Abstract
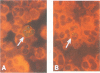
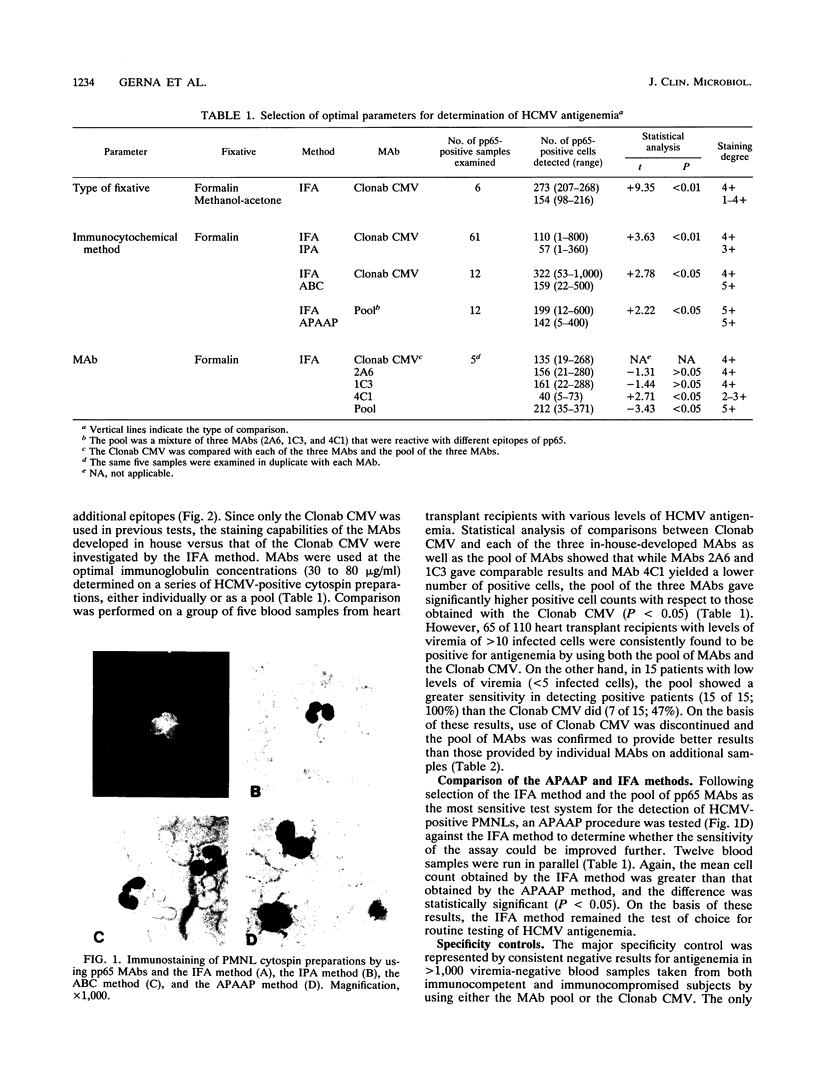
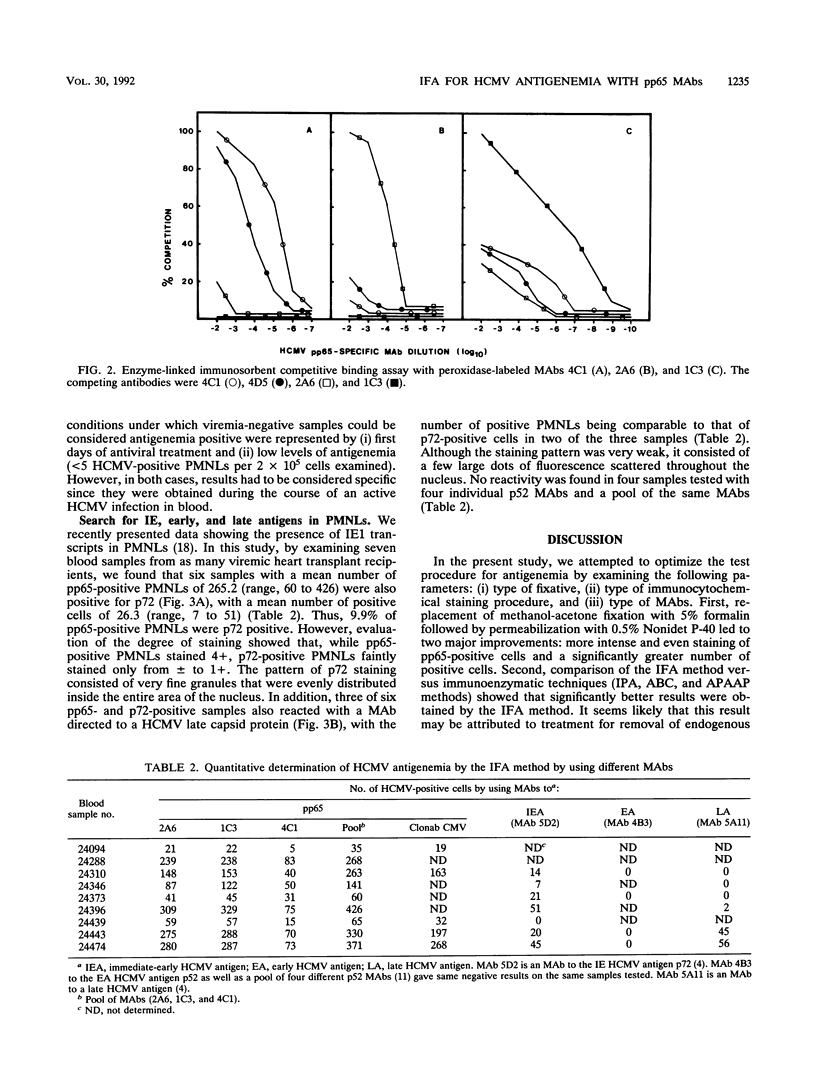
The main parameters of immunostaining techniques, i.e., the type of fixative, immunocytochemical reaction, and quality of monoclonal antibodies (MAbs), for quantitation of human cytomegalovirus (HCMV) antigenemia in peripheral blood polymorphonuclear leukocytes (currently performed by the indirect immunofluorescence or immunoperoxidase reaction by using MAbs to HCMV pp65) were investigated in order to optimize procedural steps and reagents. Significantly better results (in terms of the number of positive cells) were obtained on multiple cytospin preparations from heart transplant recipients with HCMV viremia when we used (i) formalin instead of methanol-acetone fixation and (ii) the indirect immunofluorescence reaction instead of the immunoperoxidase reaction, the avidin-biotin complex method, or the alkaline phosphatase antialkaline phosphatase procedure. In addition, comparison of the staining capabilities of three MAbs to pp65, which were developed in the laboratory and which were reactive to different epitopes of the protein, with a commercially available MAb (Clonab CMV) for determination of HCMV antigenemia showed that, while individual MAbs did not provide better results, the pool of MAbs detected a significantly higher number of positive peripheral blood polymorphonuclear leukocytes than Clonab CMV did. In addition, the sensitivity of the pool in detecting patients with low levels of viremia (less than 5/2 x 10(5) cells inoculated) as antigenemia positive was 100%, whereas the sensitivity of Clonab CMV was 47%. No differences in the specificities between the two MAb preparations were observed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bein G., Bitsch A., Hoyer J., Kirchner H. The detection of human cytomegalovirus immediate early antigen in peripheral blood leucocytes. J Immunol Methods. 1991 Mar 21;137(2):175–180. doi: 10.1016/0022-1759(91)90022-8. [DOI] [PubMed] [Google Scholar]
