Abstract
Leptin replacement rescues the phenotype of morbid obesity and hypogonadism in leptin-deficient adults. However, leptin's effects on insulin resistance are not well understood. Our objective was to evaluate the effects of leptin on insulin resistance. Three leptin-deficient adults (male, 32 yr old, BMI 23.5 kg/m2; female, 42 yr old, BMI 25.1 kg/m2; female, 46 yr old, BMI 31.7 kg/m2) with a missense mutation of the leptin gene were evaluated during treatment with recombinant methionyl human leptin (r-metHuLeptin). Insulin resistance was determined by euglycemic hyperinsulinemic clamps and by oral glucose tolerance tests (OGTTs), whereas patients were on r-metHuLeptin and after treatment was interrupted for 2–4 wk in the 4th, 5th, and 6th years of treatment. At baseline, all patients had normal insulin levels, C-peptide, and homeostatic model assessment of insulin resistance index, except for one female diagnosed with type 2 diabetes. The glucose infusion rate was significantly lower with r-metHuLeptin (12.03 ± 3.27 vs. 8.16 ± 2.77 mg·kg−1·min−1, P = 0.0016) but did not differ in the 4th, 5th, and 6th years of treatment when all results were analyzed by a mixed model [F(1,4) = 0.57 and P = 0.5951]. The female patient with type 2 diabetes became euglycemic after treatment with r-metHuLeptin and subsequent weight loss. The OGTT suggested that two patients showed decreased insulin resistance while off treatment. During an off-leptin OGTT, one of the patients developed a moderate hypoglycemic reaction attributed to increased posthepatic insulin delivery and sensitivity. We conclude that, in leptin-deficient adults, the interruption of r-metHuLeptin decreases insulin resistance in the context of rapid weight gain. Our results suggest that hyperleptinemia may contribute to mediate the increased insulin resistance of obesity.
Keywords: recombinant methionyl human leptin, euglycemic hyperinsulinemic clamp, homeostatic model assessment
leptin deficiency was the first described monogenic nonpleiotropic form of obesity. To date, 10 children (23, 25, 40, 44, 58) and three adults (46, 58) have been reported as leptin deficient in five families from Pakistan, one from Egypt, and one from Turkey. Leptin replacement decreases weight, fat mass, food intake, hyperinsulinemia, and serum lipid levels (22, 23, 25, 46). We previously described a significant reduction in food intake (32–34, 61), resulting in substantial weight and fat loss, an increase in physical activity, and the resolution of hypogonadism and type 2 diabetes mellitus in leptin-treated adults (32). Moreover, leptin replacement altered the patients' brain structure and their response to food cues (2, 38).
Although leptin and insulin have key metabolic roles, the interplay between both hormones is poorly understood. A majority of the studies suggest that leptin decreases insulin synthesis and secretion by pancreatic β-cells (9, 12, 29, 54). It is speculated that the adipoinsular axis includes a leptin-mediated inhibitory feedback loop on insulin secretion to decrease adipogenesis. Insulin, in turn, may also play a role in stimulating leptin production and secretion in adipose tissue (54).
Existing data also suggest that leptin may play a significant part in the pathophysiology of insulin resistance related to obesity. Leptin replacement reverses insulin resistance and diabetes in mice homozygous for mutations of the ob gene (42) in aP2-nSREBP-1c mice with moderate fat deficiency (56) and in severely lipoatrophic A-ZIP/F-1 mice (24). However, other animal studies in vitro and in vivo are contradictory, suggesting that leptin may increase (9, 11, 41, 59) or decrease insulin resistance (8, 9, 57). These contradictory findings may be partially explained by the fact that most of the interactions of leptin within the adipoinsular axis have been studied in vitro and in animal models. The interpretations made from in vitro studies are limited because the central effects of leptin and its integration with other metabolically active hormones are missing from these interpretations. Moreover, in human studies, the observations derive from subjects with normal or high leptin levels as opposed to leptin-deficient individuals. In such models, the assessment of the effects of leptin on insulin resistance may be biased by the presence of leptin resistance. Other human studies have evaluated the insulin-sensitizing effects of leptin replacement in patients with lipodistrophy (19, 26, 45, 48). Those results may likewise be biased by the fact that those patients whose adipoinsular axis may be disrupted lack adipose tissue. Hence, leptin-deficient humans provide a unique opportunity to evaluate the effects of leptin on insulin resistance.
To determine the effects of leptin on insulin resistance, we studied three leptin-deficient adults, while on and off leptin replacement, using euglycemic hyperinsulinemic clamps (EHC) and oral glucose tolerance tests (OGTTs).
RESEARCH DESIGN AND METHODS
Subjects.
The only three leptin-deficient patients identified at adulthood in the world to date were recruited for this study. They are from a highly consanguineous Turkish family with a nonconservative missense leptin gene mutation (Cys to Thr in codon 105). Patients started treatment with recombinant methionyl human leptin (r-metHuLeptin) in 2001 at ages 27 (male patient A), 35 (female patient B), and 40 (female patient C) yr according to a previously described protocol (32) and have been closely followed up since then. Initial leptin doses started at 0.02–0.04 mg·kg−1·day−1 at 6 PM and were designed to achieve a normal leptin concentration based on a body fat of 30% in females and 20% in males. Subsequently, doses were decreased as patients lost weight to avoid excessively rapid weight loss. Since total weight has been stable since 2004, patients have been on the same dose since that year (A = 0.15 mg/day; B = 0.2 mg/day; C = 2.5 mg/day). During the 1st-year EHC, doses of r-metHuLeptin were equal to 2.33 μg·kg−1·day−1 for patient A, 3.40 μg·kg−1·day−1 for patient B, and 33.69 μg·kg−1·day−1 for patient C. During the 2nd-year EHC, doses were 2.30 μg·kg−1·day−1 for patient A, 3.12 μg·kg−1·day−1 for patient B, and 34.12 μg·kg−1·day−1 for patient C. At the 3rd-year EHC, patient A was receiving 2.33 μg·kg−1·day−1, patient B was receiving 3.19 μg·kg−1·day−1, and patient C was receiving 23.07 μg·kg−1·day−1.
Study design.
In 2005 and 2006, the study took place at the General Clinical Research Center (GCRC) at the University of California Los Angeles (UCLA). The study protocol was approved by the Food and Drug Administration and by the UCLA Institutional Review Boards. In 2007, the study site was relocated to the University of Miami, where new approvals from the local Institutional Review Boards were obtained. Informed written consents were obtained from all subjects. R-metHuLeptin was provided by Amgen (Thousand Oaks, CA) and subsequently by Amylin Pharmaceuticals (San Diego, CA). For each patient, the researchers administered two EHC (15) on three occasions, in the 4th year (2005), in the 5th year (2006), and in the 6th year (2007). In 2005, the first clamp was performed while the patients were off leptin (18–20 days after leptin replacement was transiently stopped), and the second clamp was performed while the patients were on leptin (13–18 days after treatment was resumed). In 2006, the first clamp was performed while patients were still on leptin and was repeated 27–29 days after leptin replacement treatment was interrupted. In 2007, the first clamp was performed under leptin replacement (with infusion rates of insulin during the steady state equal to 14–15 U/h), and the second clamp was repeated 42–43 days after treatment was interrupted (with infusion rates of insulin during the steady state equal to 14–16 U/h).
