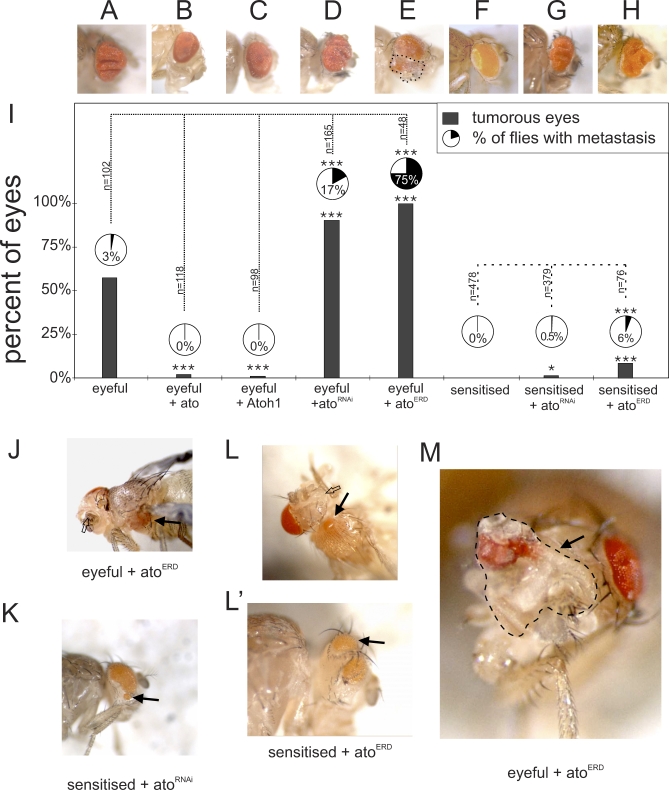
To analyze the tumor burden, each eye was scored separately. Eyes were counted as hyperplastic when the eye showed at least one fold. Metastasis can be seen as masses of amorphous red-pigmented cells outside of the eye field and are observed on the head and in the thorax and abdomen (Figure 1J–1L). Consistent with previous data, eyeful flies display excessively enlarged eyes, and eye tumors occur in 57% of the eyes, with 3% of the flies showing macroscopically visible metastases derived from the developing retina (n = 102; Figures 1A, 1I, S1A, and S1B). Overexpression of ato, or its mouse ortholog Atoh1 (Ensembl: ENSMUSG00000073043)—but not a green fluorescent protein (GFP) control transgene—in the eyeful background almost completely suppresses the formation of eye tumors (Figure 1B (ato): 2%, p < 0.0001, n = 118; Figure 1C (Atoh1): 1%, p < 0.0001, n = 98; GFP (unpublished data): p = 0.25; Figure 1I). More importantly, reduction of endogenous ato expression in the eye using an ato RNA interference (RNAi) construct (a kind gift from A. Jarman), which leads to loss of differentiated eye tissue in wild-type flies (Figure S2B), results in a dramatic increase in both tumor incidence (90%, p < 0.0001, n = 165; Figure 1D and 1I) and the number of flies with metastases (17%, p = 0.0003). These effects were not due to the overexpression of a double-stranded RNA, per se, because the expression of an RNAi construct for GFP did not change the tumor burden (unpublished data; tumors: p = 0.59, metastasis: p = 0.21).
Figure 1. atonal Gain and Loss of Function Suppress and Promote Tumor Formation, Respectively, in a Drosophila Cancer Paradigm.
(A–I) Qualitative and quantitative representation of the tumor burden in different genotypes. Each lane is a separate genotype. Gray bars show percentage of tumorous eyes, pie charts show metastasis incidence. (A) ey-GAL4, UAS-Dl, eyeful/+ fly (“+” = wild-type chromosome). (B and C) Gain of ato function suppresses tumor formation. (B) ey-GAL4, UAS-Dl, eyeful/UAS-ato. (C) ey-GAL4, UAS-Dl, eyeful/UAS-Atoh1. (D and E) Loss of ato function enhances tumor burden. (D) ey-GAL4, UAS-Dl, eyeful/+; UAS-atoRNAi/+. (E) ey-GAL4, UAS-Dl, eyeful/UAS-atoERD. Dotted line indicates undifferentiated overgrowth of the eye tumor. (F) A sensitized genotype with eye-specific Dl overexpression leading to mild eye overgrowth: ey-Gal4, UAS-Dl/+. (G and H) Loss of ato function can initiate tumor formation. (G) ey-Gal4, UAS-Dl/+, UAS-atoRNAi/+. (H) ey-Gal, UAS-Dl/UAS-atoERD.
(I) Graph showing quantification of the tumor burden in the different genotypes. Dotted lines show the comparison used for statistical analysis. A single asterisk (*) indicates p < 0.05; triple asterisks (***) indicate p < 0.001 as analyzed by the chi-square test, and ‘n' represents number of flies analyzed.
(J and K) Examples of metastasis and loss of differentiated eye tissue in eyeful and sensitized flies upon loss of ato function. (J) ey-GAL4, UAS-Dl, eyeful/UAS-atoERD: black arrow shows metastasis, open arrow shows loss of differentiated eye tissue. (K) ey-Gal4, UAS-Dl/+, UAS-atoRNAi/+ with metastasis on the head.
