Abstract
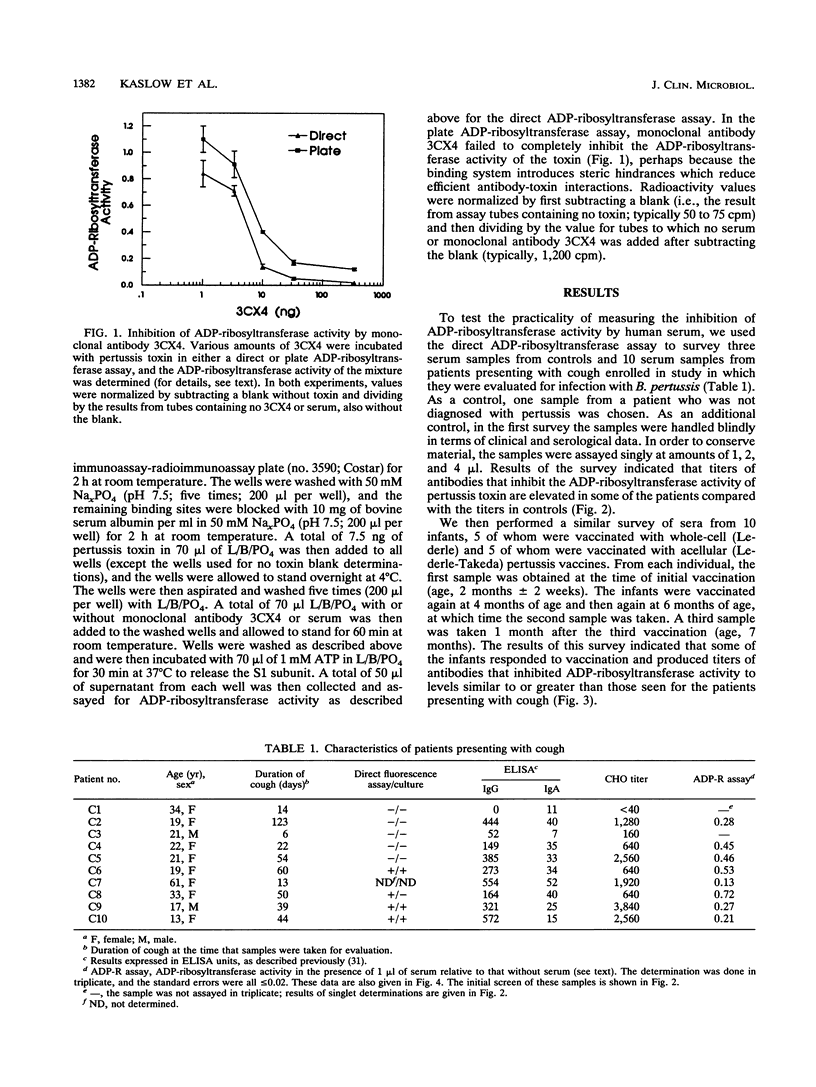
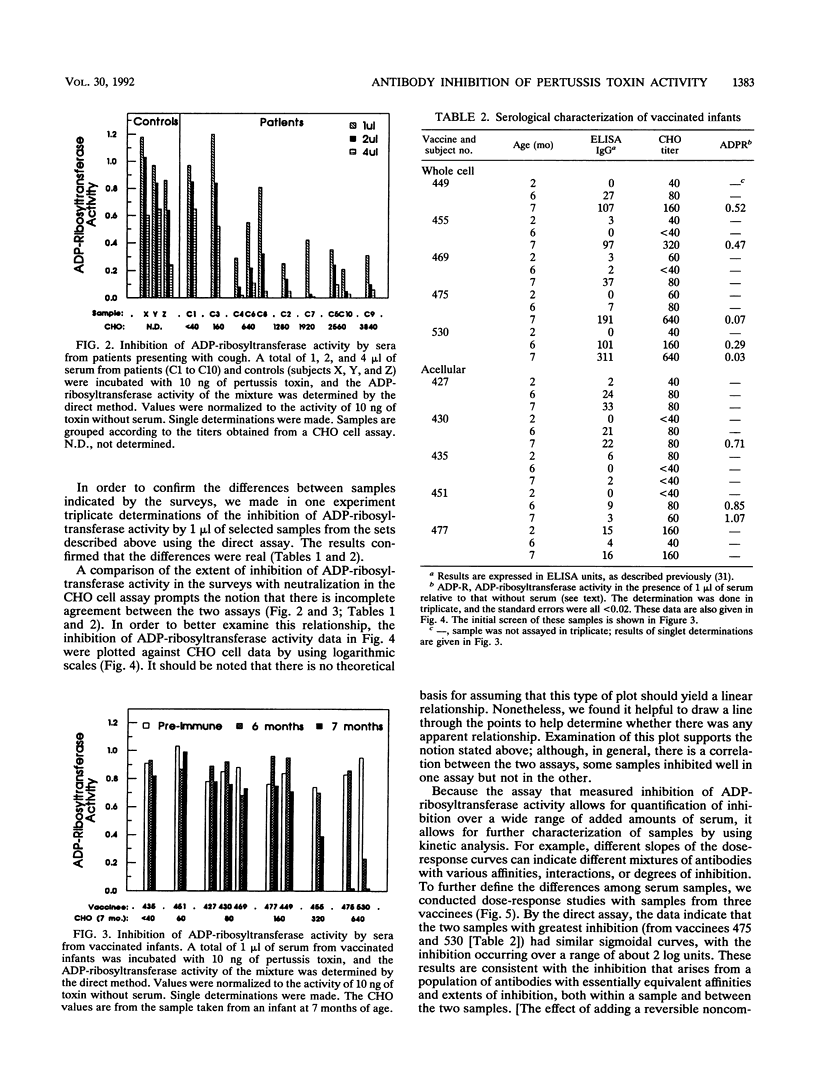
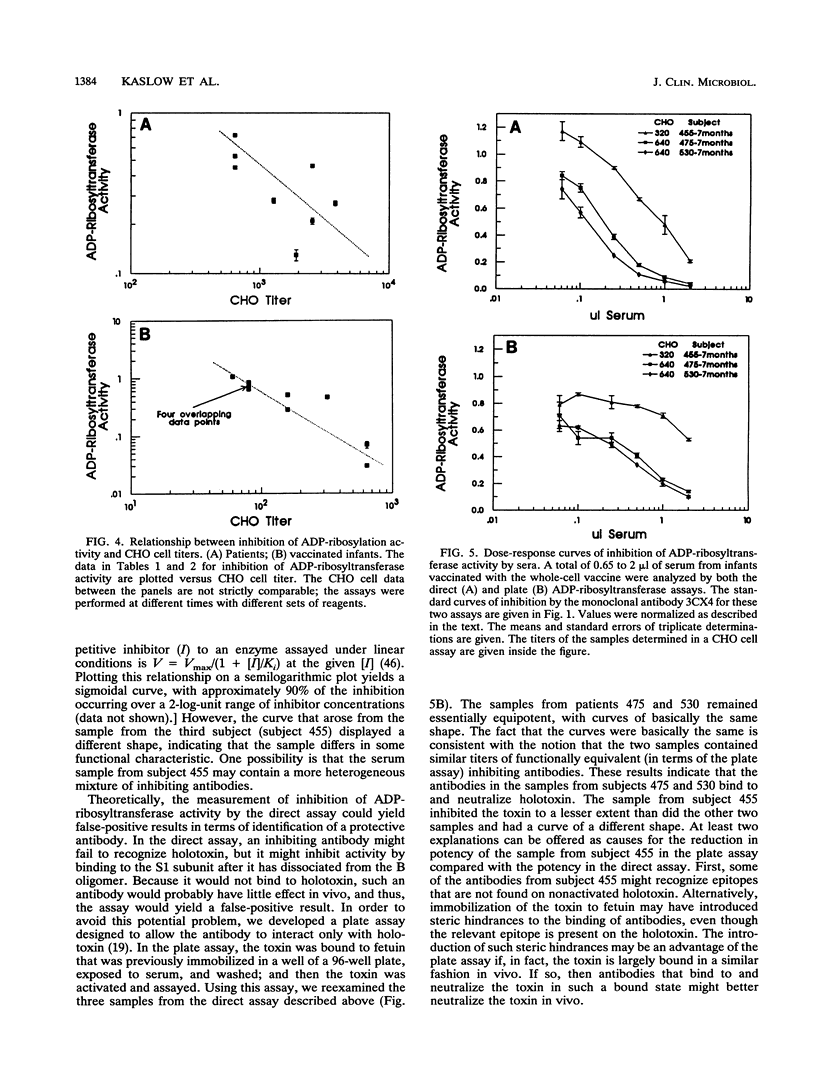
Bordetella pertussis produces a protein virulence factor termed pertussis toxin. Many candidate pertussis vaccines are based on the rationale that an immune response that neutralizes the virulence activities of this toxin, which are thought to arise from its catalytic ADP-ribosyltransferase activity, would be beneficial. The report describes two methods that quantify the inhibition of this activity by human serum. One, termed a direct assay, involves an initial incubation of toxin with serum, a second incubation that activates the toxin, and a third incubation that measures the ADP-ribosyltransferase activity of the mixture. The other assay, termed a plate assay, involves immobilization of the toxin, exposure of the immobilized toxin to serum and washing of the plate, and then activation and assay of the toxin's ADP-ribosyltransferase activity. The plate assay may be more selective than the direct assay in terms of identifying antibodies that neutralize the toxin in vivo. Sera from controls, selected patients presenting with cough, and vaccinated infants were first analyzed by the direct assay. In contrast to sera from controls, sera from several of the patients and vaccinated infants strongly inhibited activity. Dose-response curves of inhibition were determined for samples from three vaccinated infants by both the direct and plate assays. One of the samples had a dose-response curve of a different shape and thus differed not only in titer but also in functional characteristics. A comparison of inhibition of ADP-ribosyltransferase activity and neutralization in a CHO cell assay indicated that there was incomplete agreement between the two assays. Taken together, these results indicate that measurement of inhibition of ADP-ribosyltransferase activity by human serum is practical and may be useful in the evaluation of responses to pertussis vaccines.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arciniega J. L., Burns D. L., Garcia-Ortigoza E., Manclark C. R. Immune response to the B oligomer of pertussis toxin. Infect Immun. 1987 May;55(5):1132–1136. doi: 10.1128/iai.55.5.1132-1136.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bensadoun A., Weinstein D. Assay of proteins in the presence of interfering materials. Anal Biochem. 1976 Jan;70(1):241–250. doi: 10.1016/s0003-2697(76)80064-4. [DOI] [PubMed] [Google Scholar]