In addition, standard OGTTs with 75 g of glucose were performed in 2007, once while patients were on treatment and then 40 days after treatment was transiently interrupted. During these procedures, glucose, insulin, and C-peptide were assayed at 0, 30, 60, 90, and 120 min.
Levels of insulin and C-peptide, measured at 0, 30, and 60 min in the OGTT, were integrated vs. time (from t = 0 to t = 60 min) to estimate insulin secretion, hepatic extraction of insulin, and insulin delivery. Insulin secretion was determined by the deconvolution of C-peptide data according to Eaton et al. (18). Hepatic extraction and posthepatic insulin delivery were determined on the basis of the insulin kinetics model of Polonsky et al. (49). A “differential equation” method, based on the approach used by Dalla Man et al. (14), was used to estimate insulin sensitivity from the OGTT. A computer program was written according to this approach and is available by request.
In 2007, the participants were allowed to eat ad libitum, and dietary intake was assessed through food records collected for 3 days while on and off treatment. The software Food Processor SQL (Esha Research, Salem, OR) was used for nutritional analysis.
Physical activity was assessed by actigraphy (Actiwatch AW64; Mini Mitter, Bend, OR), through a wrist watch worn by the patients while on and off treatment, for 7 consecutive days.
Biochemical analysis.
In 2005 and 2006, the following immunoassays were performed in the UCLA GCRC Neuropsychiatric Institute Endocrine Core Laboratory: insulin [radioimmunoassay; Diagnostic Systems Laboratories, Webster, TX; limit of detection 1.3 μU/ml, interassay coefficient of variation (CV) 4.9%, intra-assay CV 4.9%] and C-peptide (radioimmunoassay; Diagnostic Systems Laboratories; limit of detection 0.01 ng/ml, interassay CV 4.0%, intra-assay CV 4.0%). Plasma glucose was determined during the EHC according to standard methods with the use of automated equipment. Homeostasis assessment of insulin resistance (HOMA-IR) was calculated according to the following formula: HOMA-IR = fasting insulin (μU/ml) × fasting glucose (mmol/l)/22.5.
In 2007, insulin (Siemens Healthcare Diagnostics, Deerfield, IL; limit of detection 3.0 μU/ml, interassay CV 4.6%, intra-assay CV 7.6%), C-peptide (Siemens Healthcare Diagnostics; limit of detection 0.01 ng/ml, interassay CV 3.9%, intra-assay CV 6.5%), and glucose (gluco-oxidase method; Roche Diagnostics, Indianapolis, IN; limit of detection 10.0 mg/dl, interassay CV 1.8%, intra-assay CV 2.7%) were assayed at the University of Miami Diabetes Research Institute. Commercial enzymatic tests were used to determine total cholesterol, triglycerides, and HDL cholesterol (Roche Diagnostics).
Data analysis.
The glucose infusion rate (GINF; mg·kg−1·min−1) was calculated on the basis of the findings of each EHC during the last 20 min of steady state and is presented herein as means and standard deviations. First, paired t-test was performed to test for the difference in glucose clamps between off- and on-leptin periods using the data observed in 3 yr. A mixed linear model was then employed to confirm the observed difference in glucose clamps between the off- and on-leptin periods by considering the within-subject correlation using SAS mixed procedure (SAS software, Version 9.1.3). A mixed linear model is the generalization of the standard linear model that permits the data to exhibit correlation and nonconstant variability. In the present study, GINF (response) was regressed on the leptin treatment status, intervention year, and random error effects.
RESULTS
Baseline characteristics.
All patients had normal HOMA-IR compared with the cutoff value of 2.77 proposed by Bonora et al. (6). Insulin and C-peptide levels were also normal at baseline, but patient C had clinical diagnosis of type 2 diabetes mellitus before leptin replacement (Table 1). After leptin treatment and leptin-induced weight loss, this patient's glucose and hemoglobin A1c levels normalized without any other specific treatment.
Table 1.
Basal levels of glucose homeostasis parameters
| Baseline Values | |
|---|---|
| Fasting glucose, mg/dl | |
| Patient A | 91 |
| Patient B | 88 |
| Patient C | 131 |
| Fasting insulin, μU/ml | |
| Patient A | 4.8 |
| Patient B | 3.8 |
| Patient C | 7.5 |
| Fasting C-peptide, ng/ml | |
| Patient A | 2.2 |
| Patient B | 2.0 |
| Patient C | 4.0 |
| HOMA-IR | |
| Patient A | 1.08 |
| Patient B | 0.83 |
| Patient C | 2.43 |
HOMA-IR, homeostatis model assessment of insulin resistance.
After r-metHuLeptin was started in 2001, weight loss occurred in the first 24 mo of treatment and was stable thereafter. In 2005 (after 4 yr of treatment), body mass indexes (BMIs) had decreased by 51.5% in patient A (from 51.4 to 23.5 kg/m2), 46.2% in patient B (from 46.7 to 25.1 kg/m2), and 42.8% in patient C (from 55.4 to 31.7 kg/m2). In 2006, BMIs for patients A, B, and C were 24.3, 27.5, and 31.6 kg/m2, respectively. In 2007, the patients' respective BMIs were 23.0, 26.4, and 32.3 kg/m2. During the brief periods where r-metHuLeptin was interrupted, significant weight gain was observed in all patients. Table 2 illustrates the anthropometric values during the EHC.
Table 2.
Weight, BMI, and total body fat (by DEXA) measured during on- and off-r-metHuLeptin EHC
|
2005 |
2006
|
2007
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Weight | BMI | Total fat | Weight | BMI | Total fat | Weight | BMI | Total fat | |
| Patient A | |||||||||
| On leptin | 64.7 | 23.5 | 10.4 | 66.6 | 24.3 | 13.2 | 63.5 | 23.0 | 7.0 |
| Off leptin | 65.3 | 23.7 | 72.8 | 26.4 | 73.5 | 26.7 | |||
| Patient B | |||||||||
| On leptin | 63.2 | 25.1 | 26.0 | 64.8 | 27.5 | 32.2 | 61.7 | 26.4 | 36.4 |
| Off leptin | 69.0 | 26.9 | 68.9 | 29.3 | 68.0 | 29.0 | |||
| Patient C | |||||||||
| On leptin | 72.8 | 29.8 | 31.8 | 77.3 | 31.6 | 36.6 | 75.7 | 32.3 | 45.0 |
| Off leptin | 77.5 | 31.7 | 81.4 | 33.3 | 84.8 | 36.2 | |||
BMI, body mass index; DEXA, dual-energy X-ray absorptiometry; r-metHuLeptin, recombinant methionyl human leptin; EHC, euglycemic hyperinsulinemic clamps.
