Abstract
SPARC-Related Modular Calcium Binding Protein-2 (Smoc-2) is a broadly-expressed matricellular protein which contributes to mitogenesis via activation of Integrin-Linked Kinase (ILK). Here we show that expression of Smoc2 is repressed in cultured cells following treatment with Aryl-hydrocarbon receptor (Ahr) ligands including the ubiquitous environmental pollutants Benzo[a]pyrene (B[a]P) and 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD). The Smoc2 promoter contains two consensus putative Ahr-binding sites and Smoc2 promoter-driven reporter genes are repressed in response to B[a]P in an Ahr-dependent manner in cultured cells. Using organ culture experiments we show that TCDD also represses Smoc2 mRNA expression in testes from Ahr+/+ but not Ahr−/− mice. Therefore, exposure to Ahr ligands is likely to affect Smoc2 expression in vivo. Taken together our results indicate that Smoc2 is a novel transcriptional target of activated Ahr. Perturbation of Smoc2 expression may mediate the adverse developmental effects of environmental aryl-hydrocarbon exposure.
Keywords: Smoc2, Benzo[a]pyrene, Dioxin, Ahr, testes
1. Introduction
Polycyclic aromatic hydrocarbons (PAH), typified by Benzo[a]pyrene (B[a]P) are ubiquitous environmental pollutants that are generated during the combustion of carbon-containing fuels including coal, gasoline and tobacco smoke (Baum, 1978). PAH-metabolizing cytochrome P450 enzymes are transcriptionally induced by B[a]P and other aryl-hydrocarbons and this induction is largely dependent on a transcription factor termed the Aryl-hydrocarbon Receptor, or Ahr (Whitlock, 1999). PAH such as B[a]P, as well as non-genotoxic Halogenated Aromatic Hydrocarbons (HAH, including 2,3,7,8-Tetrachlorodibenzo-p-dioxin or TCDD) are ligands that bind to and activate Ahr (Mandal, 2005). Ligand binding causes the translocation of Ahr into the nucleus where it heterodimerizes with the Ahr Nuclear Transporter (ARNT). The ligand-bound Ahr/ARNT complex binds to specific promoter elements termed Xenobiotic Response Elements (XRE) that regulate the transcription of cytochrome P450 genes including CYP1A1, CYP1A2, and others (Whitlock, 1999). B[a]P is metabolized by cytochrome P450s, to generate the DNA-damaging species and ‘ultimate carcinogen’ benzo[a]pyrene-7,8-diol-9,10-epoxide (BPDE) (Conney, 1982).
Ahr is also likely to influence cell growth and differentiation via direct mechanisms not requiring P450-mediated xenobiotic metabolism (Schmidt et al., 1996; Alexander et al., 1998; Lahvis et al., 2005). For example, studies with transgenic mice have demonstrated that Ahr and ARNT play a role in the developmental closure of a hepatic vascular shunt termed the ductus venosus (Lahvis et al., 2005). Microarray profiling experiments have shown that non-genotoxic Ahr ligands such as TCDD modulate the expression of many genes involved in signal transduction and cell cycle regulation (Hanlon et al., 2005). Thus Ahr activation by environmental PAH or HAH could result in crosstalk with developmental pathways, thereby accounting for some of the detrimental effects of aryl-hydrocarbon exposure (Puga et al., 2005).
To gain insight into possible mechanisms by which Ahr signaling influences gene expression, we performed global mRNA profiling of PAH-treated cells. Here we identify Smoc2 (SPARC-related Modular Calcium-binding protein-2) as a novel aryl-hydrocarbon-regulated gene.
Smoc-2 belongs to a family of matricellular proteins that also includes BM40/osteonectin/SPARC (Secreted Protein Acidic and Rich in Cysteine), SC1/hevin/Sparcl, tsc36/Flik/Fatl1, and Testican-1/Spock1 (Bornstein and Sage, 2002). Matricellular proteins regulate cell-matrix interactions, cell adhesion, spreading, migration, wound repair and angiogenesis during development, disease, and in response to injury (Bornstein and Sage, 2002). These biological effects result from interactions between matricellular factors and growth factors, integrins and/or other extracellular matrix proteins. Smoc-2 potentiates responses to mitogenic and angiogenic factors including FGF and VEGF (Rocnik et al., 2006). Consistent with a role for Smoc-2 in growth control, activation of Integrin-Linked Kinase (ILK) and cyclin D1 expression are Smoc-2-dependent (Liu et al., 2007). Integrins αv, β1, and β6 mediate cell adhesion to the C-terminal EF-hand of Smoc-2, also suggesting a role for Smoc-2 in integrin activation (Maier et al., 2008). SNP profiling studies have identified polymorphisms in the human SMOC2 gene with linkage to pulmonary function (Wilk et al., 2003; Wilk et al., 2007), indicating a possible role for SMOC-2 in normal growth and development. Here we demonstrate that Ahr ligands repress Smoc2 both in cultured cells and in organ culture. Ahr-mediated repression of Smoc2 expression provides a novel mechanism by which exposure to environmental agents might influence and perturb signal transduction events leading to defects in growth and development.
2. Material and Methods
2.1. Chemicals and antibodies
Benzo[a]pyrene was purchased from Sigma. TCDD was obtained from the Midwest Research Institute. All chemicals and antibodies were obtained from previously-described sources (Liu et al., 2007).
2.2. Cells and Culture
Swiss 3T3 cells were obtained from ATCC. Ahr−/− Mouse Embryonic Fibroblasts (MEFs) were provided by Dr. David Sherr (Boston University School of Medicine) and were cultured using a 3T3 protocol (Liu et al., 2007).
2.3. Microarray analysis
Quiescent Swiss 3T3 cells were stimulated with 10 % serum or 10 % serum + 1μM B[a]P for 17 hr. Total cellular RNA was harvested as described previously (Rocnik et al., 2006) and submitted to Genome Systems, Inc. (www.genomesystems.com) for labeling and hybridization to DNA chips containing 10,000 arrayed mouse expressed sequence tags (EST). Genome Systems provided a list of transcripts corresponding to arrayed EST clones that were differentially expressed between the two samples.
2.4. RNA blot analysis
20 μg samples of total RNA were electrophoresed on agarose gels, transferred to nitrocellulose filters and hybridized with random-primed 32P-labelled cDNA probes exactly as described previously (Vaziri and Faller, 1995).
2.5. Isolation of the Smoc2 promoter
A DNA fragment containing 1087 bp of the 5′ region of the mouse Smoc2 gene was amplified from 3T3 cell genomic DNA using the following primers: 5′-CGGGGTACCCCCCGTGTTGGGCTAGGGCAGGGTA-3′ (forward) and CTAGCTAGCGGTGACGCTGGAGGGGACCAAGCGA-3′ (reverse). The resulting PCR product was digested with Kpn I and NheI and ligated into the promoterless pGL2b luciferase vector (Promega).
2.6. Transfections and luciferase activity assays
Cells were seeded on 6-well plates and transfected with 4 μg of DNA using Lipofectamine 2000 (Invitrogen). Cells harvested for luciferase assays 48 hr post-transfection using a commercially available kit (Promega).
2.7. Extraction and analysis of mRNA from cultured cells
Total cellular RNA was extracted and analyzed by RT-PCR as described previously (Liu et al., 2007). The following primers were used for RT-PCR: 5′CAGGTCCAGTGTCACAGCTACAC3′ (mouse Smoc-2 forward), 5′ GGTCTTGTTCTGCCGACTCTTAAC3′ (mouse Smoc-2 reverse), 5′ GGCTACAGCTTCACCACCACAGC 3′ (mouse β-actin forward), and 5′ CCACAGGATTCCATACCCAAGAAGG3′ (mouse β-actin reverse). The amplified products were separated on 1.0 % agarose gels and visualized under an UV transilluminator.
