Abstract
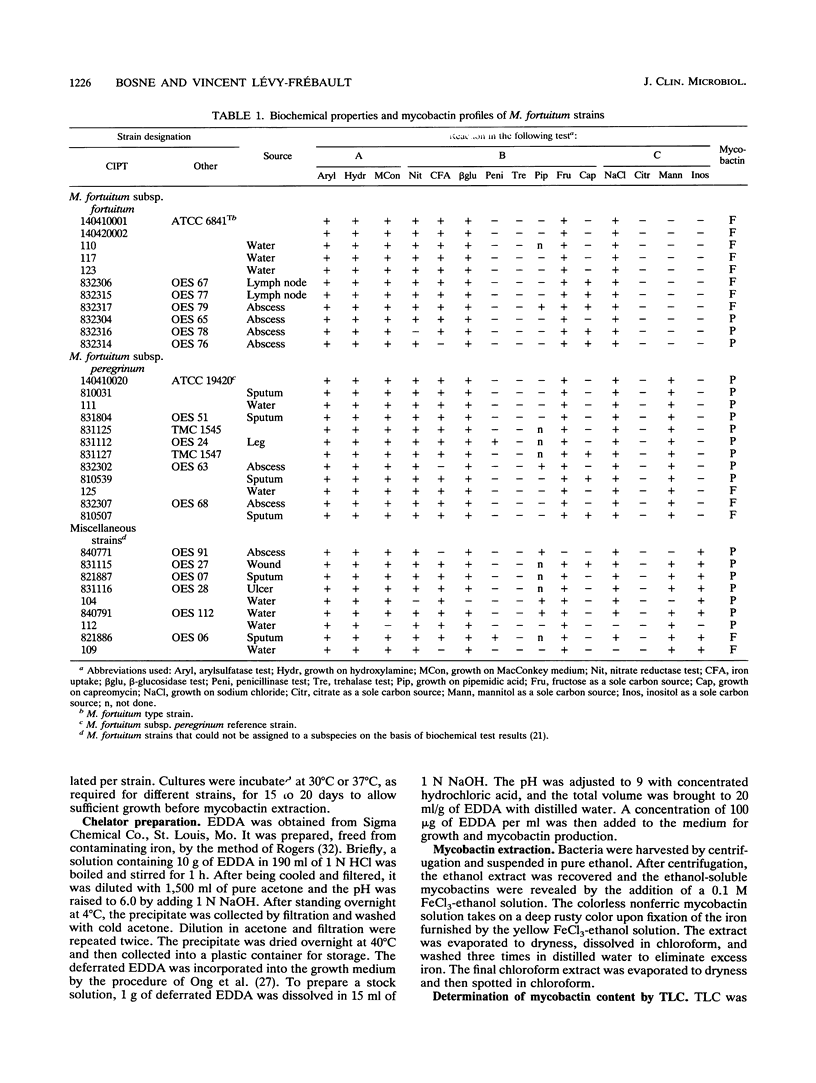
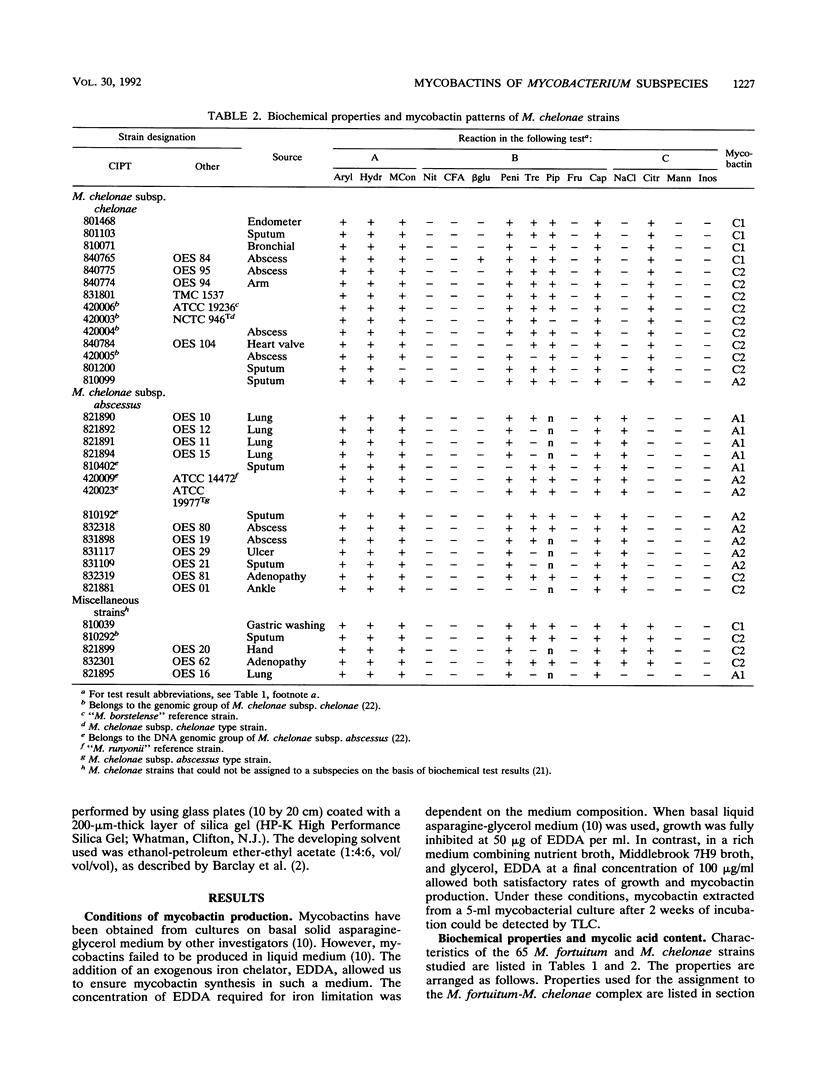
Mycobactin patterns from 65 Mycobacterium fortuitum and Mycobacterium chelonae strains have been determined by thin-layer chromatography. By use of a rich liquid medium containing an iron chelator (ethylenediamine-di-o-hydroxyphenylacetic acid [EDDA]) to ensure iron starvation, all strains were able to form mycobactins. The method developed here allows sensitive detection of mycobactin by thin-layer chromatography from as little as 5 ml of culture after a 2-week incubation. Within M. fortuitum two mycobactin patterns were identified, whereas within M. chelonae four were recognized. Comparisons with the subspecific identification performed by using biochemical tests showed that 73% of the M. fortuitum subsp. fortuitum strains shared the same mycobactin pattern (designated F), whereas 75% of the M. fortuitum subsp. peregrinum strains shared the other mycobactin pattern (designated P). Within the M. fortuitum strains that cannot be assigned to a subspecies on the basis of their biochemical features, only F and P patterns were determined. Similarly, 93% of the M. chelonae subsp. chelonae strains produced the so-called C1 and C2 patterns and 86% of the M. chelonae subsp. abscessus strains produced A1 and A2 patterns. C2 and A2 were the patterns most frequently encountered; they were represented by 65 and 50% of the M. chelonae subsp. chelonae and M. chelonae subsp. abscessus strains, respectively. Within the biochemically M. chelonae strains that did not fit any subspecies on the basis of biochemical test results, C1, C2, and A1 patterns were found. Whereas about 30% of both M. fortuitum and M. chelonae strains cannot be characterized to the subspecies level on the basis of biochemical tests, 100% of the strains of both species can be characterized on the basis of mycobactin patterns.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baess I. Deoxyribonucleic acid relatedness among species of rapidly growing mycobacteria. Acta Pathol Microbiol Immunol Scand B. 1982 Oct;90(5):371–375. doi: 10.1111/j.1699-0463.1982.tb00133.x. [DOI] [PubMed] [Google Scholar]