In the last evaluation, total cholesterol, HDL cholesterol, and triglycerides increased in all patients when off treatment. Total cholesterol increased from 107 to 135 mg/dl in patient A, from 122 to 133 mg/dl in patient B, and from 146 to 188 mg/dl in patient C. HDL cholesterol increased from 29 to 35 mg/dl in patient A, from 51 to 58 mg/dl in patient B, and from 43 to 46 mg/dl in patient C. Triglycerides increased from 67 to 150 mg/dl in patient A, from 53 to 63 mg/dl in patient B, and from 78 to 196 mg/dl in patient C.
Measurements of lifestyle.
In 2007, food intake increased for all patients after treatment was interrupted. Patient A's caloric intake increased from 2,652 ± 118 (66.6% from carbohydrates, 15.8% from fat, and 17.6% from proteins) to 3,509 ± 1,378 kcal/day (71.9% from carbohydrates, 13.6% from fat, and 14.5% from proteins), patient B's intake changed from 1,898 ± 255 (64.4% from carbohydrates, 16.7% from fat, and 18.7% from proteins) to 3,072 ± 306 kcal/day (68.4% from carbohydrates, 18.3% from fat, and 13.3% from proteins), and patient C's intake increased from 1,173 ± 340 (59.7% from carbohydrates, 20.8% from fat, and 19.5% from proteins) to 2,045 ± 872 kcal/day (61.5% from carbohydrates, 25.2% from fat, and 13.3% from proteins). Physical activity was assessed by actigraphy and increased from 114.17 ± 10.87 to 243.98 ± 11.18 counts/min for patient A and from 249.43 ± 55.03 to 304.30 ± 40.43 counts/min for patient B. Patient C had a slight decrease in physical activity after r-metHuLeptin was interrupted, from 199.19 ± 45.39 to 152.16 ± 44.32 counts/min.
EHCs.
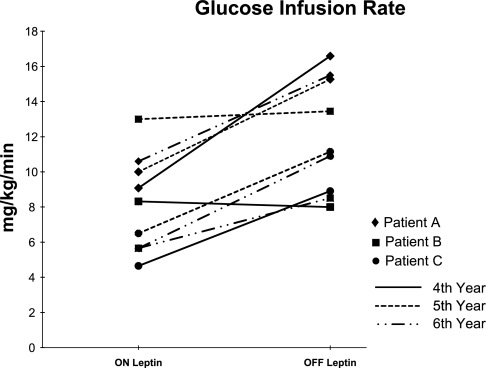
Insulinemia was measured during the EHCs performed in 2007. Peak levels of insulin at the steady state (when GINF was measured) during the on-leptin EHC were 59.9 μU/ml for patient A, 63.8 μU/ml for patient B, and 72.24 μU/ml for patient C. During the off-treatment EHC, peak levels of insulin were 71.4 μU/ml for patient A, 88.2 μU/ml for patient B, and 80.1 μU/ml for patient C. The individual GINFs, assessed by leptin treatment status and intervention year, are represented in Fig. 1. In intervention year 2005 (4 yr after r-metHuLeptin was started), the mean GINF decreased 34% after leptin treatment was resumed, from 11.17 (off leptin) to 7.35 mg·kg−1·min−1 (on leptin). Conversely, in intervention year 2006, the average GINF increased 37% when leptin treatment was interrupted, from 9.83 (on leptin) to 13.29 mg·kg−1·min−1 (off leptin). In 2007, the average GINF increased 59% when leptin treatment was interrupted, from 7.3 (on leptin) to 11.64 mg·kg−1·min−1 (off leptin). When combining the data from clamp tests performed in 2005, 2006, and 2007, the results from paired t-test supported the effect of leptin on GINFs (t = 4.68, df = 8, P = 0.0016). Mixed-model analysis showed an almost identical effect of leptin on GINFs [F(1,8) = 21.89 and P = 0.0016] when compound symmetry covariance structure option was used. No significant difference in GINF was seen across years 2005, 2006, and 2007 [F(2,6) = 0.57 and P = 0.5951]. The mean GINF while on leptin was 8.16 ± 2.77 mg·kg−1·min−1, whereas while off leptin it was 12.03 ± 3.27 mg·kg−1·min−1. Patients A and C experienced larger changes in GINFs when comparing the off- and on-leptin periods in all intervention years (patient A = +52.7% in 2006, patient C = +92.9% in 2007). On the other hand, patient B showed only a very slight change (3–4%) in clamp data between off- and on-leptin periods in 2005 and 2006, yet this patient showed a 50.3% increase in 2007, after the EHC was repeated while off leptin.
Fig. 1.
Glucose infusion rates (mg·kg−1·min−1) in leptin-deficient patients on and off leptin replacement treatment.
OGTTs.
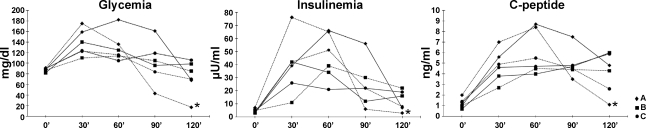
In 2007, patients underwent two OGTTs while on leptin and then 40 days after treatment was interrupted. None of the patients achieved glucose levels high enough to warrant a diagnosis of glucose intolerance or diabetes. However, between the 90th and the 120th min of the second OGTT, patient A started to develop symptoms and signs compatible with hypoglycemia. Capillary glucose was 29 mg/dl at 110′. Signs and symptoms were resolved promptly after intervention with a slow intravenous push of 25 g of glucose in D50 solution, one glucose tab per os, and two cans of a meal replacement drink. Individual values of glucose, insulin, and C-peptide are shown in Fig. 2.
Fig. 2.
Individual values of glucose, insulin, and C-peptide measured during the oral glucose tolerance test while on and off treatment with recombinant methionyl human leptin (r-metHuLeptin). *Measured at 115′. Solid lines, on r-metHuLeptin; dotted lines, off r-metHuLeptin.
Insulin sensitivity was estimated with software written according to the approach proposed by Dalla Man et al. (14). Insulin sensitivity was higher after leptin replacement was interrupted for patients A and B, whereas it was lower for patient C (Table 3).
Table 3.