2.8. Preparation of whole cell extracts and Immunoblotting
Whole cell extracts were prepared and analyzed using SDS-PAGE and immunoblotting exactly as described previously (Liu et al., 2007).
2.9. Mice
CD-1 mice were purchased from Charles River Laboratories. Ahr mutant mice were a gift of Dr. D. H. Sherr (Boston University School of Medicine). Ahr−/− mice were generated by mating Ahr+/−females to Ahr−/− males. Embryos were subsequently genotyped as previously described (Robles et al, 2000). Timed matings were used for all experiments where noon on the day of vaginal plug detection was designated as embryonic day (E) 0.5.
2.10. Extraction and analysis of RNA from cultured gonads
RNA was isolated using the RNeasy mini kit (Qiagen). cDNA was synthesized using oligo-dT primers and SuperScript II reverse transcriptase (RT) (Invitrogen). Quantitative real-time RT- PCR analysis was performed with an ABI PRISM 7900HT Sequence Detection System, using Power SYBR Green PCR Master Mix (Applied Biosystems) and the following primers: Smoc2 5′ GACCCTCTTCCTCTTCTGG3′ and 5′ TCCTTCTTGCCAATGTCTCC3′; Cyp1a1 5′AGGATGTGTCTGGTTACTTTG3′ and 5′ AGAAACATGGACATGCAAG3′; Hprt (Bouma et al., 2004). All primer pairs produced single products of the expected size, without the formation of primer dimers. Validation experiments were performed according to Applied Biosystems guidelines (AppliedBiosystems, 2004). Fold change values were calculated using the ΔΔCt method (Livak and Schmittgen, 2001). Two-tailed Student’s t-tests were performed to determine statistical significance.
2.11. Organ culture
Gonad/mesonephros complexes were dissected from E12.5 mice. One complex served as a control while the other was treated. TMTP Isopore membrane filters (5 μm, Millipore) were floated on 0.5 ml of Dulbecco’s Modified Eagle Medium (Invitrogen) supplemented with 10 % fetal bovine serum and ampicillin (50μg/ml) in 4-well cell culture plates. Isolated complexes were placed on filters and incubated at 37°C and 5% CO2. For drug treatments an appropriate volume of 1 mM TCDD stock solution in DMSO was added to give a final concentration of 1μM while an equivalent volume of DMSO was used as a control.
2.12. Wholemount in situ hybridization (WISH)
Digoxigenin (DIG)-labeled probes were generated by in vitro transcription of Smoc2- and Cyp1a1-containing plasmids using T7, SP6 or T3 RNA polymerase in the presence of DIG-labeled dUTP (Roche). Gonad-mesonephros complexes were dissected in PBS and fixed overnight at 4°C in 4 % paraformaldehyde/PBS. Whole-mount in situ hybridization was performed using standard protocols with minor modifications as described previously (Wilkinson, 1998).
3. Results
3.1. Identification of Smoc2 as a B[a]P-regulated mRNA
To gain insight into potential mechanisms by which PAH exposure affects gene expression we performed microarray experiments and determined global mRNA profiles of B[a]P-treated cells. Cultures of quiescent confluent Swiss 3T3 cells were treated with 10% serum in the presence or absence of 1μM B[a]P. Seventeen hours after serum treatment, replicate plates of control and B[a]P-treated cells were collected for analysis of RNA (by microarray and northern blotting), as described under ‘Material and Methods’.
The microarray analysis identified fewer than 100 mRNA species whose expression changed by 3-fold or more as a result of B[a]P treatment. Therefore, B[a]P did not cause global perturbation of gene expression under our experimental conditions. According to the microarray analysis, cDNAs induced by B[a]P included p21 and Mdm2, which we previously identified as B[a]P-inducible transcripts (Hsing et al., 2000). Genes encoding cyclin G (Cdk5r1), ornithine decarboxylase (Odc) and multi-drug resistant P-glycoprotein 1 (Mdr1), were also induced by B[a]P. The effect of B[a]P on Mdr1 is consistent with a previous report showing that the Mdr1 gene is Ahr-regulated (Mathieu et al., 2001). mRNAs whose abundance decreased after B[a]P treatment included T-Cadherin and IMAGE clone # 482198, which we subsequently identified as Smoc2 as described under ‘Materials and Methods’.
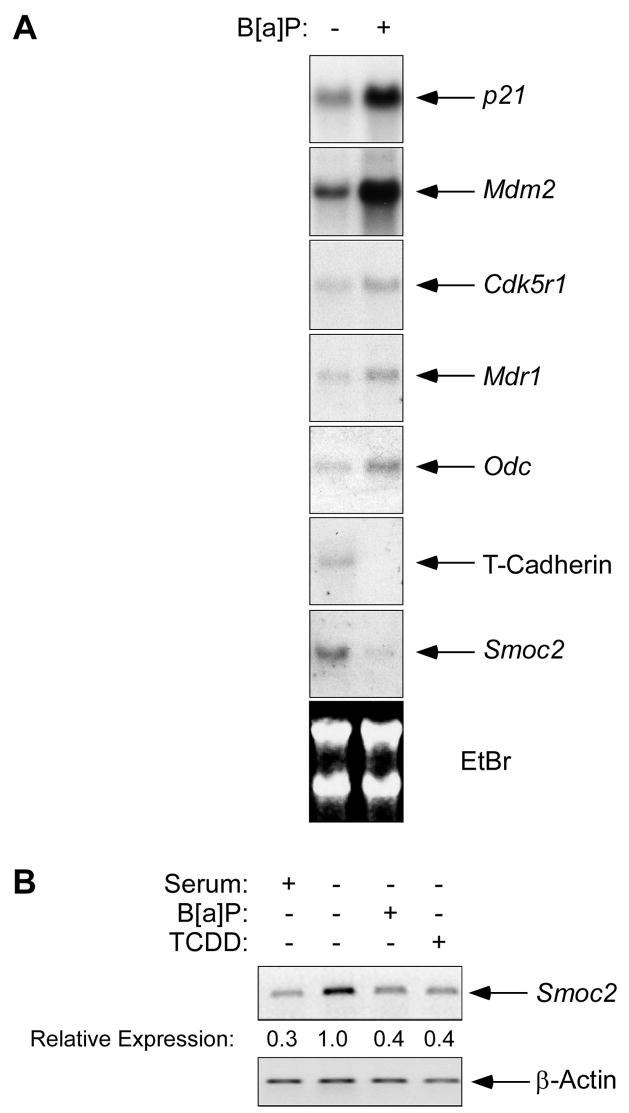
Several EST clones corresponding to B[a]P-responsive mRNAs (including p21, Mdm2, Mdr1, and Smoc2) were obtained and used as probes in northern blotting experiments to validate the microarray results. Fig. 1A shows the results of our RNA blotting experiments. For all ESTs tested, the changes in mRNA expression identified in the microarray experiment were reproduced using RNA blotting. Thus, B[a]P-induced increases in p21, Mdm2, Mdr1, Odc and decreases in T-Cadherin, and Smoc2 were readily detected, thereby validating the microarray analysis.
Fig. 1. Smoc2 mRNA is repressed in response to Ahr ligands.
(A) Samples of total RNA from control and 1 μM B[a]P-treated Swiss 3T3 cells were separated by electrophoresis on agarose gels (20μg/lane), transferred to nitrocellulose, and probed with various 32P-labelled cDNAs as indicated.
(B) Quiescent Swiss 3T3 cells were treated with 1 μM B[a]P or 10 nM TCDD for 24 hr. RNA prepared from the resulting cells was analyzed by RT-PCR using primers specific for Smoc2 and β-Actin as described under ‘Materials and Methods’.