- Gerna G., Parea M., Percivalle E., Zipeto D., Silini E., Barbarini G., Milanesi G. Human cytomegalovirus viraemia in HIV-1-seropositive patients at various clinical stages of infection. AIDS. 1990 Oct;4(10):1027–1031. doi: 10.1097/00002030-199010000-00014. [DOI] [PubMed] [Google Scholar]
- Gerna G., Revello M. G., Percivalle E., Zavattoni M., Parea M., Battaglia M. Quantification of human cytomegalovirus viremia by using monoclonal antibodies to different viral proteins. J Clin Microbiol. 1990 Dec;28(12):2681–2688. doi: 10.1128/jcm.28.12.2681-2688.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerna G., Sarasini A., Di Matteo A., Parea M., Torsellini M., Battaglia M. Rapid detection of human rotavirus strains in stools by single-sandwich enzyme-linked immunosorbent assay systems using monoclonal antibodies. J Virol Methods. 1989 Apr-May;24(1-2):43–56. doi: 10.1016/0166-0934(89)90006-2. [DOI] [PubMed] [Google Scholar]
- Gerna G., Zipeto D., Parea M., Revello M. G., Silini E., Percivalle E., Zavattoni M., Grossi P., Milanesi G. Monitoring of human cytomegalovirus infections and ganciclovir treatment in heart transplant recipients by determination of viremia, antigenemia, and DNAemia. J Infect Dis. 1991 Sep;164(3):488–498. doi: 10.1093/infdis/164.3.488. [DOI] [PubMed] [Google Scholar]
- Grazia Revello M., Percivalle E., Zannino M., Rossi V., Gerna G. Development and evaluation of a capture ELISA for IgM antibody to the human cytomegalovirus major DNA binding protein. J Virol Methods. 1991 Dec;35(3):315–329. doi: 10.1016/0166-0934(91)90073-9. [DOI] [PubMed] [Google Scholar]
- Grazia Revello M., Zavattoni M., Percivalle E., Grossi P., Gerna G. Correlation between immunofluorescent detection of human cytomegalovirus immediate early antigens in polymorphonuclear leukocytes and viremia. J Infect Dis. 1989 Jul;160(1):159–160. doi: 10.1093/infdis/160.1.159. [DOI] [PubMed] [Google Scholar]
- Martin D. C., Katzenstein D. A., Yu G. S., Jordan M. C. Cytomegalovirus viremia detected by molecular hybridization and electron microscopy. Ann Intern Med. 1984 Feb;100(2):222–225. doi: 10.7326/0003-4819-100-2-222. [DOI] [PubMed] [Google Scholar]