- Barclay R., Ewing D. F., Ratledge C. Isolation, identification, and structural analysis of the mycobactins of Mycobacterium avium, Mycobacterium intracellulare, Mycobacterium scrofulaceum, and Mycobacterium paratuberculosis. J Bacteriol. 1985 Nov;164(2):896–903. doi: 10.1128/jb.164.2.896-903.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barclay R., Ratledge C. Mycobactins and exochelins of Mycobacterium tuberculosis, M. bovis, M. africanum and other related species. J Gen Microbiol. 1988 Mar;134(3):771–776. doi: 10.1099/00221287-134-3-771. [DOI] [PubMed] [Google Scholar]
- Casal M., Rodriguez F. Etude de l'activité in vitro des bêta-lactamines et des aminosides sur Mycobacterium fortuitum. Ann Microbiol (Paris) 1981 Jul-Aug;132B(1):51–56. [PubMed] [Google Scholar]
- Daffé M., Lanéelle M. A., Asselineau C., Lévy-Frébault V., David H. Intérêt taxonomique des acides gras des mycobactéries: proposition d'une méthode d'analyse. Ann Microbiol (Paris) 1983 Sep-Oct;134B(2):241–256. [PubMed] [Google Scholar]
- David H. L., Traore I., Feuillet A. Differential identification of Mycobacterium fortuitum and Mycobacterium chelonei. J Clin Microbiol. 1981 Jan;13(1):6–9. doi: 10.1128/jcm.13.1.6-9.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall R. M., Ratledge C. Distribution and application of mycobactins for the characterization of species within the genus Rhodococcus. J Gen Microbiol. 1986 Mar;132(3):853–856. doi: 10.1099/00221287-132-3-853. [DOI] [PubMed] [Google Scholar]
- Hall R. M., Ratledge C. Equivalence of mycobactins from Mycobacterium senegalense, Mycobacterium farcinogenes and Mycobacterium fortuitum. J Gen Microbiol. 1985 Jul;131(7):1691–1696. doi: 10.1099/00221287-131-7-1691. [DOI] [PubMed] [Google Scholar]
- Hall R. M., Ratledge C. Mycobactins as chemotaxonomic characters for some rapidly growing mycobacteria. J Gen Microbiol. 1984 Aug;130(8):1883–1892. doi: 10.1099/00221287-130-8-1883. [DOI] [PubMed] [Google Scholar]
- Hull S. I., Wallace R. J., Jr, Bobey D. G., Price K. E., Goodhines R. A., Swenson J. M., Silcox V. A. Presence of aminoglycoside acetyltransferase and plasmids in Mycobacterium fortuitum. Lack of correlation with intrinsic aminoglycoside resistance. Am Rev Respir Dis. 1984 Apr;129(4):614–618. [PubMed] [Google Scholar]
- Jenkins P. A., Marks J., Schaefer W. B. Lipid chromatography and seroagglutination in the classification of rapidly growing mycobacteria. Am Rev Respir Dis. 1971 Feb;103(2):179–187. doi: 10.1164/arrd.1971.103.2.179. [DOI] [PubMed] [Google Scholar]