Because B[a]P is metabolized to genotoxic species such as BPDE in an Ahr-dependent manner in Swiss 3T3 cells (Vaziri and Faller, 1997), we considered the possibility that the changes in expression of Smoc2 (and other mRNAs shown in Fig. 1A) occurred secondarily to a DNA damage response. Indeed, the induction of p53-regulated genes such as p21 and Mdm2 following B[a]P-treatment is due to acquisition of DNA damage (Vaziri and Faller, 1997). Moreover, since Smoc2 expression is affected by cell cycle progression (Liu et al., 2007), and because PAH such as B[a]P perturb the cell cycle (Vaziri and Faller, 1997), it was possible that Smoc2 levels were affected by BaP secondarily to cell cycle changes.
To distinguish between genotoxic and non-genotoxic mechanisms of gene repression we tested the effects of the non-genotoxic Ahr ligand TCDD on Smoc2 mRNA levels. To eliminate possible effects of cell cycle status on Smoc2 expression, we performed these experiments in cells which were growth-arrested in G0 by serum-starvation. As shown by the RT-PCR analyses in Fig. 1B, Smoc2 mRNA levels were induced following serum-starvation as we reported previously (Liu et al., 2007). Interestingly, Smoc2 mRNA levels were specifically reduced in response to B[a]P or TCDD (Fig. 1B). In other experiments, we found that treatment with BPDE (a product of B[a]P metabolism which elicits DNA damage but does not activate Ahr) did not affect Smoc2 expression (P. L., data not shown). Therefore, the reduced expression of Smoc2 in B[a]P or TCDD-treated cells cannot be attributed to a DNA damage response. Moreover, the decreased Smoc2 expression following treatment with B[a]P or TCDD occured in quiescent (G0) cells and therefore was not due to Ahr ligand-induced changes in the cell cycle.
3.2. The Smoc2 promoter is B[a]P-responsive
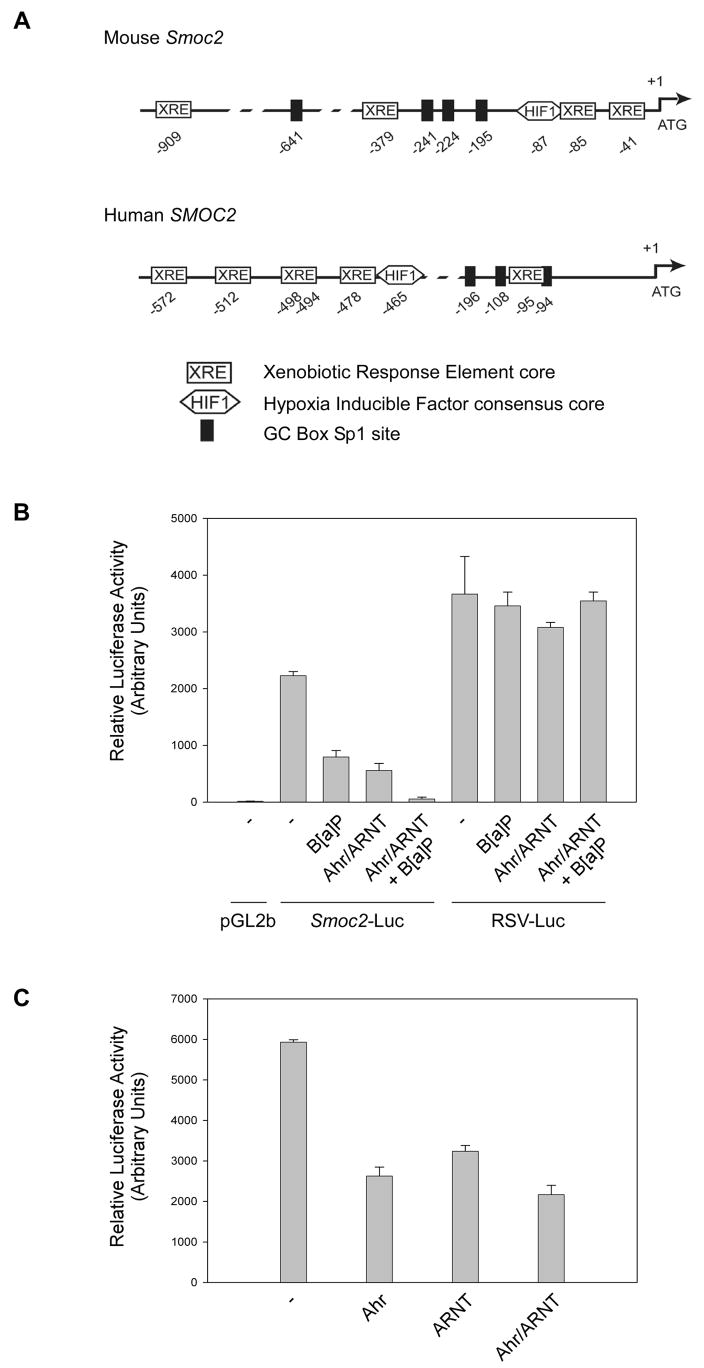
To gain insight into possible mechanisms by which Smoc2 mRNA levels are altered in response to B[a]P and TCDD we analyzed the sequence of the 5′ region of the Smoc2 gene using PROMO search tool and the TRANSFAC 8.3 database (Messeguer et al., 2002). As shown in Fig. 2A, these analyses identified multiple core XRE (GCGTG) sequences (Swanson et al., 1995), a putative Hif1α(NVNGCACGT) consensus sequence, and several GC box-Sp1 (CCGCCC) sites upstream of the translational start site. For comparison, we also analyzed the corresponding region of the human SMOC2 gene. Interestingly, putative XRE, HIF1α, and Sp1 sites were also present in the SMOC2 gene (Fig. 2A). Therefore, Smoc2 and SMOC2 genes contain XREs, potentially accounting for the altered expression of Smoc2 following treatment with Ahr ligands.
Fig. 2. The Smoc2 promoter is responsive to B[a]P and Ahr/ARNT.
(A) Putative Ahr/ARNT, Hif1α, and GC-box Sp1 binding sites identified in the mouse Smoc2 and human SMOC2 gene promoters using PROMO search tool and the TRANSFAC 8.3 database. Numbers indicate the position of core XRE (GCGTG), Hif1α(NVNGCACGT), and GC box-Sp1 (CCGCCC) elements relative to the translational start site.
(B) Smoc2-luciferase, a promoterless luciferase vector (pGL2b), and a strong constitutive RSV-luciferase reporter construct (RSV-Luc) were transiently transfected into 3T3 cells. In some transfections, Ahr and ARNT were co-expressed using CMV-driven expression plasmids. The total amount of DNA in each transfection was kept constant by including the appropriate amount of ‘empty’ pcDNA vector. 24 hr post-transfection, some cultures were treated with 1 μM B[a]P (or DMSO for controls) and incubated for an additional 24 hr prior to harvest for luciferase assays.
(C) CMV-Ahr and CMV-ARNT plasmids were transfected into 3T3 cells individually or in combination, together with Smoc2-luciferase. 48 hr post-transfection cells were harvested for luciferase assays.
To test whether transcriptional mechanisms accounted for downregulation of Smoc2 mRNA after B[a]P treatment we linked 1087 bp of the putative Smoc2 promoter to a firefly luciferase cDNA. The resulting Smoc2-luciferase construct, as well as a promoter-less luciferase vector and a RSV-luciferase construct (as negative and positive controls for promoter activity, respectively) were transfected into 3T3 cells. As shown in Fig. 2B, the 1087 bp 5′ region of the Smoc2 gene conferred a 180-fold increase in luciferase activity relative to ‘empty’ luciferase plasmid, thereby demonstrating that the Smoc2 genomic fragment has promoter activity. We used reporter gene assays to determine the effect of B[a]P on Smoc2 promoter activity. As shown in Fig. 2B, B[a]P-treatment resulted in a 65% decrease in Smoc2-dependent luciferase expression. The effect of B[a]P on the Smoc2 promoter was specific since RSV-driven luciferase activity was unaffected by B[a]P treatment in a parallel experiment. These data demonstrate that transcriptional mechanisms can account for the effect of B[a]P on endogenous Smoc2 mRNA levels.