- Miller H., Rossier E., Milk R., Thomas C. Prospective study of cytomegalovirus antigenemia in allograft recipients. J Clin Microbiol. 1991 May;29(5):1054–1055. doi: 10.1128/jcm.29.5.1054-1055.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall R. E., Dinwoodie N. Intranuclear localization of herpes simplex virus immediate-early and delayed-early proteins: evidence that ICP 4 is associated with progeny virus DNA. J Gen Virol. 1986 Oct;67(Pt 10):2163–2177. doi: 10.1099/0022-1317-67-10-2163. [DOI] [PubMed] [Google Scholar]
- Revello M. G., Percivalle E., Di Matteo A., Morini F., Gerna G. Nuclear expression of the lower matrix protein of human cytomegalovirus in peripheral blood leukocytes of immunocompromised viraemic patients. J Gen Virol. 1992 Feb;73(Pt 2):437–442. doi: 10.1099/0022-1317-73-2-437. [DOI] [PubMed] [Google Scholar]
- Revello M. G., Percivalle E., Zavattoni M., Parea M., Grossi P., Gerna G. Detection of human cytomegalovirus immediate early antigen in leukocytes as a marker of viremia in immunocompromised patients. J Med Virol. 1989 Oct;29(2):88–93. doi: 10.1002/jmv.1890290204. [DOI] [PubMed] [Google Scholar]
- Wunderli W., Kägi M. K., Grüter E., Auracher J. D. Detection of cytomegalovirus in peripheral leukocytes by different methods. J Clin Microbiol. 1989 Aug;27(8):1916–1917. doi: 10.1128/jcm.27.8.1916-1917.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Bij W., Schirm J., Torensma R., van Son W. J., Tegzess A. M., The T. H. Comparison between viremia and antigenemia for detection of cytomegalovirus in blood. J Clin Microbiol. 1988 Dec;26(12):2531–2535. doi: 10.1128/jcm.26.12.2531-2535.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Bij W., Torensma R., van Son W. J., Anema J., Schirm J., Tegzess A. M., The T. H. Rapid immunodiagnosis of active cytomegalovirus infection by monoclonal antibody staining of blood leucocytes. J Med Virol. 1988 Jun;25(2):179–188. doi: 10.1002/jmv.1890250208. [DOI] [PubMed] [Google Scholar]