Insulin sensitivity estimates based on glucose and insulin measured during OGTT
| On Leptin | Off Leptin | Off/On Ratio | |
|---|---|---|---|
| Patient A | 0.0015 | 0.0026 | 1.73 |
| Patient B | 0.0035 | 0.0047 | 1.34 |
| Patient C | 0.0041 | 0.0023 | 0.56 |
OGTT, oral glucose tolerance test.
Insulin secretion remained unchanged in patient A. However, the patient presented decreased hepatic extraction of insulin while off leptin, which resulted in higher insulin delivery. Patient B had higher insulin secretion and higher hepatic extraction while off leptin, which resulted in lower insulin delivery. Patient C presented an increase in insulin secretion with slightly higher insulin delivery while off leptin (Table 4).
Table 4.
Insulin secretion, hepatic extraction of insulin, and insulin delivery estimates during OGTT on and off leptin
|
Patient A |
Patient B
|
Patient C
|
||||
|---|---|---|---|---|---|---|
| On Leptin | Off Leptin | On Leptin | Off Leptin | On Leptin | Off Leptin | |
| Insulin secretion | 1,393.2 | 1,432.0 | 727.7 | 681.2 | 859.9 | 966.8 |
| Hepatic extraction | 813.0 | 513.8 | 139.5 | 448.7 | 527.8 | 507.6 |
| Insulin delivery | 580.1 | 918.2 | 588.2 | 232.5 | 332.0 | 459.2 |
All values are in μU/ml.
DISCUSSION
Our results document that the interruption of leptin replacement therapy decreases insulin resistance after a brief period of rapid weight gain. Although we cannot exclude the hypothesis that the rapid increase in adipocyte mass influenced the decrease in insulin resistance, the data from our OGTT studies reinforce the hypothesis that leptin may exert direct action on insulin resistance, insulin secretion, insulin hepatic extraction, and insulin delivery. According to the OGTT data, insulin resistance decreased in two patients after leptin replacement was interrupted. In patient A, the increase in sensitivity, combined with a decreased hepatic extraction of insulin, resulted in a hypoglycemic episode during the off-leptin OGTT. A hypoglycemic reaction is rarely observed in healthy patients undergoing an OGTT; its occurrence in a leptin-deficient patient further reinforces the role of leptin in insulin resistance in addition to leptin's effect on decreasing the hepatic extraction of insulin. It is puzzling that patient C presented with decreased insulin sensitivity estimated when off leptin, whereas opposite results were observed in the EHC. We believe that the assessment of insulin sensitivity using the OGTT has many limitations, and data from this test can be best used to support the findings from the EHC. Nevertheless, the approach used to estimate insulin sensitivity from the OGTT data was appropriate because it allows the analysis of cases where glucose and insulin do not return to equilibrium during the test (such as a 120-min OGTT).
Patient C's requirements of r-metHuLeptin are more than 10-fold higher than those of the other patients. We attribute this to the fact that patient C is the older patient. Therefore, this patient has been exposed to the absence of leptin and to obesity for longer. Those factors combined may contribute to the fact that the patient is more resistant to the leptin replacement.
Conflicting findings were obtained when insulin secretion, hepatic extraction of insulin, and insulin delivery were estimated from the OGTT data. Insulin delivery increased when patients A and C were off leptin, whereas it decreased in patient B. In patient A, this increase can be attributed to a substantial decrease in hepatic extraction of insulin without a significant change in insulin secretion. Patient B had decreased insulin delivery due to decreased insulin secretion and, more importantly, increased hepatic extraction. In patient C, the increase in insulin delivery is caused by an increase in insulin secretion. It is unclear why patient A presented a more marked decrease in insulin hepatic extraction. It is possible that, in a male patient, the absence of leptin leads to a more pronounced increase in visceral fat, which, in turn, reflects in increased hepatic steatosis. Consequently, the lipotoxic effect of the accumulation of lipids in the liver impairs insulin hepatic extraction. It would be useful to quantify the degree of hepatic steatosis. Further tests in these patients at that point were deemed to be excessive and were therefore not conducted. Future studies should address this point.
Meal tolerance tests (MTT) were performed on patient A pretreatment and at 1 wk, 18 mo, and 24 mo after leptin replacement was initiated (1). Our data show that the pretreatment value of insulin sensitivity in this patient was two times lower than the median and within the 10th percentile of the values found in a group of 88 healthy subjects (13). Sensitivity slightly increased by 10% 1 wk after leptin replacement started, by 150% after 18 mo, and 10-fold after 24 mo of treatment.
These results differ from those of our present study. It is worthwhile to note that, in the MTT, we evaluated the chronic effects of leptin on insulin sensitivity (18 and 24 mo after initiation of therapy) and compared the results with a state of morbid obesity, low insulin sensitivity, and higher leptin dose in a single male patient. In this present study, we evaluate the acute effects (2–4 wk after interruption of therapy) in a state of lower BMI, higher insulin sensitivity, and lower leptin dose. Therefore, it is possible that leptin exerts differential effects, depending on the length of administration, dose, and BMI. An alternative hypothesis is that there are divergent acute and chronic effects of leptin; acutely, leptin may increase insulin resistance, whereas chronically, it may exert the opposite effect. Moreover, discordant results may arise from the OGTT and the MTT due to the different methodologies applied to assess our patients, which makes the comparison between those tests less than ideal.
The observed negative effects of leptin on insulin sensitivity can be explained by experimental models in vitro exposed to low and chronic physiological doses of leptin (41, 54). However, data on insulin resistance and leptin in animal models and humans are conflicting. It appears that leptin exerts direct inhibitory effects on insulin production and secretion by pancreatic β-cells (9, 12, 20, 30, 50, 54, 55). In vitro, human islets chronically exposed to leptin present decreased production of interleukin-1 receptor antagonist by the β-cells, which induces IL-1β release from islet preparation. As a result, IL-1β leads to impaired β-cell function and apoptosis through the activation of the c-Jun NH2-terminal kinase pathway (36, 37). In addition, leptin attenuates insulin secretion by maintaining the patency of the β-cell KATP channels, thereby hyperpolarizing the plasma membrane (43). However, in overweight Hispanic adolescents, leptin was not associated with insulin secretion or β-cell function (31). The effect on insulin resistance is even less clear. There is evidence to support a role of leptin to increase, decrease, and have no effects on insulin resistance on the basis of different study models (9). Some studies have shown that leptin treatment decreases insulin resistance in normal (57) and in diabetic rats (10), and it can also reverse diabetes in leptin-deficient ob/ob mice (42). In our study, the absence of leptin determined an increase in insulin resistance, which can be explained by leptin's direct action on glucose uptake. However, we cannot rule out the hypothesis that the observed higher glucose uptake could have been caused at least in part by the rapid increase in adipocyte mass after leptin withdrawal.