- Blumberg D. A., Mink C. M., Cherry J. D., Johnson C., Garber R., Plotkin S. A., Watson B., Ballanco G. A., Daum R. S., Sullivan B. Comparison of acellular and whole-cell pertussis-component diphtheria-tetanus-pertussis vaccines in infants. The APDT Vaccine Study Group. J Pediatr. 1991 Aug;119(2):194–204. doi: 10.1016/s0022-3476(05)80727-9. [DOI] [PubMed] [Google Scholar]
- Burns D. L., Kenimer J. G., Manclark C. R. Role of the A subunit of pertussis toxin in alteration of Chinese hamster ovary cell morphology. Infect Immun. 1987 Jan;55(1):24–28. doi: 10.1128/iai.55.1.24-28.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns D. L., Manclark C. R. Adenine nucleotides promote dissociation of pertussis toxin subunits. J Biol Chem. 1986 Mar 25;261(9):4324–4327. [PubMed] [Google Scholar]
- Christodoulides M., Parton R., Stewart-Tull D. E. Optimal conditions for the toxoiding of pertussis toxin with 1-ethyl-3(3-dimethylaminopropyl) carbodiimide.HCl. FEMS Microbiol Immunol. 1989 Dec;1(8-9):425–435. doi: 10.1111/j.1574-6968.1989.tb02434.x. [DOI] [PubMed] [Google Scholar]
- Cody C. L., Baraff L. J., Cherry J. D., Marcy S. M., Manclark C. R. Nature and rates of adverse reactions associated with DTP and DT immunizations in infants and children. Pediatrics. 1981 Nov;68(5):650–660. [PubMed] [Google Scholar]
- Gillenius P., Jätmaa E., Askelöf P., Granström M., Tiru M. The standardization of an assay for pertussis toxin and antitoxin in microplate culture of Chinese hamster ovary cells. J Biol Stand. 1985 Jan;13(1):61–66. doi: 10.1016/s0092-1157(85)80034-2. [DOI] [PubMed] [Google Scholar]
- Halperin S. A., Issekutz T. B., Kasina A. Modulation of Bordetella pertussis infection with monoclonal antibodies to pertussis toxin. J Infect Dis. 1991 Feb;163(2):355–361. doi: 10.1093/infdis/163.2.355. [DOI] [PubMed] [Google Scholar]
- Hausman S. Z., Burns D. L., Sickler V. C., Manclark C. R. Immune response to dimeric subunits of the pertussis toxin B oligomer. Infect Immun. 1989 Jun;57(6):1760–1764. doi: 10.1128/iai.57.6.1760-1764.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewlett E. L., Sauer K. T., Myers G. A., Cowell J. L., Guerrant R. L. Induction of a novel morphological response in Chinese hamster ovary cells by pertussis toxin. Infect Immun. 1983 Jun;40(3):1198–1203. doi: 10.1128/iai.40.3.1198-1203.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaslow H. R., Lesikar D. D. Sulfhydryl-alkylating reagents inactivate the NAD glycohydrolase activity of pertussis toxin. Biochemistry. 1987 Jul 14;26(14):4397–4402. doi: 10.1021/bi00388a031. [DOI] [PubMed] [Google Scholar]