(L and L′) ey-Gal, UAS-Dl/UAS-atoERD showing metastasis in the thorax (black arrows) and on the head and loss of differentiated retina (open arrow).
(M) ey-Gal, UAS-Dl/UAS-atoERD showing a massive undifferentiated tumor (dotted line) on the fly head.
Since Ato is known as a transcriptional activator, we asked whether its role in eye tumors is mediated by its ability to activate gene expression. We constructed a repressor form of Ato by fusing it to an Engrailed repressor domain (AtoERD) [16]. Expression of AtoERD in the developing eye leads to loss of differentiated eye tissue mimicking ato loss-of-function mutations and the atoRNAi construct (Figure S2A–S2C). Expression of AtoERD in eyeful flies results in both the loss of differentiated eye tissue in 30% of the eye fields (Figure 1J, open arrow), as well as in 100% tumors in the remaining eyes (p < 0.0001, n = 48; Figure 1E and 1I). Importantly, these tumors include large patches of undifferentiated tissue, showing that loss of ato's differentiation function is linked to its anti-oncogenic function (Figure 1E and 1M, dotted line). Expression of AtoERD in the eyeful flies also results in 75% of the flies showing metastasis (Figure 1J, black arrow, and Figure 1I; p < 0.0001). These data suggest that ato is a key regulator of tumor progression in Drosophila and that it may perform this function by regulating the differentiation status of the transformed tissue.
Ato Acts as a Switch for Tumor Initiation
Loss of ato in a wild-type background abrogates retinal differentiation and causes subsequent loss of the entire tissue [4]. If loss of differentiation is an early causal event in cancer, a key anti-oncogenic role for ato requires that its loss act as a switch for tumor initiation in a pre-oncogenic background. To this end, we used the sensitized genetic background that was used to generate the eyeful model, namely eye-specific Dl overexpression [14]. This genotype results in an increase in proliferation, leading in turn to a slight overgrowth of the eye, but no tumors are observed (n = 478; Figure 1F) [17]. Inhibition of ato function by AtoERD leads, as it does in wild-type flies, to loss of retinal differentiation (36% of the eye fields, empty arrow, Figure 1L), and to a 9% de novo tumor incidence in the remaining eye fields (p < 0.0001, n = 76; Figure 1H, 1L, and 1L′), with 6% of the flies showing metastasis (p = 0.0003). Similarly, ato knockdown using atoRNAi in this sensitized background leads to eye tumors in 0.5% of the eyes (p = 0.0333, n = 379; Figure 1G and 1K), and 0.3% of the flies have metastasis (p = 0.1953). The metastases in the sensitized flies upon loss of ato function are mostly present in the thorax and on the head. The metastases on the head (atoRNAi = 75%; atoERD = 43%) show a high resemblance to ectopically induced eyes as seen by Kurata and colleagues upon overactivation of Notch signaling [18]. The loss of ato might increase Notch signaling and as such interfere with patterning and determination. We note, however, that we never see any head metastasis when overexpressing Dl, indicating that loss of ato creates new phenotypes including the metastasis in the thorax, which cannot be explained by an increase in Notch activity alone. In either case, these data support the hypothesis that loss of ato function is sufficient to transform a sensitized lesion into a metastatic tumor, possibly by interfering with patterning and determination.
Ato Regulates Apoptosis and Proliferation in Drosophila Eye Tumors
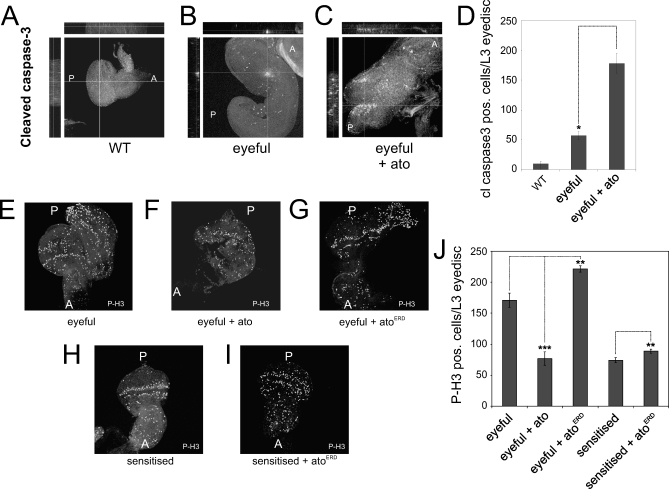
ato loss- and gain-of-function analyses suggest a decisive role in tumor formation in the fly retina. The growth of tumors is a balance between cell proliferation and cell death. We asked whether ato regulates either or both of these processes in the context of eyeful tumors, during the development of these tumors. To this end, we examined third instar larval eye discs, the Drosophila eye anlage, for markers of apoptosis and proliferation. Overexpression of Ato in the eyeful fly results in dramatically increased levels of the apoptotic regulator caspase-3 (FlyBase ID: FBgn0028381) in eyeful eye discs (3-fold, p = 0.011; Figure 2A–2D). This explains, at least in part, the suppression of the eyeful tumors in the adult flies.
Figure 2. Apoptosis and Proliferation upon ato Loss and Gain of Function.