The effect of leptin on insulin sensitivity can be explained by three mechanisms. First, leptin cross-talks with the insulin-signaling pathways at the levels of the insulin receptor substrate (IRS) and phosphatidylinositol 3-kinase (PI 3-kinase) through the activation of JAK-2, which induces tyrosine phosphorylation of IRS-2 and leads to activation of PI 3-kinase and an increase in glucose uptake (28). Second, leptin has a direct thermogenic effect that, mediated by PI 3-kinase signaling, influences glucose metabolism (17). Finally, leptin activates the α2 catalytic subunit of 5′-AMP-activated protein kinase (AMPK) in skeletal muscle, which in turn promotes catabolism and inhibits anabolic pathways in response to a fall in the ATP/AMP ratio by phosphorylating key enzymes of intermediary metabolism (39). The activation of AMPK subsequently increases PI 3-kinase binding via IRS-1, enhancing glucose uptake (27). However, it also has been reported that leptin increases insulin resistance in isolated rat adipocytes (41) and skeletal muscle (59) and in human hepatic cell lines (11). Additionally, circadian peaks of leptin are positively correlated with insulin resistance in humans (5), and leptin concentrations are higher in insulin-resistant than insulin-sensitive men, regardless of adiposity (53). In other studies, no effects of leptin on insulin sensitivity were observed (60, 63).
It is noteworthy that leptin acts as a signal of peripheral nutritional status to the hypothalamus. This action modulates neurotransmitter systems that regulate energy expenditure and glucose homeostasis (4, 51, 52). Through its central actions, leptin increases sympathetic stimulation of peripheral tissues, thereby augmenting the resting metabolic rate in animals. By altering the signal transducer and activator of transcription 3 (STAT3) signaling pathway, long-term leptin infusion to the hypothalamus may be involved in food intake and glucose regulation in rodents (7). These effects may also be explained by other non-STAT3 leptin signals (3). In addition, leptin administration regulates hepatic glucose flux in wild-type as well as ob/ob mice (35).
Humans with lipoatrophic disorders, who exhibit very low levels of leptin and increased insulin resistance and hyperinsulinemia, experience a decrease in insulin resistance and an increase in insulin secretion after treatment with r-metHuLeptin (19, 26, 45, 48). Fasting leptin levels in normal subjects are inversely related to insulin sensitivity when measured by the EHC, regardless of the insulin area under the curve, sex, and adiposity. The data are consistent with the view that plasma leptin may have an independent effect on insulin resistance, which is not entirely explained by adiposity or hyperinsulinemia (16). Yet the reasons remain unclear as to why leptin has different effects on insulin resistance and whether they can be attributed directly to leptin or through interactions with incretins, cytokines, and adipocytokines.
We acknowledge that our study has limitations. First, the diverse baseline characteristics (such as age, sex, BMI, and leptin dose) could have contributed to the conflicting findings. Nevertheless, our patients had important similarities, such as previous history of morbid obesity caused by genetically based leptin deficiency, normal basal levels of insulin, and normal indexes of insulin resistance before treatment, as well as substantial weight loss. Second, our study is limited by its small number. However, these are the only three leptin-deficient patients identified at adulthood in the world at the present time. Third, the duration of the periods on and off leptin was different for clamps performed in 2005, 2006, and 2007. However, leptin dose and BMI did not differ significantly between the three years. Fourth, the fact that EHC were not performed before leptin replacement was initiated prevents us from showing the different actions of leptin over time (before vs. after treatment, on vs. off). Finally, the presence of controls would support our findings. Hypoleptinemic patients with anorexia nervosa submitted to the administration of r-metHuLeptin would be ideal controls who unfortunately are notoriously hard to recruit and adequately treat. Although scientifically that would be the ideal control group, it would not be ethical to treat anorexia nervosa patients with a hormone that suppresses food intake.
On the basis of our previous clinical experience with these patients, we maintained the patients off treatment during 2–4 wk. Since r-metHuLeptin has a short half-life of ∼3–4 h (62), it was our conclusion that increasing the period without treatment would bring no additional benefit to the patients. In the future, we plan to perform the evaluations after the drug is interrupted for a shorter period of time.
By assessing caloric intake and by measuring physical activity, we observed that the interruption of r-metHuLeptin led to an increase in the caloric intake of all patients, with a concomitant increase in physical activity in two of them. This may suggest that two of our patients increased their physical activity to avoid excessive weight gain after the drug was stopped. This is a confounding factor that could have contributed to decrease insulin resistance. However, it is unlikely that this hypothesis is true, since all patients increased their caloric intake and gained weight, which leads to an increase in insulin resistance. The results from the lipid profiles measured in 2007 (mainly total cholesterol and triglycerides) are a reflection of weight gain due to the absence of leptin, of increased fat intake while off leptin (patients B and C), and of increased de novo lipogenesis due to the absence of leptin. The effect of the absence of leptin on lipogenesis was confirmed in another study. By measuring nonsterified fatty acids during EHCs, we showed that the absence of leptin enhanced the insulin-mediated stimulation of lipogenesis (47). Additionally, higher levels of insulinemia were achieved during the off-treatment EHC. This is a possible confounding factor that leads to the overestimation of insulin sensitivity. In our study, patient B did not experience significant changes in insulin resistance during periods on and off leptin treatment in 2005 and 2006. This patient had the lowest basal levels of insulin, C-peptide, and HOMA-IR, which could reflect a state of higher basal insulin sensitivity and a smaller likelihood that leptin influences insulin in this patient.
Insulin sensitivity is determined by myriad factors, and not only by leptinemia. It is possible that, in our patients, the absence of leptin could have influenced insulin sensitivity not only through a direct effect, but also indirectly via its effects on the levels of thyroid hormones, glucocorticoids, catecholamines, free fatty acids, and triglycerides. Since all those factors are interdependent, it is not possible to evaluate their effects separately in living humans. Complex interactions between those factors result in insulin sensitivity or in insulin resistance and are responsible for the differences observed in humans and in ob/ob mice. Moreover, as stated before, the decrease in insulin resistance after leptin withdrawal could have been determined at least in part by increased adipocyte mass, and not only by leptin's direct actions on glucose uptake. Although we agree that the weight gain between the on- and off-leptin assessments is an important confounding factor to our study, it is virtually impossible to keep leptin-deficient patients at a stable weight after r-metHuLeptin withdrawal.
In the EHC we performed in these patients in 2007, insulin was measured during the time that GINF was assessed. Higher insulin levels were obtained while patients were off r-metHuLeptin. This could have been attributed to the effects of leptin on insulin hepatic extraction. According to the OGTT data, the absence of leptin decreased hepatic extraction in patients A and C, which could lead to higher insulin levels. This would require increased infusion of glucose, which could be misinterpreted as increased insulin sensitivity. The design of our study did not allow us to access whether endogenous insulin secretion was completely blocked during the steady state. Since we followed the standard clamp technique described in the literature, we thought it would reasonable to assume that endogenous insulin secretion was blocked in our studies.