3.3. Role of Ahr in regulation of Smoc2 expression
The repression of Smoc2 by Ahr ligands, together with the presence of consensus core XREs in the Smoc2 promoter (Fig. 2A) suggested a possible role for Ahr-ARNT in transcriptional regulation of this gene. Therefore, we tested the effect of over-expressed Ahr-ARNT on Smoc2-driven luciferase activity. As shown in Fig. 2B, co-transfection of CMV-Ahr and CMV-ARNT vectors repressed Smoc2-dependent luciferase expression by 75%, even in the absence of B[a]P treatment. In CMV-Ahr/ARNT-transfected cells that additionally received B[a]P, Smoc2-dependent luciferase activity was reduced by 98%. RSV-driven luciferase activity was unaffected by co-transfected CMV-Ahr/ARNT and B[a]P. We also determined the effects of individual and combinatorial over-expression of Ahr and ARNT on Smoc2-Luciferase expression. In a representative experiment shown in Fig. 2D, ARNT and Ahr inhibited Smoc2-dependent luciferase activity by 45 % and 56 % respectively and combined expression of Ahr and ARNT led to a slight further increase in repression (61 % inhibition). Taken together, these data indicate that the Smoc2 gene is a potential target of repression by Ahr-ARNT-containing complexes. However, given the potential for dimerization of Ahr and ARNT with other PAS family members, we do not exclude the possibility that Smoc2 (or any other XRE-containing gene) is regulated by heterodimeric bHLH complexes containing additional PAS family members.
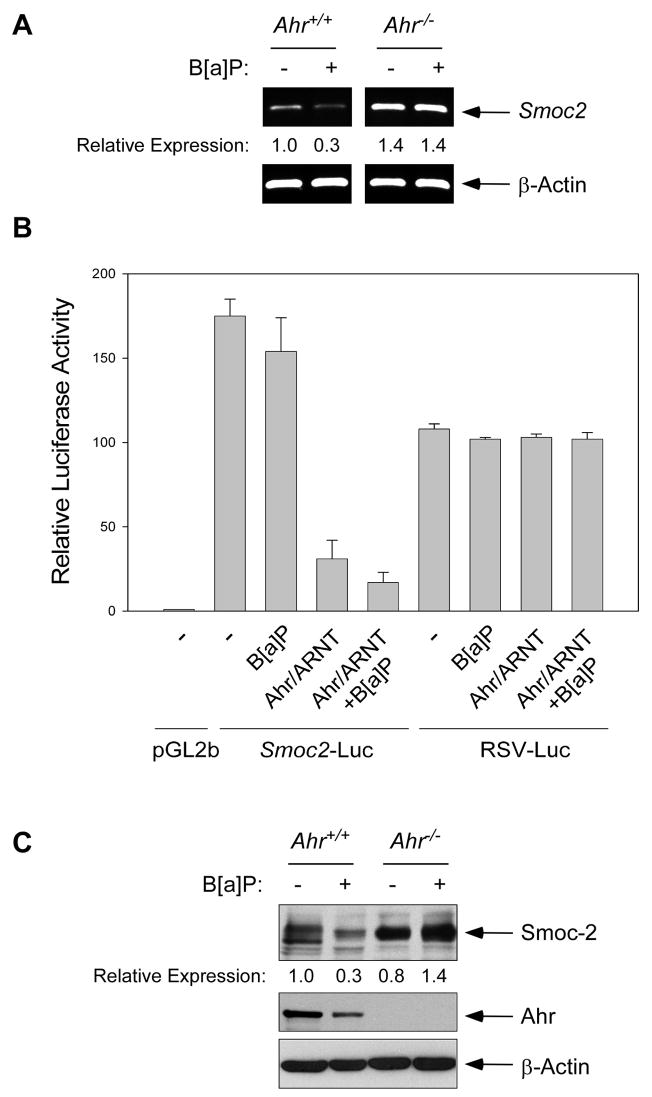
Since the results of Fig. 2 indicated a potential role for Ahr in regulating the Smoc2 promoter we asked whether Ahr is specifically required for B[a]P-induced changes in Smoc2 expression. Therefore, we compared the effect of B[a]P on Smoc2 mRNA levels in Ahr+/+ (3T3) cells and Ahr−/−3T3 fibroblasts from Ahr-null mice. As shown in Fig. 3A, B[a]P-treatment decreased Smoc2 mRNA levels in Ahr+/+, but not Ahr−/− cells, suggesting that Ahr mediates down-regulation of Smoc2 by B[a]P. To further test a role for Ahr in Smoc2 regulation, we determined the effect of B[a]P on Smoc2 promoter-driven luciferase expression in Ahr−/− cells. As shown in Fig. 3B, Smoc2-Luciferease activity was not significantly affected by B[a]P-treatment in Ahr−/− cells. However, when we reconstituted Ahr expression in Ahr−/− cells using CMV-Ahr, Smoc2-Luciferase activity was repressed by 83%. Reconstitution of Ahr in Ahr−/− MEFS also conferred responsiveness to B[a]P (Fig. 3B). Taken together these data show that Smoc2 expression is down-regulated in response to B[a]P via an Ahr-dependent mechanism.
Fig. 3. Repression of Smoc2 expression in B[a]P-treated cultured cells is Ahr-dependent.
(A) Quiescent cultures of Ahr+/+ and Ahr−/− fibroblasts were treated with 1 μM B[a]P. After 24 hr, total RNA was extracted and analyzed by RT-PCR using primers specific for Smoc2 or β-Actin.
(B) Ahr−/− cells were transiently transfected with Smoc2-luciferase, pGl2b, RSV-Luc and CMV-Ahr + CMV-ARNT. 24 hr post-transfection, some cultures were treated with 1 μM B[a]P (or received DMSO for controls) and incubated for an additional 24 hr prior to harvest for luciferase assays.
(C) Quiescent Ahr+/+ and Ahr−/− fibroblasts were treated with 1 μM B[a]P for 48 hr. Protein extracts were analyzed by SDS-PAGE and immunoblotting using antibodies against SMOC-2, Ahr, and β-Actin.
As shown in Fig. 2A, the Smoc2 promoter contains 2 consensus Ahr/ARNT sites. Deletion analyses of the Smoc2 promoter showed that a minimal 424 bp promoter element containing two consensus Ahr/ARNT-binding sites retained responsiveness to B[a]P treatment (data not shown). Deletion of both Ahr/ARNT sequences resulted in abrogation of Smoc2-luciferase activity (data not shown) both basally and in B[a]P-treated cells. Therefore, we have not been able to determine whether the Ahr/ARNT sites are specifically required for repression of the Smoc2 promoter in B[a]P-treated cells. Nevertheless, our data show that the Smoc2 promoter is negatively regulated by B[a]P in an Ahr-dependent manner.
It was of interest to test whether the Ahr-mediated changes in Smoc2-luciferase expression and Smoc2 mRNA were reflected by decreases in Smoc-2 protein levels. Therefore, we treated quiescent cells with B[a]P for varying times and performed immunoblot analysis. As shown in Fig. 3C, B[a]P-treatment decreased Smoc-2 levels in Ahr-expressing 3T3 cells (but not in Ahr−/− MEFs). In these immunoblotting experiments the reduced expression of Smoc-2 was not evident until 2 days post-B[a]P treatment, most likely reflecting the long half-life of Smoc-2 protein.