(A–C) Staining for cleaved caspase-3 indicates an increase in apoptosis upon overexpression of ato in the eyeful flies. (A) Immunohistochemistry for cleaved capsase-3 in wild type.
(B) Cleaved caspase-3 staining in ey-GAL4, UAS-Dl, eyeful/+.
(C) ey-GAL4, UAS-Dl, eyeful/UAS-ato.
(D) Quantification of cleaved caspase-3–positive cells per eye disc. Error bars indicate the standard error of the mean, and double asterisks (**) indicate p < 0.05.
(E–I) Representative images from third instar eye discs in different genetic backgrounds stained for phospho-Histone H3 (P-H3). (E) ey-GAL4, UAS-Dl, eyeful/+. (F) ey-GAL4, UAS-Dl, eyeful/UAS-ato. (G) ey-GAL4, UAS-Dl, eyeful/UAS-atoERD. (H) ey-GAL4, UAS-Dl/+. (I) ey-GAL4, UAS-Dl/UAS-atoERD. (J) Quantification of proliferating cells in the eyeful and sensitized background upon loss or gain of ato function. Quantification was done for a minimum of ten eye discs per genotype. The chi-square test was used to analyze for significance. Dotted lines connect compared genotypes. Triple asterisks (***) indicate p < 0.001; double asterisks (**) indicate p < 0.01.
Next, we examined proliferation in eyeful eye discs under gain and loss of ato function conditions, using phospho-HistoneH3 (FlyBase ID:FBtr0071345) as a marker. Proliferation in the third instar eye disc normally occurs anterior to the morphogenetic furrow, where all the cells are still undifferentiated. Additionally, approximately two rows of undifferentiated cells posterior to the furrow, called the second mitotic wave (SMW), also proliferate. In the eyeful discs, proliferating cells are not restricted to these two domains but are also present posterior to the SMW (Figure 2E). Expression of ato in the eyeful disc reduces this ectopic proliferation (Figure 2F), whereas inhibition of ato activity increases ectopic proliferation (Figure 2G). As suppression of Ato activity can initiate de novo tumor formation in a sensitized background, we examined proliferation upon expression of AtoERD in the Dl-sensitized background. In the sensitized eye discs, proliferating cells are mostly restricted anterior to the furrow and the SMW (Figure 2H), whereas loss of ato function leads to the appearance of proliferating cells in the posterior region of the disc (Figure 2I).
The total number of cell divisions in a tissue determines the overall size of that tissue. We therefore quantified the total number of phospho-HistoneH3–positive cells per disc. Overexpression of Ato in eyeful eye discs results in a significant decrease in number of cells expressing the mitotic marker phosphorylated HistoneH3 (p = 0.00004; Figure 2J). Conversely, expression of the dominant-negative AtoERD leads to a significant up-regulation of proliferation in the eyeful eye discs (p = 0.004; Figure 2J). Thus, Ato limits number of cell divisions in the eyeful tumors. Expression of AtoERD in the Dl-sensitized eye discs results in a significant increase in phosphorylated HistoneH3 expression in the developing eye discs (p = 0.002; Figure 2J), explaining the induction of tumors by loss of ato.
Our analysis suggests that ato regulates both proliferation and death of retinal precursors during tumor formation in the Drosophila eye.
Ato Regulates Tissue Differentiation and Patterning in Drosophila Eye Tumors
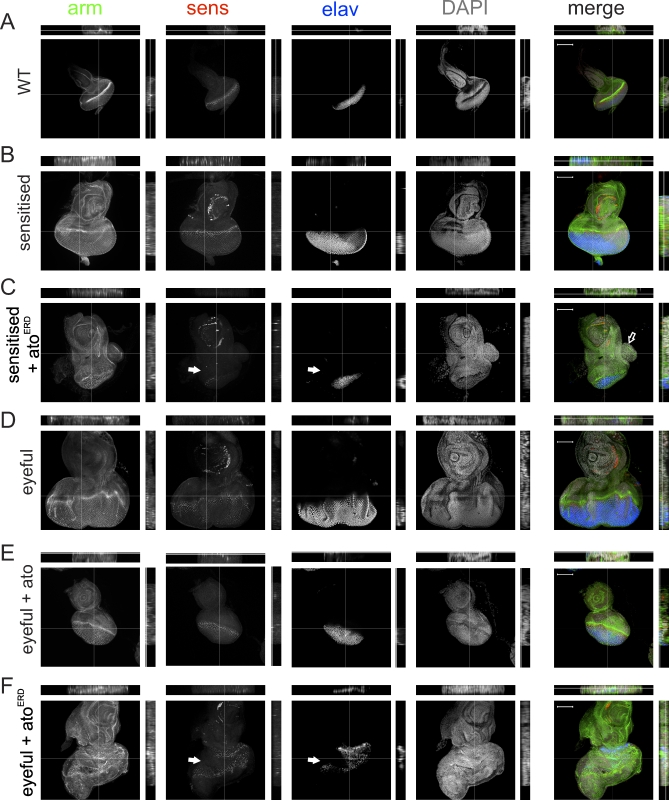
During normal development, Ato is required for the correct differentiation of retinal cells and the proper patterning of the eye disc. If Ato's function in suppressing eye tumors is related to its activity as a differentiation factor, we might expect to observe Ato-dependent alterations in tissue differentiation and organization upon manipulation of Ato activity in a tumor context. To test this prediction, we examined the expression of the early differentiation and R8 marker Senseless (Sens, CG32120), the general retinal photoreceptor marker embryonic lethal, abnormal vision (ELAV, CG4262), and the epithelial marker Armadillo/β-Catenin (Arm, CG11579) following manipulation of Ato activity.