- Kaslow H. R., Lim L. K., Moss J., Lesikar D. D. Structure-activity analysis of the activation of pertussis toxin. Biochemistry. 1987 Jan 13;26(1):123–127. doi: 10.1021/bi00375a018. [DOI] [PubMed] [Google Scholar]
- Kaslow H. R., Schlotterbeck J. D., Gotto J. Evaluation of antibodies elicited by immunization with pertussis toxin. Dev Biol Stand. 1991;73:143–150. [PubMed] [Google Scholar]
- Kaslow H. R., Schlotterbeck J. D., Kenimer J. G. Monoclonal antibodies that inhibit ADP-ribosyltransferase but not NAD-glycohydrolase activity of pertussis toxin. Infect Immun. 1990 Mar;58(3):746–752. doi: 10.1128/iai.58.3.746-752.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaslow H. R., Schlotterbeck J. D., Mar V. L., Burnette W. N. Alkylation of cysteine 41, but not cysteine 200, decreases the ADP-ribosyltransferase activity of the S1 subunit of pertussis toxin. J Biol Chem. 1989 Apr 15;264(11):6386–6390. [PubMed] [Google Scholar]
- Katada T., Tamura M., Ui M. The A protomer of islet-activating protein, pertussis toxin, as an active peptide catalyzing ADP-ribosylation of a membrane protein. Arch Biochem Biophys. 1983 Jul 1;224(1):290–298. doi: 10.1016/0003-9861(83)90212-6. [DOI] [PubMed] [Google Scholar]
- Katada T., Ui M. ADP ribosylation of the specific membrane protein of C6 cells by islet-activating protein associated with modification of adenylate cyclase activity. J Biol Chem. 1982 Jun 25;257(12):7210–7216. [PubMed] [Google Scholar]
- Katada T., Ui M. Direct modification of the membrane adenylate cyclase system by islet-activating protein due to ADP-ribosylation of a membrane protein. Proc Natl Acad Sci U S A. 1982 May;79(10):3129–3133. doi: 10.1073/pnas.79.10.3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katada T., Ui M. Islet-activating protein. A modifier of receptor-mediated regulation of rat islet adenylate cyclase. J Biol Chem. 1981 Aug 25;256(16):8310–8317. [PubMed] [Google Scholar]
- Kenimer J. G., Kim K. J., Probst P. G., Manclark C. R., Burstyn D. G., Cowell J. L. Monoclonal antibodies to pertussis toxin: utilization as probes of toxin function. Hybridoma. 1989 Feb;8(1):37–51. doi: 10.1089/hyb.1989.8.37. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lim L. K., Sekura R. D., Kaslow H. R. Adenine nucleotides directly stimulate pertussis toxin. J Biol Chem. 1985 Mar 10;260(5):2585–2588. [PubMed] [Google Scholar]
- Locht C., Keith J. M. Pertussis toxin gene: nucleotide sequence and genetic organization. Science. 1986 Jun 6;232(4755):1258–1264. doi: 10.1126/science.3704651. [DOI] [PubMed] [Google Scholar]