- Kubica G. P., Baess I., Gordon R. E., Jenkins P. A., Kwapinski J. B., McDurmont C., Pattyn S. R., Saito H., Silcox V., Stanford J. L. A co-operative numerical analysis of rapidly growing mycobacteria. J Gen Microbiol. 1972 Nov;73(1):55–70. doi: 10.1099/00221287-73-1-55. [DOI] [PubMed] [Google Scholar]
- Luquin M., Ausina V., López Calahorra F., Belda F., García Barceló M., Celma C., Prats G. Evaluation of practical chromatographic procedures for identification of clinical isolates of mycobacteria. J Clin Microbiol. 1991 Jan;29(1):120–130. doi: 10.1128/jcm.29.1.120-130.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lévy-Frébault V., Daffé M., Goh K. S., Lanéelle M. A., Asselineau C., David H. L. Identification of Mycobacterium fortuitum and Mycobacterium chelonei. J Clin Microbiol. 1983 May;17(5):744–752. doi: 10.1128/jcm.17.5.744-752.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lévy-Frébault V., Daffé M., Restrepo E., Grimont F., Grimont P. A., David H. L. Differentiation of Mycobacterium thermoresistibile from Mycobacterium phlei and other rapidly growing mycobacteria. Ann Inst Pasteur Microbiol. 1986 Mar-Apr;137A(2):143–151. doi: 10.1016/s0769-2609(86)80019-9. [DOI] [PubMed] [Google Scholar]
- Minnikin D. E., Minnikin S. M., Goodfellow M., Stanford J. L. The mycolic acids of Mycobacterium chelonei. J Gen Microbiol. 1982 Apr;128(4):817–822. doi: 10.1099/00221287-128-4-817. [DOI] [PubMed] [Google Scholar]
- Ong S. A., Peterson T., Neilands J. B. Agrobactin, a siderophore from Agrobacterium tumefaciens. J Biol Chem. 1979 Mar 25;254(6):1860–1865. [PubMed] [Google Scholar]
- Pattyn S. R., Magnusson M., Stanford J. L., Grange J. M. A study of Mycobacterium fortuitum (ranae). J Med Microbiol. 1974 Feb;7(1):67–76. doi: 10.1099/00222615-7-1-67. [DOI] [PubMed] [Google Scholar]
- Ratledge C., Patel P. V. Isolation, properties and taxonomic relevance of lipid-soluble, iron-binding compounds (the nocobactins) from Nocardia. J Gen Microbiol. 1976 Mar;93(1):141–152. doi: 10.1099/00221287-93-1-141. [DOI] [PubMed] [Google Scholar]
- Rogall T., Wolters J., Flohr T., Böttger E. C. Towards a phylogeny and definition of species at the molecular level within the genus Mycobacterium. Int J Syst Bacteriol. 1990 Oct;40(4):323–330. doi: 10.1099/00207713-40-4-323. [DOI] [PubMed] [Google Scholar]