In wild-type and Dl-sensitized eye discs Arm, ELAV, and Sens reveal the regular and stereotypical differentiation and epithelial organization of the developing retina, although the Dl-sensitized discs are clearly larger (Figure 3A and 3B). Loss of Ato activity in the Dl-sensitized eye discs (Figure 3C) results in the disruption of the regular pattern of Arm expression, suggesting defects in the organization of the retinal epithelium. This is accompanied by severe reduction in Sens and ELAV staining, suggesting lack of differentiated photoreceptors. The proportion of undifferentiated to differentiated cells is increased, indicating that the initial steps of retinal differentiation, namely the specification of the Ato-dependent R8 cell, are compromised (Figure 3C, white arrows). In some instances, lobes of proliferative and undifferentiated tissue are observed in these eye discs (Figure 3C, open arrow), correlating with the appearance of tumors in the adult flies. In the eyeful eye discs, disorganization of the epithelium as well as defects in the pattern of differentiated cells are apparent (Figure 3D). Overexpression of Ato in the eyeful eye discs restores both the size and all three markers to essentially wild-type patterns of expression (Figure 3E), explaining the appearance of normal adult eyes in this background. Conversely, expression of AtoERD severely disrupts retinal patterning and the expression pattern of all three markers (Figure 3F). Differentiation markers are not only reduced, but also appear in a highly disruptive pattern to the extent that the morphogenetic furrow is difficult to discriminate (Figure 3F).
Figure 3. Analysis of Tissue Patterning and Differentiation in Third Instar Eye Discs.
Immunohistochemistry was used for armadillo (arm, green), embryonic lethal, abnormal vision (elav, blue), senseless (sens, red), and diamidinophenylindole (dapi, grey)
(A) Wild-type eye disc.
(B) ey-GAL4, UAS-Dl/+ shows enlarged discs with wild-type patterning.
(C) ey-GAL4, UAS-Dl/UAS-atoERD show disrupted patterning with expansion of the undifferentiated domain (white arrows). Proliferative outgrowth is indicated with an open arrow.
(D) ey-GAL4, UAS-Dl, eyeful/+.
(E) Gain of ato function in the eyeful background leads to restoration of the pattern of differentiation: ey-GAL4, UAS-Dl, eyeful/UAS-ato shows almost normal appearance of all markers.
(F) Loss of ato function in an eyeful background leads to loss of uniform arm staining and a loss of and abnormal pattern of differentiation (elav and sens, white arrows). ey-GAL4, UAS-Dl, eyeful/UAS-atoERD.
Images and orthogonal sections are shown. All images were taken at the depth of the nuclei. Scale bars represent 100 μm.
In summary, loss- and gain-of-function analyses in Drosophila support a critical and early role for the loss of ato in tumor initiation and progression. This effect is likely mediated by alteration in the expression of downstream genes required for retinal differentiation, as such perturbing proliferation, apoptosis, and tissue organization.
Ato Functions via JNK-Dependent Mechanism
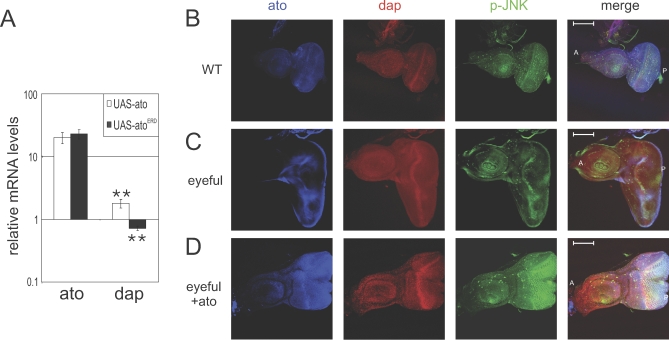
To better understand the role of that Ato plays in tumor formation, we sought to determine the genetic mechanism by which it acts to suppress the formation and progression of tumors. Gain- and loss-of-function analysis indicated an ato-dependent regulation of proliferation in the Drosophila eye. Recently, the Drosophila ortholog of the gene encoding the cell cycle inhibitor p21waf1, dacapo (dap, CG1772), was reported to be a target gene of ato in the eye [19]. Consistent with this, overexpression of wild-type ato in the eye disc leads to significant up-regulation of Dap mRNA (∼80%, p = 0.018), whereas expression of AtoERD leads to significant down-regulation of Dap mRNA (28%, p = 0.014; Figure 4A). Ato expression also results in earlier onset and elevated Dap levels in the eyeful and wild-type eye discs, in agreement with the reduction in pH3 levels observed in the same discs (Figures 4B–4D and S3).
Figure 4. Ato Up-Regulates Dap and p-JNK.