On the basis of our findings, we conclude that leptin replacement leads to a significant increase in insulin resistance of leptin-deficient patients who are not morbidly obese. Conversely, interrupting leptin treatment decreases insulin resistance, which can be explained by leptin's direct effect on insulin resistance or by the subsequent rapid weight gain determined by the r-metHuLeptin withdrawal. Nevertheless, the increase in insulin resistance caused by this withdrawal has no pathophysiological effects during these short periods off leptin, as patients remained clinically normoglycemic.
Our findings might not apply to obese patients who do not have a mutation in the leptin gene; they are typically hyperleptinemic and leptin resistant (21). In such patients, additional exogenous leptin may have no effect on insulin sensitivity due to leptin resistance. However, their resistance to leptin may be primarily central and not peripheral. If that is the case, we would suggest that hyperleptinemia contributes to the metabolic syndrome by increasing peripheral insulin resistance and decreasing hepatic insulin extraction. If our findings are confirmed in a context of stable weight after r-metHuLeptin withdrawal, the hypothesis that the hyperleptinemia of obesity contributes to increased insulin resistance and to increased posthepatic delivery of insulin (due to decreased hepatic extraction) should therefore be tested in future studies.
GRANTS
This work was supported by National Institutes of Health Grants RR-16996, HG-002500, DK-58851, RR-017611, and K24-RR-016996 to J. Licinio and DK-063240, RR-017365, MH-062777, RR-000865, and K24-RR-017365 to M. -L. Wong and awards from the National Alliance for Research on Schizophrenia and Depression to M. -L. Wong. This study is registered at clinicaltrials.gov: ID No. NCT00657605.
Acknowledgments
During the course of this study, Amgen and Amylin Pharmaceuticals graciously provided r-MetHuLeptin. Neither Amgen nor Amylin Pharmaceuticals contributed to the design, analysis, or writing of this study. We are also grateful to the members of the UCLA GCRC, the University of Miami GCRC, and the University of Miami Behavioral Medicine Research Center, to Drs. João Vicente Busnello and Luciana Ribeiro for their contributions to the clinical care of the leptin-deficient patients, and to Arie Zakaryan for assistance in the preparation of this manuscript.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Andreev VP, Paz-Filho G, Wong ML, Licinio J. Deconvolution of insulin secretion, insulin hepatic extraction, post-hepatic delivery rates and sensitivity during 24-h standardized meals: time course of glucose homeostasis in leptin replacement treatment. Horm Metab Res. In press. [DOI] [PubMed]
- 2.Baicy K, London ED, Monterosso J, Wong ML, Delibasi T, Sharma A, Licinio J. Leptin replacement alters brain response to food cues in genetically leptin-deficient adults. Proc Natl Acad Sci USA 104: 18276–18279, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bates SH, Kulkarni RN, Seifert M, Myers MG Jr. Roles for leptin receptor/STAT3-dependent and -independent signals in the regulation of glucose homeostasis. Cell Metab 1: 169–178, 2005. [DOI] [PubMed] [Google Scholar]
- 4.Bjorbaek C, Kahn BB. Leptin signaling in the central nervous system and the periphery. Recent Prog Horm Res 59: 305–331, 2004. [DOI] [PubMed] [Google Scholar]
- 5.Boden G, Chen X, Kolaczynski JW, Polansky M. Effects of prolonged hyperinsulinemia on serum leptin in normal human subjects. J Clin Invest 100: 1107–1113, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonora E, Kiechl S, Willeit J, Oberhollenzer F, Egger G, Targher G, Alberiche M, Bonadonna RC, Muggeo M. Prevalence of insulin resistance in metabolic disorders: the Bruneck Study. Diabetes 47: 1643–1649, 1998. [DOI] [PubMed] [Google Scholar]
- 7.Buettner C, Pocai A, Muse ED, Etgen AM, Myers MG Jr, Rossetti L. Critical role of STAT3 in leptin's metabolic actions. Cell Metab 4: 49–60, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ceddia RB Direct metabolic regulation in skeletal muscle and fat tissue by leptin: implications for glucose and fatty acids homeostasis. Int J Obes (Lond) 29: 1175–1183, 2005. [DOI] [PubMed] [Google Scholar]
- 9.Ceddia RB, Koistinen HA, Zierath JR, Sweeney G. Analysis of paradoxical observations on the association between leptin and insulin resistance. FASEB J 16: 1163–1176, 2002. [DOI] [PubMed] [Google Scholar]
- 10.Chinookoswong N, Wang JL, Shi ZQ. Leptin restores euglycemia and normalizes glucose turnover in insulin-deficient diabetes in the rat. Diabetes 48: 1487–1492, 1999. [DOI] [PubMed] [Google Scholar]
- 11.Cohen B, Novick D, Rubinstein M. Modulation of insulin activities by leptin. Science 274: 1185–1188, 1996. [DOI] [PubMed] [Google Scholar]
- 12.Covey SD, Wideman RD, McDonald C, Unniappan S, Huynh F, Asadi A, Speck M, Webber T, Chua SC, Kieffer TJ. The pancreatic beta cell is a key site for mediating the effects of leptin on glucose homeostasis. Cell Metab 4: 291–302, 2006. [DOI] [PubMed] [Google Scholar]
- 13.Dalla Man C, Caumo A, Basu R, Rizza R, Toffolo G, Cobelli C. Minimal model estimation of glucose absorption and insulin sensitivity from oral test: validation with a tracer method. Am J Physiol Endocrinol Metab 287: E637–E643, 2004. [DOI] [PubMed] [Google Scholar]
- 14.Dalla Man C, Caumo A, Cobelli C. The oral glucose minimal model: estimation of insulin sensitivity from a meal test. IEEE Trans Biomed Eng 49: 419–429, 2002. [DOI] [PubMed] [Google Scholar]
- 15.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol Endocrinol Metab Gastrointest Physiol 237: E214–E223, 1979. [DOI] [PubMed] [Google Scholar]