- Magnusson R., Larsson U., Bartfai T., Askelöf P. A sensitive method for measuring neutralizing antibodies to Bordetella pertussis toxin: optimized ADP-ribosylation of transducin. Biologicals. 1991 Jan;19(1):9–15. doi: 10.1016/1045-1056(91)90018-f. [DOI] [PubMed] [Google Scholar]
- Miller D. L., Ross E. M., Alderslade R., Bellman M. H., Rawson N. S. Pertussis immunisation and serious acute neurological illness in children. Br Med J (Clin Res Ed) 1981 May 16;282(6276):1595–1599. doi: 10.1136/bmj.282.6276.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mink C. M., Cherry J. D., Christenson P., Lewis K., Pineda E., Shlian D., Dawson J. A., Blumberg D. A. A search for Bordetella pertussis infection in university students. Clin Infect Dis. 1992 Feb;14(2):464–471. doi: 10.1093/clinids/14.2.464. [DOI] [PubMed] [Google Scholar]
- Moss J., Stanley S. J., Burns D. L., Hsia J. A., Yost D. A., Myers G. A., Hewlett E. L. Activation by thiol of the latent NAD glycohydrolase and ADP-ribosyltransferase activities of Bordetella pertussis toxin (islet-activating protein). J Biol Chem. 1983 Oct 10;258(19):11879–11882. [PubMed] [Google Scholar]
- Moss J., Stanley S. J., Watkins P. A., Burns D. L., Manclark C. R., Kaslow H. R., Hewlett E. L. Stimulation of the thiol-dependent ADP-ribosyltransferase and NAD glycohydrolase activities of Bordetella pertussis toxin by adenine nucleotides, phospholipids, and detergents. Biochemistry. 1986 May 6;25(9):2720–2725. doi: 10.1021/bi00357a066. [DOI] [PubMed] [Google Scholar]
- Nicosia A., Perugini M., Franzini C., Casagli M. C., Borri M. G., Antoni G., Almoni M., Neri P., Ratti G., Rappuoli R. Cloning and sequencing of the pertussis toxin genes: operon structure and gene duplication. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4631–4635. doi: 10.1073/pnas.83.13.4631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda M., Cowell J. L., Burstyn D. G., Manclark C. R. Protective activities of the filamentous hemagglutinin and the lymphocytosis-promoting factor of Bordetella pertussis in mice. J Infect Dis. 1984 Dec;150(6):823–833. doi: 10.1093/infdis/150.6.823. [DOI] [PubMed] [Google Scholar]
- Sato H., Ito A., Chiba J., Sato Y. Monoclonal antibody against pertussis toxin: effect on toxin activity and pertussis infections. Infect Immun. 1984 Nov;46(2):422–428. doi: 10.1128/iai.46.2.422-428.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato H., Sato Y., Ito A., Ohishi I. Effect of monoclonal antibody to pertussis toxin on toxin activity. Infect Immun. 1987 Apr;55(4):909–915. doi: 10.1128/iai.55.4.909-915.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato H., Sato Y., Ohishi I. Comparison of pertussis toxin (PT)-neutralizing activities and mouse-protective activities of anti-PT mouse monoclonal antibodies. Infect Immun. 