3.4. AhR-dependent repression of Smoc2 expression in an organ culture model
Potentially, changes in Smoc2 expression could mediate some of the effects of exposure to environmental Ahr ligands. It is important therefore, to determine whether Smoc2 expression is sensitive to PAH/HAH in Ahr-expressing tissues. We have found that embryonic mouse testes express Smoc2 in the interstitium, the region where the steroid-producing Leydig cells develop (Fig. 4A). Additionally, Smoc2 is expressed in the mesonephros, in the region of the Müllerian and Wolffian ducts (Fig. 4A). In females, the Müllerian ducts develop into the oviducts, uteri, and upper vagina, while in males, the Wolffian ducts develop into the epididymides, vas deferentia, and seminal vesicles. Developmental and reproductive defects caused by in utero TCDD exposure are well documented, but the mechanisms of action are poorly understood. However, it was recently shown that pregnant female rats treated with TCDD on E11 produce male offspring with decreased testosterone levels on E19.5 due to decreased testicular steriodogenesis (Adamsson et al., 2008). This exposure tended to decrease the expression of StAR, p450scc, and 3β-HSD, which are expressed in Leydig cells. Since Smoc2 is expressed in the testis, probably in Leydig cells, and because the testis is a target of TCDD action, we investigated the effects of TCDD exposure and Ahr status on Smoc2 expression in this organ.
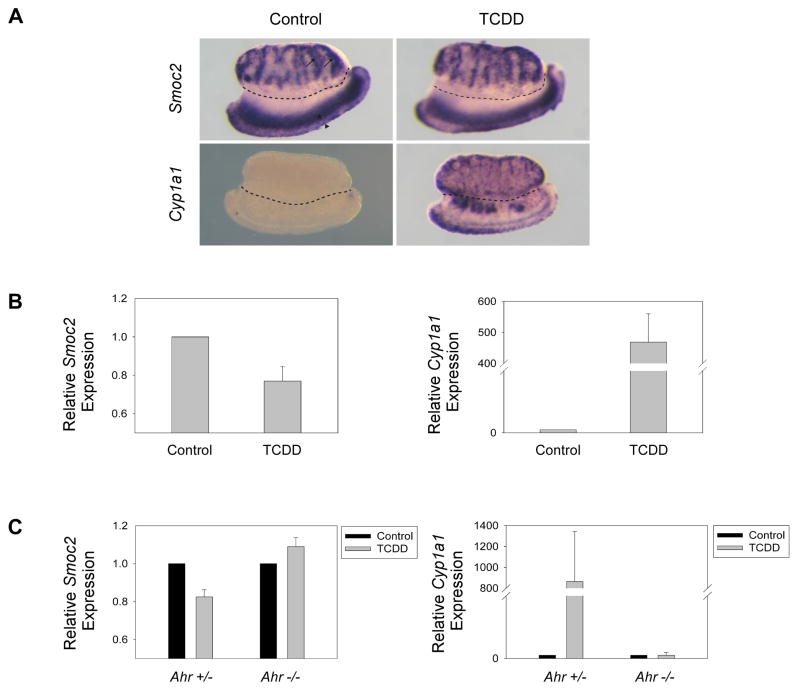
Fig. 4. Smoc2 expression is downregulated in response to TCDD in testis/mesonephros complexes in an Ahr-dependent manner.
(A) WISH analysis of Smoc2 and Cyp1a1 expression in gonad/mesonephros complexes treated with 1μM TCDD for 24 hours. Gonads are above the dotted line, mesonephroi are below. arrow=interstitium, arrowhead=Wolffian duct, asterisk=Mullerian duct.
(B) Real time RT-PCR analysis of Smoc2 (left) and Cyp1a1 (right) expression in wild-type gonad/mesonephros complexes treated with 1μM TCDD for 24 hours.
(C) Real time RT-PCR analysis of Smoc2 (left) and Cyp1a1 (right) expression in Ahr+/− and Ahr−/−testis/mesonephros complexes treated with 1μM TCDD for 24 hours.
Gonad/mesonephros complexes were dissected from E12.5 CD-1 mouse embryos. This time point was chosen because it is coincident with the onset of Smoc2 testicular expression. Isolated gonad/mesonephros complexes were cultured in the presence of 1 μM TCDD (or DMSO for controls) for 24 hours. Analysis of gene expression by quantitative real-time RT-PCR revealed a significant decrease of 24% (n=11, p<0.01) in Smoc2 expression in testis/mesonephros complexes in response to TCDD (Fig. 4B). WISH analysis revealed that the suppression of Smoc2 expression was not localized to a specific area (i.e. the testicular interstitium or mesonephros), although this finding does not preclude the possibility that Smoc2 is downregulated in a specific cell type (Fig. 4B). Testis/mesonephros complexes express Ahr (data not shown) and as a control for Ahr activation in these experiments we examined expression of Cyp1a1 (a known Ahr target gene) in parallel cultures of isolated gonads. As expected, TCDD induced an approximately 450-fold expression of Cyp1a1 as determined by quantitative real-time PCR (Fig. 4B). Interestingly, Cyp1a1 expression was induced in regions of the testis and the mesonephros that overlap with Smoc2 expression (Fig. 4A), consistent with a role for Ahr in repression of Smoc2 expression in the testes.
To test whether AhR mediates the repression of Smoc2 in TCDD-treated gonads, testes harvested from E12.5 Ahr+/− and Ahr−/− mice were cultured in 1μM TCDD for 24 hours and analyzed for Smoc2 expression. Similar to wild-type CD-1 mice, Smoc2 expression was downregulated by 18% (n=5, p<0.005) in Ahr+/− mice in response to TCDD (Fig. 4C). However, Smoc2 expression in Ahr−/− gonad/mesonephros complexes was unchanged (n=7, p=0.48). Similar to previous reports (Shimizu et al., 2000), Cyp1a1 induction by TCDD only occurred in the AhR-expressing gonad/mesonephros complexes (Fig. 4C). We conclude that TCDD represses Smoc2 mRNA levels in an Ahr-dependent manner in cultured embryonic testes.
4. Discussion
In this study we have identified Smoc2 as an mRNA that is downregulated in response to Ahr ligands both in vitro and in vivo. Smoc-2 is a matricellular protein, which promotes cell cycle progression in mesenchymal, endothelial, and possibly other cell types (Rocnik et al., 2006; Liu et al., 2007), most likely by facilitating integrin-ILK-dependent signaling cascades (Liu et al., 2007). Our finding that Smoc2 is repressed in response to Ahr ligands provides a novel link between environmental PAH/HAH exposure and growth control.
Increasingly, it appears likely that inappropriate Ahr activation by environmental agents may perturb growth and development via transcriptional regulation of genes involved in signal transduction and cell cycle regulation (Fisher et al., 2004; Hanlon et al., 2005; Thackaberry et al., 2005). For example, Hanlon et al. identified glypican 1 as a TCDD-responsive gene in a recent microarray screen (Hanlon et al., 2005). Glypican is a membrane proteoglycan that affects growth factor signaling (Fransson, 2003). Poellinger and colleagues showed that the ECM component osteopontin is downregulated in mice expressing constitutively active form of Ahr (Kuznetsov et al., 2005). Our finding that Ahr regulates Smoc2 expression provides an additional link between PAH exposure, Ahr activation and control of cell growth by the ECM. Smoc2 shows a defined pattern of expression in the mouse embryo, suggestive of specific developmental roles (Liu et al., 2007). Ahr-mediated changes in expression of Smoc2 (or other ECM components such as or glypican 1) could perturb growth factor signaling and cell cycle progression, thereby accounting for some of the detrimental consequences of of aryl-hydrocarbon exposure during development (Puga et al., 2005). Indeed we show that Smoc2 is repressed in Ahr ligand-treated cultured embryonic mouse gonads.
Although our results show that Ahr mediates B[a]P-induced changes in Smoc2 expression, further studies are necessary to determine whether the Smoc2 promoter is directly repressed by Ahr. Potentially, ligand-activated Ahr could act directly on XREs or might regulate gene expression via indirect mechanisms. For example, Ahr appears to activate c-Ha-ras via a redox-sensitive mechanism (Enan et al., 1998; Kerzee and Ramos, 2000) and can also activate ERK, p38 MAP kinase (Park et al., 2005) and the src tyrosine kinase (Enan and Matsumura, 1996). Clearly, regulation of protein kinase cascades by Ahr could affect gene expression. Ahr also interacts physically with the Rb tumor suppressor and modulates E2F activity (Puga et al., 2000; Strobeck et al., 2000; Marlowe et al., 2004), thereby providing a link between Ahr and E2F-regulated cell cycle genes.