- 16.Donahue RP, Prineas RJ, Donahue RD, Zimmet P, Bean JA, De Courten M, Collier G, Goldberg RB, Skyler JS, Schneiderman N. Is fasting leptin associated with insulin resistance among nondiabetic individuals? The Miami Community Health Study. Diabetes Care 22: 1092–1096, 1999. [DOI] [PubMed] [Google Scholar]
- 17.Dulloo AG, Stock MJ, Solinas G, Boss O, Montani JP, Seydoux J. Leptin directly stimulates thermogenesis in skeletal muscle. FEBS Lett 515: 109–113, 2002. [DOI] [PubMed] [Google Scholar]
- 18.Eaton RP, Allen RC, Schade DS, Erickson KM, Standefer J. Prehepatic insulin production in man: kinetic analysis using peripheral connecting peptide behavior. J Clin Endocrinol Metab 51: 520–528, 1980. [DOI] [PubMed] [Google Scholar]
- 19.Ebihara K, Kusakabe T, Hirata M, Masuzaki H, Miyanaga F, Kobayashi N, Tanaka T, Chusho H, Miyazawa T, Hayashi T, Hosoda K, Ogawa Y, DePaoli AM, Fukushima M, Nakao K. Efficacy and safety of leptin-replacement therapy and possible mechanisms of leptin actions in patients with generalized lipodystrophy. J Clin Endocrinol Metab 92: 532–541, 2007. [DOI] [PubMed] [Google Scholar]
- 20.Emilsson V, Liu YL, Cawthorne MA, Morton NM, Davenport M. Expression of the functional leptin receptor mRNA in pancreatic islets and direct inhibitory action of leptin on insulin secretion. Diabetes 46: 313–316, 1997. [DOI] [PubMed] [Google Scholar]
- 21.Enriori PJ, Evans AE, Sinnayah P, Cowley MA. Leptin resistance and obesity. Obesity (Silver Spring) 14, Suppl 5: 254S–258S, 2006. [DOI] [PubMed] [Google Scholar]
- 22.Farooqi IS, Jebb SA, Langmack G, Lawrence E, Cheetham CH, Prentice AM, Hughes IA, McCamish MA, O'Rahilly S. Effects of recombinant leptin therapy in a child with congenital leptin deficiency. N Engl J Med 341: 879–884, 1999. [DOI] [PubMed] [Google Scholar]
- 23.Farooqi IS, Matarese G, Lord GM, Keogh JM, Lawrence E, Agwu C, Sanna V, Jebb SA, Perna F, Fontana S, Lechler RI, DePaoli AM, O'Rahilly S. Beneficial effects of leptin on obesity, T cell hyporesponsiveness, and neuroendocrine/metabolic dysfunction of human congenital leptin deficiency. J Clin Invest 110: 1093–1103, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gavrilova O, Marcus-Samuels B, Leon LR, Vinson C, Reitman ML. Leptin and diabetes in lipoatrophic mice. Nature 403: 850; discussion 850–851, 2000. [DOI] [PubMed] [Google Scholar]
- 25.Gibson WT, Farooqi IS, Moreau M, DePaoli AM, Lawrence E, O'Rahilly S, Trussell RA. Congenital leptin deficiency due to homozygosity for the Delta133G mutation: report of another case and evaluation of response to four years of leptin therapy. J Clin Endocrinol Metab 89: 4821–4826, 2004. [DOI] [PubMed] [Google Scholar]
- 26.Javor ED, Cochran EK, Musso C, Young JR, Depaoli AM, Gorden P. Long-term efficacy of leptin replacement in patients with generalized lipodystrophy. Diabetes 54: 1994–2002, 2005. [DOI] [PubMed] [Google Scholar]
- 27.Kahn BB, Alquier T, Carling D, Hardie DG. AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab 1: 15–25, 2005. [DOI] [PubMed] [Google Scholar]
- 28.Kellerer M, Koch M, Metzinger E, Mushack J, Capp E, Haring HU. Leptin activates PI-3 kinase in C2C12 myotubes via janus kinase-2 (JAK-2) and insulin receptor substrate-2 (IRS-2) dependent pathways. Diabetologia 40: 1358–1362, 1997. [DOI] [PubMed] [Google Scholar]
- 29.Kieffer TJ, Habener JF. The adipoinsular axis: effects of leptin on pancreatic β-cells. Am J Physiol Endocrinol Metab 278: E1–E14, 2000. [DOI] [PubMed] [Google Scholar]
- 30.Kieffer TJ, Heller RS, Leech CA, Holz GG, Habener JF. Leptin suppression of insulin secretion by the activation of ATP-sensitive K+ channels in pancreatic beta-cells. Diabetes 46: 1087–1093, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koebnick C, Roberts CK, Shaibi GQ, Kelly LA, Lane CJ, Toledo-Corral CM, Davis JN, Ventura EE, Alexander K, Weigensberg MJ, Goran MI. Adiponectin and leptin are independently associated with insulin sensitivity, but not with insulin secretion or beta-cell function in overweight hispanic adolescents. Horm Metab Res 40: 708–712, 2008. [DOI] [PubMed] [Google Scholar]
- 32.Licinio J, Caglayan S, Ozata M, Yildiz BO, de Miranda PB, O'Kirwan F, Whitby R, Liang L, Cohen P, Bhasin S, Krauss RM, Veldhuis JD, Wagner AJ, DePaoli AM, McCann SM, Wong ML. Phenotypic effects of leptin replacement on morbid obesity, diabetes mellitus, hypogonadism, and behavior in leptin-deficient adults. Proc Natl Acad Sci USA 101: 4531–4536, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Licinio J, Milane M, Thakur S, Whelan F, Yildiz BO, Delibasi T, de Miranda PB, Ozata M, Bolu E, Depaoli A, Wong ML. Effects of leptin on intake of specific micro- and macronutrients in a woman with leptin gene deficiency studied off and on leptin at stable body weight. Appetite 49: 594–599, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Licinio J, Ribeiro L, Busnello JV, Delibasi T, Thakur S, Elashoff RM, Sharma A, Jardack PM, Depaoli AM, Wong ML. Effects of leptin replacement on macro- and micronutrient preferences. Int J Obes (Lond) 31: 1859–1863, 2007. [DOI] [PubMed] [Google Scholar]
- 35.Liu L, Karkanias GB, Morales JC, Hawkins M, Barzilai N, Wang J, Rossetti L. Intracerebroventricular leptin regulates hepatic but not peripheral glucose fluxes. J Biol Chem 273: 31160–31167, 1998. [DOI] [PubMed] [Google Scholar]
- 36.Maedler K, Schulthess FT, Bielman C, Berney T, Bonny C, Prentki M, Donath MY, Roduit R. Glucose and leptin induce apoptosis in human beta-cells and impair glucose-stimulated insulin secretion through activation of c-Jun N-terminal kinases. FASEB J 22: 1905–1913, 2008. [DOI] [PubMed] [Google Scholar]
- 37.Maedler K, Sergeev P, Ehses JA, Mathe Z, Bosco D, Berney T, Dayer JM, Reinecke M, Halban PA, Donath MY. Leptin modulates beta cell expression of IL-1 receptor antagonist and release of IL-1beta in human islets. Proc Natl Acad Sci USA 101: 8138–8143, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Matochik JA, London ED, Yildiz BO, Ozata M, Caglayan S, DePaoli AM, Wong ML, Licinio J. Effect of leptin replacement on brain structure in genetically leptin-deficient adults. J Clin Endocrinol Metab 90: 2851–2854, 2005. [DOI] [PubMed] [Google Scholar]
