Abstract
Neuropeptide S (NPSa), the endogenous ligand of a previously orphan receptor now named NPSR, regulates various biological functions in the brain, including arousal, locomotion, anxiety, and food intake. Here we report on a focused structure-activity study of Gly5 which has been replaced with L and D amino acids. Fifteen NPS related peptides were synthesized and pharmacologically tested for intracellular calcium mobilization using HEK293 cells stably expressing the mouse NPSR. The results of this study demonstrated that peptide potency is inversely related to the side chain size while peptide efficacy strongly depends on the relative L and D configuration with the L aminoacids favoring agonist while D aminoacids displaying antagonist pharmacological activity. [D-Val5]NPS behaved as NPSR pure antagonist in HEK293mNPSR cells showing the highest potency (pKB 7.56) among this series of peptides. The antagonist action of [D-Val5]NPS was confirmed in vivo in mice where the peptide at a dose of 10 nmoles completely blocked the stimulatory effect of 0.1 nmole NPS on locomotor activity.
Introduction
Neuropeptide S (NPS) has been recently identified as the endogenous peptide ligand of the orphan GPCR GPR154 now referred to as NPSR1 The human NPS peptide has the following primary sequence: SFRNGVGTGMKKTSFQRAKS.1 The NPS peptide precursor gene is present in all vertebrates, with the exception of fish, and displays a high level of sequence conservation, especially at the amino terminus2. The NPSR primary structure shows low homology to other members of the GPCR family. In situ hybridization studies revealed that NPSR mRNA is widely expressed throughout the nervous system while the NPS precursor mRNA is strongly expressed only in some brainstem nuclei including the pericoerulear area.1,3 Using cells expressing the recombinant NPSR it has been demonstrated that NPSR is coupled with both Gq and Gs proteins4, since at nanomolar concentrations NPS is able to stimulate intracellular calcium levels and cAMP accumulation. In vivo, supraspinal administration of NPS in rodents produces a wide range of biological effects including stimulation of wakefulness1, 5, hyperlocomotion1, 5-7, inhibition of food intake7, 8, and anxiolytic-like effects.1, 5, 9 In addition, recent elegant studies demonstrated that the amygdala may likely represents the crucial brain area for NPS anxiolytic-like effects and inhibitory action on aversive memories.10, 11
Since the formal identification of the NPS / NPSR system we started a structure-activity relationship project aimed at the identification of novel NPSR ligands. These tools, especially selective and pure antagonists are required for understanding the physiological and pathological roles of this peptidergic system and for foreseeing the therapeutic value of molecules interacting selectively with NPSR. Ala- and D-scans together with N- and C-terminal truncation studies demonstrated that the N terminal portion of NPS, in particular the sequence Phe2-Arg3-Asn4, is crucial for bioactivity.6, 12 These residues were then subjected to systematic replacement with coded and non-coded amino acids. A study focused on position 2 demonstrated that lipophilicity but not aromaticity is crucial, that both the size of the side chain and its distance from the peptide backbone are important for biological activity, and that this position plays a role in both receptor binding and activation.13 Investigation of position 3 revealed that the guanidine moiety and its basic character are not crucial requirements and that an aliphatic amino acid with a linear three carbon atom long side chain is sufficient to bind and fully activate NPSR14, while the study on position 4 suggested a pivotal role of the Asn4 side chain for NPS bioactivity since all other amino acid replacements investigated produced either an important decrease of biological activity or generated inactive derivatives.14 In parallel, we also performed a conformation-activity relationship study15 that demonstrated that helicity can be tolerated in the C-terminal part of NPS but not around Gly7, a results which is only in part in line with the model of a nascent helix spanning residues 5 through 13 proposed by Bernier et al.12 In the context of the same study we identified [Aib5]NPS and [D-Ala5]NPS as partial agonists at NPSR.15 These results indicate that conformational changes induced by substituting Gly5 with the achiral alpha helix promoting amino acid Aib or with D-Ala are capable of reducing agonist efficacy.