(A) Quantitative RT-PCR for Ato (first two bars) and Dap (second two bars) upon Ato (white bars) and AtoERD (gray bars) expression, standardized to wild type and driver controls and to expression of three housekeeping genes. Error bars indicate the standard error of the mean. Double asterisks (**) indicate p < 0.01 (t-test).
(B–D) Expression of ato in ey-GAL4, UAS-Dl, eyeful flies up-regulates Dap and phosphorylated JNK. (B) Third instar eye disc from wild type. (C) Third instar eye disc from ey-GAL4, UAS-Dl, eyeful/+. (D) Third instar eye discs from ey-GAL4, UAS-Dl, eyeful/UAS-ato. A indicates anterior; P indicates posterior.
We have shown that Ato regulates apoptosis and that it restores proper differentiation in the eyeful eye discs. We reasoned that tumorous eyeful cells may interpret the Ato differentiation signal as cellular stress and, as a result, commit suicide. A major regulator of cell death in response to stress is the JNK pathway. We therefore examined the expression of phosphorylated (i.e., activated) form of the Drosophila JNK (pJNK), Basket (Bsk, CG5680). Eyeful discs show reduced pJNK levels. Overexpression of Ato in this background, as well as wild-type eye discs, results in dramatic up-regulation of pJNK levels (Figures 4B–4D and S3).
These data suggest that Ato regulates the expression and activity of major regulators of cell proliferation and death. We therefore tested whether these genes also play a role in the eyeful tumors. Dap overexpression leads to a significant inhibition of tumor occurrence (22%, p < 0.0001, n = 107; Figure 5C and 5I), but only a partial reduction in metastasis (1%, p = 0.1145). Thus, whereas Dap regulation appears to contribute to tumor suppression by Ato, it is unlikely to explain the full effect of ato expression.
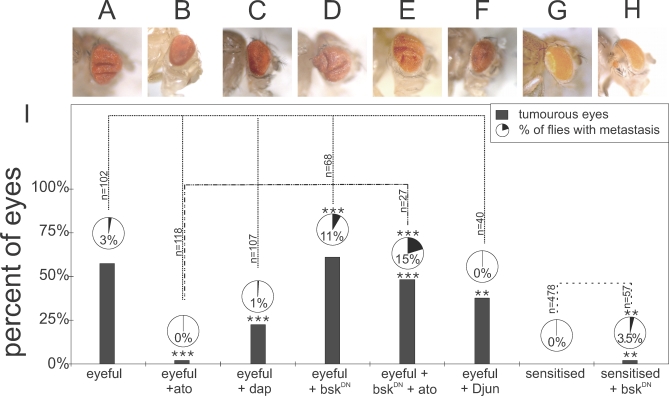
Figure 5. ato Functions by a JNK-Dependent Mechanism.
(A) ey-GAL4, UAS-Dl, eyeful/+ fly.
(B) ey-GAL4, UAS-Dl, eyeful/UAS-ato.
(C) ey-GAL4, UAS-Dl, eyeful/UAS-dap.
(D) bskDN/X; ey-GAL4, UAS-Dl, eyeful/+.
(E) ato function depends on JNK activity: bskDN/X; ey-GAL4, UAS-Dl, eyeful/UAS-ato.
(F) ey-GAL4, UAS-Dl, eyeful/UAS-Djun.
(G) A sensitized genotype with eye-specific Dl overexpression leading to mild eye overgrowth: ey-Gal4, UAS-Dl/+.
(H) bskDN/X; ey-GAL4, UAS-Dl/+.
(I) Quantitative representation of the tumor burden in different genotypes. Each lane is a separate genotype. Gray bars show percentage of tumorous eyes; pie charts give metastasis incidence. Double asterisks (**) indicate p < 0.01; triple asterisks (***) indicate p < 0.001 as analyzed by chi-square test, and n represents number of flies analyzed.
To analyze whether the elevated activity of JNK signaling upon ato expression is functionally relevant, we inhibited JNK signaling using a dominant-negative form of Bsk (BskDN). This partially mimics down-regulation of ato in the eyeful model and results in tumors in 61% of the eyes (p = 0.574; Figure 5D and 5I) and an approximately7-fold increase in metastasis (p = 0.0003). Furthermore, expression of BskDN in the Dl-sensitized background leads to the induction of tumors (2%, p = 0.0113, n = 57; Figure 5H and 5I) and metastasis (3.5%, p = 0.0112). Conversely, overexpression of Djun (Jra, CG2275), the transcriptional effector of the JNK pathway, leads to reduction of the tumor burden (38% tumors, p = 0.0036, n = 40; 0% metastasis, p = 0.559; Figure 5F and 5I), partially mimicking overexpression of Ato expression.
Next, we tested genetic epistasis between ato and JNK by overexpressing Ato while simultaneously inhibiting JNK signaling. This leads to a suppression of the inhibitory effects of ato on the eyeful flies and restores tumor formation (48%, p < 0.001), as well as enhances the metastatic phenotype (15%, p = 0.001, n = 27; Figure 5E and 5I). This indicates that JNK signaling is downstream of ato and that ato requires active JNK signaling to inhibit cancer formation.