1991 Oct;59(10):3832–3835. doi: 10.1128/iai.59.10.3832-3835.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato H., Sato Y. Protective activities in mice of monoclonal antibodies against pertussis toxin. Infect Immun. 1990 Oct;58(10):3369–3374. doi: 10.1128/iai.58.10.3369-3374.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato Y., Arai H., Suzuki K. Leukocytosis-promoting factor of Bordetella pertussis. 3. Its identity with protective antigen. Infect Immun. 1974 May;9(5):801–810. doi: 10.1128/iai.9.5.801-810.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekura R. D., Fish F., Manclark C. R., Meade B., Zhang Y. L. Pertussis toxin. Affinity purification of a new ADP-ribosyltransferase. J Biol Chem. 1983 Dec 10;258(23):14647–14651. [PubMed] [Google Scholar]
- Sekura R. D., Zhang Y. L., Roberson R., Acton B., Trollfors B., Tolson N., Shiloach J., Bryla D., Muir-Nash J., Koeller D. Clinical, metabolic, and antibody responses of adult volunteers to an investigational vaccine composed of pertussis toxin inactivated by hydrogen peroxide. J Pediatr. 1988 Nov;113(5):806–813. doi: 10.1016/s0022-3476(88)80005-2. [DOI] [PubMed] [Google Scholar]
- Shahin R. D., Brennan M. J., Li Z. M., Meade B. D., Manclark C. R. Characterization of the protective capacity and immunogenicity of the 69-kD outer membrane protein of Bordetella pertussis. J Exp Med. 1990 Jan 1;171(1):63–73. doi: 10.1084/jem.171.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahin R. D., Witvliet M. H., Manclark C. R. Mechanism of pertussis toxin B oligomer-mediated protection against Bordetella pertussis respiratory infection. Infect Immun. 1990 Dec;58(12):4063–4068. doi: 10.1128/iai.58.12.4063-4068.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura M., Nogimori K., Murai S., Yajima M., Ito K., Katada T., Ui M., Ishii S. Subunit structure of islet-activating protein, pertussis toxin, in conformity with the A-B model. Biochemistry. 1982 Oct 26;21(22):5516–5522. doi: 10.1021/bi00265a021. [DOI] [PubMed] [Google Scholar]
- Weiss A. A., Hewlett E. L. Virulence factors of Bordetella pertussis. Annu Rev Microbiol. 1986;40:661–686. doi: 10.1146/annurev.mi.40.100186.003305. [DOI] [PubMed] [Google Scholar]
- Wessling-Resnick M., Johnson G. L. Kinetic and hydrodynamic properties of transducin: comparison of physical and structural parameters for GTP-binding regulatory proteins. Biochemistry. 1987 Jul 14;26(14):4316–4323. doi: 10.1021/bi00388a020. [DOI] [PubMed] [Google Scholar]