However, based on the presence of consensus Ahr/ARNT-binding sites in the Smoc2 gene, we consider it likely that Ahr regulates Smoc2 expression directly. Regulation of cellular genes such as Cyp1A1 (encoding Cytochrome P-450 1A1) is one of the best-characterized responses to ligand-activated Ahr. Cyp1A1 activation is mediated via direct binding of the ligand/Ahr/ARNT complex to XREs located in the 5′-flanking region of the gene. However, some XRE-containing genes are repressed by aryl-hydrocarbons, albeit via poorly-understood mechanisms. For example, the rat male-specific constitutive hepatic Cyp2c11 gene is repressed by aryl-hydrocarbons at least in part via mechanisms involving changes in gene transcription (Lee and Riddick, 2000; Bhathena et al., 2002; Riddick et al., 2004).
Surprisingly, although Ahr binds to XREs in the Cyp2c11 5′-flanking region, Cyp2c11 promoter-luciferase reporter constructs containing XREs were not repressed in response to treatment with Ahr ligands (Bhathena et al., 2002; Sawaya and Riddick, 2008). Therefore, Ahr ligands may down-regulate Cyp2c11 by a negative transcriptional mechanism that is not solely due to Ahr binding to an identified XRE-like sequence (Bhathena et al., 2002). Clearly, transient transfections of luciferase-linked promoter fragments may not fully recapitulate the complexity of Cyp2c11 regulation by Ahr in vivo.
In contrast with the Cyp2c11 promoter, we have shown that Smoc2-luciferase constructs are repressed by B[a]P in an Ahr-dependent manner, thereby distinguishing the mechanisms of Smoc2 and CYP2C11 repression after aryl-hydrocarbon treatment. Resink and colleagues showed that the gene encoding T-cadherin (a glycosylphosphatidylinositol-modified cadherin subtype) contains a 5′ regulatory XRE and is repressed in an Ahr-dependent manner (Niermann et al., 2003). Interestingly, our microarray analysis also identified T-cadherin as a B[a]P-suppressed transcript. Based on the study by Resink and colleagues, it is likely that the downregulation of T-cadherin and Smoc2 in B[a]P-treated mesenchymal cells occurs via Ahr-mediated repression.
Similar to Ahr, other ligand-activated nuclear receptors such as the estrogen receptor (ER) are able to induce or repress gene transcription. ER signaling is complex and subject to modulation by a large number of co-repressors or co-activators (Tremblay and Giguere, 2002). For example, AhR dissociates ERα-Sp1 interactions thereby inhibiting transcription of the CAD promoter (Khan et al., 2006). Therefore, co-regulator proteins and promoter context could determine whether ligand-activated Ahr induces or suppresses gene expression. There is mounting evidence that Ahr-mediated gene regulation involves co-activators and co-repressors. For instance, Puga and colleagues have shown that Ahr interacts with RB, a transcriptional repressor (Puga et al., 2000). Potentially, Ahr-mediated suppression of Smoc2 expression could involve transcriptional co-repression.
Our data are consistent with repression of Smoc2 by Ahr-ARNT complexes. However, as with any XRE-containing gene there exists the potential for other PAS proteins to heterodimerize with Ahr and/or ARNT to regulate Smoc2. As shown in Fig. 2A, Smoc2 and SMOC2 genes possess consensus HIF1αsites in addition to XREs. In unpublished experiments we have found that the endogenous Smoc2 mRNA is downregulated in hypoxic cells and that Smoc2 promoter-driven luciferase expression is repressed in response to both hypoxia and ectopically-expressed HIF1α(data not shown). Therefore, the Smoc2 promoter is repressed by Ahr, HIF1α, ARNT, and perhaps bHLH complexes containing other PAS family members. Further experiments are underway to test roles for PAS family members and other transcriptional co-regulators in Ahr-mediated repression of the Smoc2 promoter.
Specific elements that regulate the expression of the human SMOC2 gene have yet to be demonstrated. However, the occurrence of six XREs in 575 bases immediately flanking the 5′ end of the coding sequence (Fig. 2A) provides circumstantial evidence that human SMOC2 is regulated by the Ahr/ARNT heterodimer. These XREs are adjacent to or overlap GC-rich Sp1 sites (Fig. 2A), which are involved in the transcriptional regulation other Ahr/ARNT targets (Kobayashi et al., 1996; Wang et al., 1998). Environmentally-induced changes in SMOC-2 expression might impact human health. For example, a recent analysis of patient samples from the Framingham Heart Study demonstrated genetic associations between pulmonary function measures and human SMOC2 (Wilk et al., 2007). Prior to the study of this patient group, the only genetic defect known to cause obstructive pulmonary disease involved the serine protease inhibitor alpha-1anti-trypsin (Tobin et al., 1983). SMOC-2 contains Kazal serine protease inhibitory motifs (Vannahme et al., 2003) and it is possible that putative SMOC-2-dependent protease inhibitory activity is similarly important for pulmonary function. Regardless of the mechanism by which SMOC-2 contributes to normal pulmonary function, repression of SMOC2 provides a possible means by which environmental agents could perturb the normal physiology of the lung or other organs.
Our studies suggest that the testes are a target organ that may be adversely affected by environmental agents via AhR-dependent changes in Smoc2 expression. Several reports indicate that in utero exposure to dioxin is associated with abnormalities in reproductive tract development and reproductive functions including steroidogenesis (Cooke et al., 1998; Hurst et al., 2002)). We show here that Smoc2 is expressed in fetal testicular Leydig cells (steroid producing cells) and in the primordial reproductive tract. Moreover, we have demonstrated that the embryonic testis/mesonephros complex responds to dioxin exposure by reducing Smoc2 expression. The role of Smoc2 in embryonic gonad development has yet to be determined. However, our previous in vitro data suggests that SMOC2 is involved in mediating the mitogenic and angiogenic effects of growth factors such as VEGF, PDGF, and FGF (Rocnik et al., 2006; Liu et al., 2007). Intriguingly, members of these growth factors families are known to be essential for normal embryonic testis development (Colvin et al., 2001; Brennan et al., 2003; Bott et al., 2006). If Smoc2 does contribute to signaling by these growth factors in the testis, then its reduction in response to TCDD may compromise the development of this organ. Clearly further studies are necessary to identify the developmental role(s) of Smoc2 and the consequences of reduced Smoc2 expression following aryl-hydrocarbon exposure in vivo.
Acknowledgments
This research was supported by NIEHS grant R01 ES09558 (to C. V.) and NICHD grant R01 HD42779 and ACS Research Scholar Grant DDC-109233 (to K. H. A.). We thank David Sherr for providing Ahr−/− mice.