Discussion
Our data support a function for ato in oncogenesis. Loss of ato promotes tumor formation and progression and might, as such, be selected for during oncogenesis. This indicates that tumor formation and progression might not only require maintenance of self-renewal capacity, but also loss of the capacity to induce cell fate commitment and differentiation. Therefore, genes that act precisely at the junction of the transition from a proliferating progenitor to a committed cell ought to show anti-oncogenic behavior. Losing ato in the absence of any other compounding factor is neutral towards tumor formation. However, loss of ato in a sensitized background is sufficient to initiate and enhance tumor formation. In our experiments, we used activation of the Notch signaling pathway as a sensitizing factor, but other pathways also lead to the formation of tumors when ato is lost [13]. Therefore, loss of differentiation factors might “tip the balance” towards malignancy, regardless of what the additional oncogenic event may be. It will be interesting to investigate what the different pathways are that interact with loss of ato to enhance cancer formation and how they switch an ato mutation from neutral to tumor progression to oncogenic.
The induction of cellular differentiation acts on two levels: first, the cell cycle is inhibited by the expression of cell cycle inhibitors; and second, gene expression is modulated to instruct a specific fate and function. Several lines of evidence suggest that both levels of ato activity are important in its anti-oncogenic function. First, ato regulates the expression of dap—itself a direct target gene of ato during normal differentiation—during eye tumor formation. Second, loss of ato leads to more proliferation in the sensitized and cancerous tissue in a Drosophila model. Third, loss of ato leads to the disruption of retinal differentiation and patterning, correlating with the formation of tumors that include overgrowth of undifferentiated tissue in the fly eye. Together, these data support the idea that Ato exerts its anti-oncogenic function by activation of its developmental target genes and pathways. Finally, earlier reports suggest that, under certain conditions, proliferation can be uncoupled from the induction of differentiation as double-mutant cells for retinoblastoma and dacapo in the developing Drosophila eye keep proliferating although they start to differentiate [20]. Our data suggest that the inhibition of proliferation is not the only mechanism by which differentiation factors might suppress tumor formation, as ato is also able to induce apoptosis in an eyeful eye disc.
The function of JNK in the Drosophila eye has been described as both tumor promoting and anti-oncogenic. Igaki and colleagues describe a role for JNK in invasion upon loss of cell polarity [21], and Uhlirova et al. describe how JNK cooperates with Ras to induce tumor overgrowth in the eye [22]. In the overgrowth-sensitized setting of scribble mutant cells, however, JNK is necessary to remove these cells by apoptosis [22]. This shows that the molecular environment in which JNK acts decides the outcome. We propose that the status of differentiation might be an important factor in the decision of the outcome of JNK activity. ato function might divert JNK from an oncogenic function to a tumor suppressor function in which JNK will reduce the size of the overgrowth and, as such, reduce the number of metastases. Our data indicate that although JNK is necessary for the anti-oncogenic function of Ato, it is not sufficient, because inhibition of JNK signaling does not completely mimic the loss of ato function in the eyeful flies. This suggests JNK as a permissive, rather than instructive, factor for ato's function and indicates that ato might also modulate tumor formation by JNK-independent mechanisms.
In summary, we present the first evidence that a master regulator of tissue-specific differentiation is a key regulator of tumor initiation and progression. The evidence that the human ortholog of Ato is a tumor suppressor gene in colorectal cancer, the largest cause of cancer deaths world-wide [13], as well as the absolute functional conservation between fly and mouse Ato [23] underscore the importance of understanding the fundamental molecular and genetic mechanisms of the function of this group of key developmental regulators.
Materials and Methods
Drosophila husbandry.
Fly strains used were ey-GAL4, GS88A8, UAS-Dl/Cyo (called eyeful flies in the text) and ey-GAL4, UAS-Dl/Cyo flies (a gift from M. Domiguez), UAS-atoRNAi3B and UAS-atoRNAi 4E (gift from A. P. Jarman), UAS-ato, UAS-Atoh1, w1118 P{UAS-bsk.DN}2, UAS-Djun, UAS-dacapo (a gift from A. Hidalgo), CantonS, and yw. All flies were raised at 25 °C on standard fly food.
Immunohistochemistry.
Eye discs of wandering third instar larva were dissected and processed as described [24]. ato antibody (kind gift from A. Jarman and P. zur Lage), Dap antibody (Developmental Studies Hybridoma Bank), P-JNK (Cell Signaling Technologies), phospho-HistoneH3 (Upstate Biotechnologies), and cleaved caspase-3 (Cell Signaling Technology).
Generation of UAS-atoERD transgenic flies.
Uas-atoERD was generated by fusing the full-length Atonal ORF to a fragment encoding a Myc-tagged Engrailed repression domain (amino acids 2–298) [25] using the pUAST vector [26]. Seven uas-atoERD transgenic lines were obtained using standard Drosophila transformation protocols.
Quantitative reverse-transcriptase PCR on Drosophila larval eye-antennal discs.
Crosses between ato-GAL4 (P{GawB}NP6558 obtained from Drosophila Genetic Resource Center, Kyoto) or Gal4–7 and UAS-ato or UAS-atoERD were performed at 18 °C and shifted to 28 °C at third larval instar stage. Eye-antennal discs were dissected in RNA later (Ambion). RNA extraction was performed with Mini RNA Isolation kit (Zymo Research). act79B, gadph, and Rpl32 were used as control housekeeping genes (ΔCT), and Canton S and UAS-Ato as control RNA (ΔΔCT).