- Rogers H. J. Iron-Binding Catechols and Virulence in Escherichia coli. Infect Immun. 1973 Mar;7(3):445–456. doi: 10.1128/iai.7.3.445-456.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silcox V. A., Good R. C., Floyd M. M. Identification of clinically significant Mycobacterium fortuitum complex isolates. J Clin Microbiol. 1981 Dec;14(6):686–691. doi: 10.1128/jcm.14.6.686-691.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snow G. A. Mycobactins: iron-chelating growth factors from mycobacteria. Bacteriol Rev. 1970 Jun;34(2):99–125. doi: 10.1128/br.34.2.99-125.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snow G. A., White A. J. Chemical and biological properties of mycobactins isolated from various mycobacteria. Biochem J. 1969 Dec;115(5):1031–1050. doi: 10.1042/bj1151031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanford J. L., Gunthorpe W. J. Serological and bacteriological investigation of Mycobacterium ranae (fortuitum). J Bacteriol. 1969 May;98(2):375–383. doi: 10.1128/jb.98.2.375-383.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeya K., Nakayama Y., Muraoka S. Specificity in skin reaction to tuberculin protein prepared from rapidly growing mycobacteria and some nocardia. Am Rev Respir Dis. 1970 Dec;102(6):982–986. doi: 10.1164/arrd.1970.102.6.982. [DOI] [PubMed] [Google Scholar]
- Tsukamura M. Differentiation between Mycobacterium abscessus and Mycobacterium borstelense. Am Rev Respir Dis. 1970 Mar;101(3):426–428. doi: 10.1164/arrd.1970.101.3.426. [DOI] [PubMed] [Google Scholar]
- Wallace R. J., Jr, Brown B. A., Silcox V. A., Tsukamura M., Nash D. R., Steele L. C., Steingrube V. A., Smith J., Sumter G., Zhang Y. S. Clinical disease, drug susceptibility, and biochemical patterns of the unnamed third biovariant complex of Mycobacterium fortuitum. J Infect Dis. 1991 Mar;163(3):598–603. doi: 10.1093/infdis/163.3.598. [DOI] [PubMed] [Google Scholar]
- Wallace R. J., Jr, Swenson J. M., Silcox V. A., Bullen M. G. Treatment of nonpulmonary infections due to Mycobacterium fortuitum and Mycobacterium chelonei on the basis of in vitro susceptibilities. J Infect Dis. 1985 Sep;152(3):500–514. doi: 10.1093/infdis/152.3.500. [DOI] [PubMed] [Google Scholar]
- Wallace R. J., Jr, Swenson J. M., Silcox V. A., Good R. C., Tschen J. A., Stone M. S. Spectrum of disease due to rapidly growing mycobacteria. Rev Infect Dis. 1983 Jul-Aug;5(4):657–679. doi: 10.1093/clinids/5.4.657. [DOI] [PubMed] [Google Scholar]
- Wolinsky E. Nontuberculous mycobacteria and associated diseases. Am Rev Respir Dis. 1979 Jan;119(1):107–159. doi: 10.1164/arrd.1979.119.1.107. [DOI] [PubMed] [Google Scholar]