- 39.Minokoshi Y, Kim YB, Peroni OD, Fryer LG, Muller C, Carling D, Kahn BB. Leptin stimulates fatty-acid oxidation by activating AMP-activated protein kinase. Nature 415: 339–343, 2002. [DOI] [PubMed] [Google Scholar]
- 40.Montague CT, Farooqi IS, Whitehead JP, Soos MA, Rau H, Wareham NJ, Sewter CP, Digby JE, Mohammed SN, Hurst JA, Cheetham CH, Earley AR, Barnett AH, Prins JB, O'Rahilly S. Congenital leptin deficiency is associated with severe early-onset obesity in humans. Nature 387: 903–908, 1997. [DOI] [PubMed] [Google Scholar]
- 41.Muller G, Ertl J, Gerl M, Preibisch G. Leptin impairs metabolic actions of insulin in isolated rat adipocytes. J Biol Chem 272: 10585–10593, 1997. [DOI] [PubMed] [Google Scholar]
- 42.Muzzin P, Eisensmith RC, Copeland KC, Woo SL. Correction of obesity and diabetes in genetically obese mice by leptin gene therapy. Proc Natl Acad Sci USA 93: 14804–14808, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Niswender KD, Magnuson MA. Obesity and the beta cell: lessons from leptin. J Clin Invest 117: 2753–2756, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.O'Rahilly S Human obesity and insulin resistance: lessons from experiments of nature. Biochem Soc Trans 35: 33–36, 2007. [DOI] [PubMed] [Google Scholar]
- 45.Oral EA, Simha V, Ruiz E, Andewelt A, Premkumar A, Snell P, Wagner AJ, DePaoli AM, Reitman ML, Taylor SI, Gorden P, Garg A. Leptin-replacement therapy for lipodystrophy. N Engl J Med 346: 570–578, 2002. [DOI] [PubMed] [Google Scholar]
- 46.Ozata M, Ozdemir IC, Licinio J. Human leptin deficiency caused by a missense mutation: multiple endocrine defects, decreased sympathetic tone, and immune system dysfunction indicate new targets for leptin action, greater central than peripheral resistance to the effects of leptin, and spontaneous correction of leptin-mediated defects. J Clin Endocrinol Metab 84: 3686–3695, 1999. [DOI] [PubMed] [Google Scholar]
- 47.Paz-Filho GJ, Ayala A, Esposito K, Erol HK, Delibasi T, Barry EH, Wong ML, Licinio J. Effects of leptin on lipid metabolism. Horm Metab Res 40: 572–574, 2008. [DOI] [PubMed] [Google Scholar]
- 48.Petersen KF, Oral EA, Dufour S, Befroy D, Ariyan C, Yu C, Cline GW, DePaoli AM, Taylor SI, Gorden P, Shulman GI. Leptin reverses insulin resistance and hepatic steatosis in patients with severe lipodystrophy. J Clin Invest 109: 1345–1350, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Polonsky KS, Licinio-Paixao J, Given BD, Pugh W, Rue P, Galloway J, Karrison T, Frank B. Use of biosynthetic human C-peptide in the measurement of insulin secretion rates in normal volunteers and type I diabetic patients. J Clin Invest 77: 98–105, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Roduit R, Thorens B. Inhibition of glucose-induced insulin secretion by long-term preexposure of pancreatic islets to leptin. FEBS Lett 415: 179–182, 1997. [DOI] [PubMed] [Google Scholar]
- 51.Sahu A Minireview: A hypothalamic role in energy balance with special emphasis on leptin. Endocrinology 145: 2613–2620, 2004. [DOI] [PubMed] [Google Scholar]
- 52.Sandoval DA, Davis SN. Leptin: metabolic control and regulation. J Diabetes Complications 17: 108–113, 2003. [DOI] [PubMed] [Google Scholar]
- 53.Segal KR, Landt M, Klein S. Relationship between insulin sensitivity and plasma leptin concentration in lean and obese men. Diabetes 45: 988–991, 1996. [DOI] [PubMed] [Google Scholar]
- 54.Seufert J Leptin effects on pancreatic beta-cell gene expression and function. Diabetes 53, Suppl 1: S152–S158, 2004. [DOI] [PubMed] [Google Scholar]
- 55.Seufert J, Kieffer TJ, Habener JF. Leptin inhibits insulin gene transcription and reverses hyperinsulinemia in leptin-deficient ob/ob mice. Proc Natl Acad Sci USA 96: 674–679, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shimomura I, Hammer RE, Ikemoto S, Brown MS, Goldstein JL. Leptin reverses insulin resistance and diabetes mellitus in mice with congenital lipodystrophy. Nature 401: 73–76, 1999. [DOI] [PubMed] [Google Scholar]
- 57.Sivitz WI, Walsh SA, Morgan DA, Thomas MJ, Haynes WG. Effects of leptin on insulin sensitivity in normal rats. Endocrinology 138: 3395–3401, 1997. [DOI] [PubMed] [Google Scholar]
- 58.Strobel A, Issad T, Camoin L, Ozata M, Strosberg AD. A leptin missense mutation associated with hypogonadism and morbid obesity. Nat Genet 18: 213–215, 1998. [DOI] [PubMed] [Google Scholar]
- 59.Sweeney G, Keen J, Somwar R, Konrad D, Garg R, Klip A. High leptin levels acutely inhibit insulin-stimulated glucose uptake without affecting glucose transporter 4 translocation in l6 rat skeletal muscle cells. Endocrinology 142: 4806–4812, 2001. [DOI] [PubMed] [Google Scholar]
- 60.Widdowson PS, Upton R, Pickavance L, Buckingham R, Tadayyon M, Arch J, Williams G. Acute hyperleptinemia does not modify insulin sensitivity in vivo in the rat. Horm Metab Res 30: 259–262, 1998. [DOI] [PubMed] [Google Scholar]
- 61.Williamson DA, Ravussin E, Wong ML, Wagner A, Dipaoli A, Caglayan S, Ozata M, Martin C, Walden H, Arnett C, Licinio J. Microanalysis of eating behavior of three leptin deficient adults treated with leptin therapy. Appetite 45: 75–80, 2005. [DOI] [PubMed] [Google Scholar]
- 62.Wong SL, DePaoli AM, Lee JH, Mantzoros CS. Leptin hormonal kinetics in the fed state: effects of adiposity, age, and gender on endogenous leptin production and clearance rates. J Clin Endocrinol Metab 89: 2672–2677, 2004. [DOI] [PubMed] [Google Scholar]
- 63.Zierath JR, Frevert EU, Ryder JW, Berggren PO, Kahn BB. Evidence against a direct effect of leptin on glucose transport in skeletal muscle and adipocytes. Diabetes 47: 1–4, 1998. [DOI] [PubMed] [Google Scholar]