Image quantification of proliferating and apoptotic cells.
The number of proliferating cells per eye disc was quantified using the “analyse particle” function in ImageJ with the parameters 5 to 60 for size and 0.5 to 1.0 for circularity.
Supporting Information
(A) Third instar eye disc of ey-Gal4>Dl>eyeful/+. Confocal section of antibody-stained eye disc for senseless (red; indicating R8 photoreceptors), armadillo (green; indicating cell cortexes), and elav (blue; marker of mature neurons). Respective z-stacks are indicated next to the main image. White line indicates normal outline of eye-antennal imaginal disc. The malignant outgrowth (striped square) is enlarged in (B).
(B) Enlarged image from (A). Respective z-stacks are indicated next to the main image. White arrowhead indicates morphogenetic furrow in eye disc outgrowth. The different cell types present in a normal eye are also present in the outgrowth, indicating that the outgrowth originates from undifferentiated normal eye disc tissue.
(4.60 MB PDF)
(A) Representative picture ey-Gal4.
(B) Representative picture of UAS-atoRNAi driven by ey-Gal4. atoRNAi construct is active since the expression in the developing eye leads to a decrease in eye size.
(C) Representative picture of UAS-atoERD driven by ey-Gal4. The atoERD leads to a phenocopy of the loss of ato.
(378 KB PDF)
Expression of ato in wild-type flies up-regulates Dap and phosphorylated JNK. Third instar eye disc from eyeless-Gal4/UAS-ato is shown.
(2.06 MB PDF)
Acknowledgments
We thank S. Reeve, S. Claerhout, P. Vanderhaeghen, P. Verstreken, J.C. Marine, and P. Carmeliet for critical reading of the manuscript. We also thank M. Dominguez, A. Hidalgo, and H. J. Bellen for sharing flies and antibodies; and especially A. P. Jarman for sharing the unpublished ato RNAi lines.
Abbreviations
- Arm
Armadillo
- Ato
Atonal
- Bsk
Basket
- Dl
Delta
- ELAV
embryonic lethal, abnormal vision
- JNK
Jun N-terminal kinase
- pJNK
phosphorylated Jun N-terminal kinase
- RNAi
RNA interference
- Sens
Senseless
Footnotes
Author contributions. WB and BAH conceived and designed the experiments. WB, NDG, SA, and IL performed the experiments. WB and BAH analyzed the data. PM contributed reagents/materials/analysis tools. WB and BAH wrote the paper.
Funding. This work was supported by VIB, a Stichting Emmanuel Van der Scheuren pre-doctoral fellowship to WB, Fonds voor Wetenschappelijk Onderzoek–Vlaanderen (FWO) postdoctoral fellowship to SA, the European Molecular Biology Organization (EMBO) Young Investigator Program, Impuls, CREA, and Geconcerteerd Onderzoeksacties (GOA) grants from K.U. Leuven, and FWO grants G.0542.08N and G.0543.08N to BAH. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Competing interests. The authors have declared that no competing interests exist.
References
- Hassan BA, Bellen HJ. Doing the MATH: is the mouse a good model for fly development? Genes Dev. 2000;14:1852–1865. [PubMed] [Google Scholar]
- Quan XJ, Hassan BA. From skin to nerve: flies, vertebrates and the first helix. Cell Mol Life Sci. 2005;62:2036–2049. doi: 10.1007/s00018-005-5124-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarman AP, Grau Y, Jan LY, Jan YN. atonal is a proneural gene that directs chordotonal organ formation in the Drosophila peripheral nervous system. Cell. 1993;73:1307–1321. doi: 10.1016/0092-8674(93)90358-w. [DOI] [PubMed] [Google Scholar]
- Jarman AP, Grell EH, Ackerman L, Jan LY, Jan YN. Atonal is the proneural gene for Drosophila photoreceptors. Nature. 1994;369:398–400. doi: 10.1038/369398a0. [DOI] [PubMed] [Google Scholar]
- Jarman AP, Sun Y, Jan LY, Jan YN. Role of the proneural gene, atonal, in formation of Drosophila chordotonal organs and photoreceptors. Development. 1995;121:2019–2030. doi: 10.1242/dev.121.7.2019. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- Conheim J. Congenitales, quergestreiftes Muskelsarkon der Nieren. Virchows Arch. 1875;65:64. [Google Scholar]
- Harris H. Tumor suppression: putting on the brakes. Nature. 2004;427:201. doi: 10.1038/427201a. [DOI] [PubMed] [Google Scholar]
- Harris H. Suppression of malignancy in hybrid cells: the mechanism. J Cell Sci. 1985;79:83–94. doi: 10.1242/jcs.79.1.83. [DOI] [PubMed] [Google Scholar]
- Clarke MF, Fuller M. Stem cells and cancer: two faces of eve. Cell. 2006;124:1111–1115. doi: 10.1016/j.cell.2006.03.011. [DOI] [PubMed] [Google Scholar]
- Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nature. 2005;434:843–850. doi: 10.1038/nature03319. [DOI] [PubMed] [Google Scholar]