Abbreviations
- SPARC
secreted protein acidic and rich in cysteine
- Smoc2
SPARC-related modular calcium binding protein-2
- ILK
integrin-linked kinase
- Ahr
aryl-hydrocarbon receptor
- B[a]P
benzo[a]pyrene
- TCDD
2,3,7,8-tetrachlorodibenzo-p-dioxin
- PAH
polycyclic aromatic hydrocarbons
- HAH
halogenated aromatic hydrocarbons
- ARNT
Ahr nuclear transporter
- XRE
xenobiotic response elements
- BPDE
benzo[a]pyrene-7,8-diol-9,10-epoxide
- EST
expressed sequence tags
- WISH
wholemount in situ hybridization
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adamsson A, Simanainen U, Viluksela M, Paranko J, Toppari J. The effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin on foetal male rat steroidogenesis. Int J Androl. 2008 doi: 10.1111/j.1365-2605.2008.00900.x. [DOI] [PubMed] [Google Scholar]
- Alexander DL, Ganem LG, Fernandez-Salguero P, Gonzalez F, Jefcoate CR. Aryl-hydrocarbon receptor is an inhibitory regulator of lipid synthesis and of commitment to adipogenesis. J Cell Sci. 1998;111(Pt 22):3311–22. doi: 10.1242/jcs.111.22.3311. [DOI] [PubMed] [Google Scholar]
- Baum EJ. Occurrence and surveillance of polycyclic aromatic hydrocarbons. Academic Press; NY: 1978. [Google Scholar]
- Bhathena A, Lee C, Riddick DS. Suppression of cytochrome P450 2C11 by aromatic hydrocarbons: mechanistic insights from studies of the 5′-flanking region of the CYP2C11 gene. Drug Metab Dispos. 2002;30:1385–92. doi: 10.1124/dmd.30.12.1385. [DOI] [PubMed] [Google Scholar]
- Bornstein P, Sage EH. Matricellular proteins: extracellular modulators of cell function. Curr Opin Cell Biol. 2002;14:608–16. doi: 10.1016/s0955-0674(02)00361-7. [DOI] [PubMed] [Google Scholar]
- Bott RC, McFee RM, Clopton DT, Toombs C, Cupp AS. Vascular endothelial growth factor and kinase domain region receptor are involved in both seminiferous cord formation and vascular development during testis morphogenesis in the rat. Biol Reprod. 2006;75:56–67. doi: 10.1095/biolreprod.105.047225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouma GJ, Hart GT, Washburn LL, Recknagel AK, Eicher EM. Using real time RT-PCR analysis to determine multiple gene expression patterns during XX and XY mouse fetal gonad development. Gene Expr Patterns. 2004;5:141–9. doi: 10.1016/j.modgep.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Brennan J, Tilmann C, Capel B. Pdgfr-alpha mediates testis cord organization and fetal Leydig cell development in the XY gonad. Genes Dev. 2003;17:800–10. doi: 10.1101/gad.1052503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colvin JS, Green RP, Schmahl J, Capel B, Ornitz DM. Male-to-female sex reversal in mice lacking fibroblast growth factor 9. Cell. 2001;104:875–89. doi: 10.1016/s0092-8674(01)00284-7. [DOI] [PubMed] [Google Scholar]
- Conney AH. Induction of microsomal enzymes by foreign chemicals and carcinogenesis by polycyclic aromatic hydrocarbons: G. H. A. Clowes Memorial Lecture. Cancer Res. 1982;42:4875–917. [PubMed] [Google Scholar]
- Cooke GM, Price CA, Oko RJ. Effects of in utero and lactational exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) on serum androgens and steroidogenic enzyme activities in the male rat reproductive tract. J Steroid Biochem Mol Biol. 1998;67:347–54. doi: 10.1016/s0960-0760(98)00127-7. [DOI] [PubMed] [Google Scholar]
- Enan E, El-Sabeawy F, Scott M, Overstreet J, Lasley B. Alterations in the growth factor signal transduction pathways and modulators of the cell cycle in endocervical cells from macaques exposed to TCDD. Toxicol Appl Pharmacol. 1998;151:283–93. doi: 10.1006/taap.1998.8470. [DOI] [PubMed] [Google Scholar]
- Enan E, Matsumura F. Identification of c-Src as the integral component of the cytosolic Ah receptor complex, transducing the signal of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) through the protein phosphorylation pathway. Biochem Pharmacol. 1996;52:1599–612. doi: 10.1016/s0006-2952(96)00566-7. [DOI] [PubMed] [Google Scholar]
- Fisher MT, Nagarkatti M, Nagarkatti PS. Combined screening of thymocytes using apoptosis-specific cDNA array and promoter analysis yields novel gene targets mediating TCDD-induced toxicity. Toxicol Sci. 2004;78:116–24. doi: 10.1093/toxsci/kfh058. [DOI] [PubMed] [Google Scholar]
- Fransson LA. Glypicans. Int J Biochem Cell Biol. 2003;35:125–9. doi: 10.1016/s1357-2725(02)00095-x. [DOI] [PubMed] [Google Scholar]
- Hanlon PR, Zheng W, Ko AY, Jefcoate CR. Identification of novel TCDD-regulated genes by microarray analysis. Toxicol Appl Pharmacol. 2005;202:215–28. doi: 10.1016/j.taap.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Hsing A, Faller DV, Vaziri C. DNA-damaging aryl hydrocarbons induce Mdm2 expression via p53-independent post-transcriptional mechanisms. J Biol Chem. 2000;275:26024–31. doi: 10.1074/jbc.M002455200. [DOI] [PubMed] [Google Scholar]
- Hurst CH, Abbott B, Schmid JE, Birnbaum LS. 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) disrupts early morphogenetic events that form the lower reproductive tract in female rat fetuses. Toxicol Sci. 2002;65:87–98. doi: 10.1093/toxsci/65.1.87. [DOI] [PubMed] [Google Scholar]
- Kerzee JK, Ramos KS. Activation of c-Ha-ras by benzo(a)pyrene in vascular smooth muscle cells involves redox stress and aryl hydrocarbon receptor. Mol Pharmacol. 2000;58:152–8. doi: 10.1124/mol.58.1.152. [DOI] [PubMed] [Google Scholar]
- Khan S, Barhoumi R, Burghardt R, Liu S, Kim K, Safe S. Molecular mechanism of inhibitory aryl hydrocarbon receptor-estrogen receptor/Sp1 cross talk in breast cancer cells. Mol Endocrinol. 2006;20:2199–214. doi: 10.1210/me.2006-0100. [DOI] [PubMed] [Google Scholar]
- Kobayashi A, Sogawa K, Fujii-Kuriyama Y. Cooperative interaction between AhR. Arnt and Sp1 for the drug-inducible expression of CYP1A1 gene. J Biol Chem. 1996;271:12310–6. doi: 10.1074/jbc.271.21.12310. [DOI] [PubMed] [Google Scholar]
- Kuznetsov NV, Andersson P, Gradin K, Stein P, Dieckmann A, Pettersson S, Hanberg A, Poellinger L. The dioxin/aryl hydrocarbon receptor mediates downregulation of osteopontin gene expression in a mouse model of gastric tumourigenesis. Oncogene. 2005;24:3216–22. doi: 10.1038/sj.onc.1208529. [DOI] [PubMed] [Google Scholar]
- Lahvis GP, Pyzalski RW, Glover E, Pitot HC, McElwee MK, Bradfield CA. The aryl hydrocarbon receptor is required for developmental closure of the ductus venosus in the neonatal mouse. Mol Pharmacol. 2005;67:714–20. doi: 10.1124/mol.104.008888. [DOI] [PubMed] [Google Scholar]
- Lee C, Riddick DS. Transcriptional suppression of cytochrome P450 2C11 gene expression by 3-methylcholanthrene. Biochem Pharmacol. 2000;59:1417–23. doi: 10.1016/s0006-2952(00)00249-5. [DOI] [PubMed] [Google Scholar]
- Liu P, Lu J, Cardoso WV, Vaziri C. The SPARC-related Factor SMOC-2 Promotes Growth Factor-induced Cyclin D1 Expression and DNA Synthesis via Integrin-linked Kinase (ILK) Mol Biol Cell. 2007 doi: 10.1091/mbc.E07-05-0510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Maier S, Paulsson M, Hartmann U. The widely expressed extracellular matrix protein SMOC-2 promotes keratinocyte attachment and migration. Exp Cell Res. 2008 doi: 10.1016/j.yexcr.2008.05.020. [DOI] [PubMed] [Google Scholar]
- Mandal PK. Dioxin: a review of its environmental effects and its aryl hydrocarbon receptor biology. J Comp Physiol [B] 2005;175:221–30. doi: 10.1007/s00360-005-0483-3. [DOI] [PubMed] [Google Scholar]
- Marlowe JL, Knudsen ES, Schwemberger S, Puga A. The aryl hydrocarbon receptor displaces p300 from E2F-dependent promoters and represses S phase-specific gene expression. J Biol Chem. 2004;279:29013–22. doi: 10.1074/jbc.M404315200. [DOI] [PubMed] [Google Scholar]
- Mathieu MC, Lapierre I, Brault K, Raymond M. Aromatic hydrocarbon receptor (AhR). AhR nuclear translocator- and p53-mediated induction of the murine multidrug resistance mdr1 gene by 3-methylcholanthrene and benzo(a)pyrene in hepatoma cells. J Biol Chem. 2001;276:4819–27. doi: 10.1074/jbc.M008495200. [DOI] [PubMed] [Google Scholar]
- Messeguer X, Escudero R, Farre D, Nunez O, Martinez J, Alba MM. PROMO: detection of known transcription regulatory elements using species-tailored searches. Bioinformatics. 2002;18:333–4. doi: 10.1093/bioinformatics/18.2.333. [DOI] [PubMed] [Google Scholar]
- Niermann T, Schmutz S, Erne P, Resink T. Aryl hydrocarbon receptor ligands repress T-cadherin expression in vascular smooth muscle cells. Biochem Biophys Res Commun. 2003;300:943–9. doi: 10.1016/s0006-291x(02)02970-4. [DOI] [PubMed] [Google Scholar]
- Park SJ, Yoon WK, Kim HJ, Son HY, Cho SW, Jeong KS, Kim TH, Kim SH, Kim SR, Ryu SY. 2,3,7,8-Tetrachlorodibenzo-p-dioxin activates ERK and p38 mitogen-activated protein kinases in RAW 264.7 cells. Anticancer Res. 2005;25:2831–6. [PubMed] [Google Scholar]
- Puga A, Barnes SJ, Dalton TP, Chang C, Knudsen ES, Maier MA. Aromatic hydrocarbon receptor interaction with the retinoblastoma protein potentiates repression of E2F-dependent transcription and cell cycle arrest. J Biol Chem. 2000;275:2943–50. doi: 10.1074/jbc.275.4.2943. [DOI] [PubMed] [Google Scholar]
- Puga A, Tomlinson CR, Xia Y. Ah receptor signals cross-talk with multiple developmental pathways. Biochem Pharmacol. 2005;69:199–207. doi: 10.1016/j.bcp.2004.06.043. [DOI] [PubMed] [Google Scholar]
- Riddick DS, Lee C, Bhathena A, Timsit YE, Cheng PY, Morgan ET, Prough RA, Ripp SL, Miller KK, Jahan A, Chiang JY. Transcriptional suppression of cytochrome P450 genes by endogenous and exogenous chemicals. Drug Metab Dispos. 2004;32:367–75. doi: 10.1124/dmd.32.4.367. [DOI] [PubMed] [Google Scholar]
- Rocnik EF, Liu P, Sato K, Walsh K, Vaziri C. The Novel SPARC Family Member SMOC-2 Potentiates Angiogenic Growth Factor Activity. J Biol Chem. 2006;281:22855–64. doi: 10.1074/jbc.M513463200. [DOI] [PubMed] [Google Scholar]
- Sawaya RM, Riddick DS. Cytochrome P450 2C11 5′-flanking region and promoter: Regulation by aromatic hydrocarbons in vitro. Toxicology. 2008 doi: 10.1016/j.tox.2008.03.011. [DOI] [PubMed] [Google Scholar]
- Schmidt JV, Su GH, Reddy JK, Simon MC, Bradfield CA. Characterization of a murine Ahr null allele: involvement of the Ah receptor in hepatic growth and development. Proc Natl Acad Sci U S A. 1996;93:6731–6. doi: 10.1073/pnas.93.13.6731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu Y, Nakatsuru Y, Ichinose M, Takahashi Y, Kume H, Mimura J, Fujii-Kuriyama Y, Ishikawa T. Benzo[a]pyrene carcinogenicity is lost in mice lacking the aryl hydrocarbon receptor. Proc Natl Acad Sci U S A. 2000;97:779–82. doi: 10.1073/pnas.97.2.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strobeck MW, Fribourg AF, Puga A, Knudsen ES. Restoration of retinoblastoma mediated signaling to Cdk2 results in cell cycle arrest. Oncogene. 2000;19:1857–67. doi: 10.1038/sj.onc.1203510. [DOI] [PubMed] [Google Scholar]
- Swanson HI, Chan WK, Bradfield CA. DNA binding specificities and pairing rules of the Ah receptor, ARNT, and SIM proteins. J Biol Chem. 1995;270:26292–302. doi: 10.1074/jbc.270.44.26292. [DOI] [PubMed] [Google Scholar]
- Thackaberry EA, Jiang Z, Johnson CD, Ramos KS, Walker MK. Toxicogenomic profile of 2,3,7,8-tetrachlorodibenzo-p-dioxin in the murine fetal heart: modulation of cell cycle and extracellular matrix genes. Toxicol Sci. 2005;88:231–41. doi: 10.1093/toxsci/kfi301. [DOI] [PubMed] [Google Scholar]
- Tobin MJ, Cook PJ, Hutchison DC. Alpha 1 antitrypsin deficiency: the clinical and physiological features of pulmonary emphysema in subjects homozygous for Pi type Z. A survey by the British Thoracic Association. Br J Dis Chest. 1983;77:14–27. doi: 10.1016/0007-0971(83)90002-5. [DOI] [PubMed] [Google Scholar]
- Tremblay GB, Giguere V. Coregulators of estrogen receptor action. Crit Rev Eukaryot Gene Expr. 2002;12:1–22. doi: 10.1615/critreveukaryotgeneexpr.v12.i1.10. [DOI] [PubMed] [Google Scholar]
- Vannahme C, Gosling S, Paulsson M, Maurer P, Hartmann U. Characterization of SMOC-2, a modular extracellular calcium-binding protein. Biochem J. 2003;373:805–14. doi: 10.1042/BJ20030532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaziri C, Faller DV. Repression of platelet-derived growth factor beta-receptor expression by mitogenic growth factors and transforming oncogenes in murine 3T3 fibroblasts. Mol Cell Biol. 1995;15:1244–53. doi: 10.1128/mcb.15.3.1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaziri C, Faller DV. A benzo[a]pyrene-induced cell cycle checkpoint resulting in p53-independent G1 arrest in 3T3 fibroblasts. J Biol Chem. 1997;272:2762–9. doi: 10.1074/jbc.272.5.2762. [DOI] [PubMed] [Google Scholar]
- Wang F, Hoivik D, Pollenz R, Safe S. Functional and physical interactions between the estrogen receptor Sp1 and nuclear aryl hydrocarbon receptor complexes. Nucleic Acids Res. 1998;26:3044–52. doi: 10.1093/nar/26.12.3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitlock JP., Jr Induction of cytochrome P4501A1. Annu Rev Pharmacol Toxicol. 1999;39:103–25. doi: 10.1146/annurev.pharmtox.39.1.103. [DOI] [PubMed] [Google Scholar]
- Wilk JB, DeStefano AL, Joost O, Myers RH, Cupples LA, Slater K, Atwood LD, Heard-Costa NL, Herbert A, O’Connor GT, Gottlieb DJ. Linkage and association with pulmonary function measures on chromosome 6q27 in the Framingham Heart Study. Hum Mol Genet. 2003;12:2745–51. doi: 10.1093/hmg/ddg311. [DOI] [PubMed] [Google Scholar]
- Wilk JB, Herbert A, Shoemaker CM, Gottlieb DJ, Karamohamed S. Secreted modular calcium-binding protein 2 haplotypes are associated with pulmonary function. Am J Respir Crit Care Med. 2007;175:554–60. doi: 10.1164/rccm.200601-110OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson D. In: In Situ Hybridization: A Practical Approach. 2. Wilkinson DG, editor. New York: Oxford University Press; 1998. p. 224. [Google Scholar]