- Roy M, Pear WS, Aster JC. The multifaceted role of Notch in cancer. Curr Opin Genet Dev. 2007;17:52–59. doi: 10.1016/j.gde.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Bossuyt W, Kazanjian A, de Geest N, Van Kelst S, De Hertogh G, et al. Atonal homolog 1 is a tumor suppressor gene. PLoS Biol. 2009;7:e1000039. doi: 10.1371/journal.pbio.1000039. doi: 10.1371/journal.pbio.1000039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferres-Marco D, Gutierrez-Garcia I, Vallejo DM, Bolivar J, Gutierrez-Aviño FJ, et al. Epigenetic silencers and Notch collaborate to promote malignant tumors by Rb silencing. Nature. 2006;439:430–436. doi: 10.1038/nature04376. [DOI] [PubMed] [Google Scholar]
- Palomero T, Sulis ML, Cortina M, Real PJ, Barnes K, et al. Mutational loss of PTEN induces resistance to NOTCH1 inhibition in T-cell leukemia. Nat Med. 2007;13:1203–1210. doi: 10.1038/nm1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John A, Smith ST, Jaynes JB. Inserting the Ftz homeodomain into engrailed creates a dominant transcriptional repressor that specifically turns off Ftz target genes in vivo. Development. 1995;121:1801–1813. doi: 10.1242/dev.121.6.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domínguez M, Casares F. Organ specification-growth control connection: new in-sights from the Drosophila eye-antennal disc. Dev Dyn. 2005;232:673–684. doi: 10.1002/dvdy.20311. [DOI] [PubMed] [Google Scholar]
- Kurata S, Go MJ, Artavanis-Tsakonas S, Gehring WJ. Notch signaling and the determination of appendage identity. Proc Natl Acad Sci U S A. 2000;97:2117–2122. doi: 10.1073/pnas.040556497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukhanova M, Deb DK, Gordon GM, Matakatsu MT, Du W. Proneural bHLH proteins and EGFR signaling coordinately regulate cell type specification and Cdk inhibitor expression during development. Mol Cell Biol. 2007;27:2987–2996. doi: 10.1128/MCB.01685-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firth LC, Baker NE. Extracellular signals responsible for spatially regulated proliferation in the differentiating Drosophila eye. Dev Cell. 2005;8:541–551. doi: 10.1016/j.devcel.2005.01.017. [DOI] [PubMed] [Google Scholar]
- Igaki T, Pagliarini RA, Xu T. Loss of cell polarity drives tumor growth and invasion through JNK activation in Drosophila. Curr Biol. 2006;16:1139–1146. doi: 10.1016/j.cub.2006.04.042. [DOI] [PubMed] [Google Scholar]
- Uhlirova M, Jasper H, Bohmann D. Non-cell-autonomous induction of tissue overgrowth by JNK/Ras cooperation in a Drosophila tumor model. Proc Natl Acad Sci U S A. 2005;102:13123–13128. doi: 10.1073/pnas.0504170102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang VY, Hassan BA, Bellen HJ, Zoghbi HY. Drosophila atonal fully rescues the phenotype of Math1 null mice: new functions evolve in new cellular contexts. Curr Biol. 2002;12:1611–1616. doi: 10.1016/s0960-9822(02)01144-2. [DOI] [PubMed] [Google Scholar]
- Hassan BA, Bermingham NA, He Y, Sun Y, Jan YN, et al. atonal regulates neurite arborization but does not act as a proneural gene in the Drosophila brain. Neuron. 2000;25:549–561. doi: 10.1016/s0896-6273(00)81059-4. [DOI] [PubMed] [Google Scholar]
- Conlon FL, Sedgwick SG, Weston KM, Smith JC. Inhibition of Xbra transcription activation causes defects in mesodermal patterning and reveals autoregulation of Xbra in dorsal mesoderm. Development. 1996;122:2427–2435. doi: 10.1242/dev.122.8.2427. [DOI] [PubMed] [Google Scholar]
- Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Third instar eye disc of ey-Gal4>Dl>eyeful/+. Confocal section of antibody-stained eye disc for senseless (red; indicating R8 photoreceptors), armadillo (green; indicating cell cortexes), and elav (blue; marker of mature neurons). Respective z-stacks are indicated next to the main image. White line indicates normal outline of eye-antennal imaginal disc. The malignant outgrowth (striped square) is enlarged in (B).
(B) Enlarged image from (A). Respective z-stacks are indicated next to the main image. White arrowhead indicates morphogenetic furrow in eye disc outgrowth. The different cell types present in a normal eye are also present in the outgrowth, indicating that the outgrowth originates from undifferentiated normal eye disc tissue.
(4.60 MB PDF)
(A) Representative picture ey-Gal4.
(B) Representative picture of UAS-atoRNAi driven by ey-Gal4. atoRNAi construct is active since the expression in the developing eye leads to a decrease in eye size.
(C) Representative picture of UAS-atoERD driven by ey-Gal4. The atoERD leads to a phenocopy of the loss of ato.
(378 KB PDF)
Expression of ato in wild-type flies up-regulates Dap and phosphorylated JNK. Third instar eye disc from eyeless-Gal4/UAS-ato is shown.
(2.06 MB PDF)