On this basis we planned the present SAR study focusing on Gly5 and replaced it with a series of L and D amino acids characterized by hydrophobic aromatic and aliphatic side chains including some Cys derivatives protected on the sulfhydryl group.
15 novel human NPS analogues were synthesized and pharmacologically evaluated in a calcium mobilization assay using HEK293 cells stably expressing mouse NPSR (HEK293mNPSR) and the fluorometric imaging plate reader FlexStation.
Results and Discussion
In the calcium mobilization assay NPS increased the intracellular calcium concentrations in a concentration-dependent manner with pEC50 and Emax values of 8.65 and 295% over basal, respectively (Table 1). This result is in line with those reported in the literature.4, 13, 15 The substitution of Gly5 with natural amino acids containing hydrophobic aromatic side chains (compound 1 and 2) produced a drastic decrease (> 300-fold) in peptide potency and, in the case of [Phe5]NPS, also an important reduction of efficacy. On the other hand, the replacement of Gly5 with natural amino acids with hydrophobic aliphatic side chains (compounds 3-6) generated NPSR full agonists with moderate to high potency. In particular [Cys5]NPS was found to be only 6 fold less potent than the natural peptide, while increasing the size of the amino acid side chain produced a progressive decrease in potency with [Leu5]NPS being 100-fold less potent than NPS. These results suggest that position 5 can tolerate substitutions with amino acids characterized by small side chains while larger side chains reduce agonist potency. Previous results obtained with [Ala5]NPS which behaves as a high potency NPSR full agonist6, 15 corroborate this suggestion. It should be emphasized that these analogs differ from the natural peptide not only by their position 5 side chain but also by the insertion of an L chiral center which substitutes the achiral Cα of Gly5. This chiral insertion, however, does not seem to significantly affect the pharmacological activity of the peptide analogs. Interestingly, this modification is compatible with the nascent helix spanning residues 5 through 13 recently proposed as the bioactive conformation of NPS.12
Table 1.
Effects of NPS and NPS analogues modified in position 5 in HEK293 cells expressing the mouse NPSR.
| N | Compound | Agonist | Antagonist | |
|---|---|---|---|---|
| pEC50 (CL95%) | Emax ± sem | pKB (CL95%) | ||
| NPS | 8.65 (8.55-8.75) | 295 ± 22% | ND | |
| 1 | [Phe5]NPS | 6.11 (5.35-6.90) | 69 ± 8%* | > 6 |
| 2 | [Trp5]NPS | Crc incomplete 10 μM: 30 ± 9 % | > 6 | |
| 3 | [Leu5]NPS | 6.64 (6.47-6.81) | 214 ± 26% | ND |
| 4 | [Val5]NPS | 7.18 (6.57-7.79) | 231 ± 68% | ND |
| 5 | [Met5]NPS | 7.06 (6.69-7.43) | 240 ± 19% | ND |
| 6 | For[Cys5]NPS | 7.86 (7.25-8.47) | 280 ± 37% | ND |
| 7 | [D-Phe5]NPS | Inactive up to 10 μM | 6.27 (5.88-6.66) | |
| 8 | [D-Trp5]NPS | Inactive up to 10 μM | 6.79 (6.21-7.37) | |
| 9 | [D-Leu5]NPS | 7.05 (6.53-7.57) | 118 ± 33%* | 7.44 (6.96 – 7.94) |
| 10 | [D-Val5]NPS | Inactive up to 10 μM | 7.56 (7.12-8.00) | |
| 11 | [D-Met5]NPS | Inactive up to 10 μM | 7.09 (6.31-7.87) | |
| 12 | [D-Cys5]NPS | 7.15 (6.39-7.91) | 59 ± 13%* | 7.84 (7.52-8.16) |
| 13 | [D-Cys(Acm)5]NPS | Inactive up to 10 μM | 6.47 (5.27-7.67) | |
| 14 | [D-Cys(Bzl)5]NPS | Inactive up to 10 μM | 7.22 (7.01-7.43) | |
| 15 | [D-Cys(tBu)5]NPS | Inactive up to 10 μM | 6.62 (6.40-6.84) | |
pEC50 : the negative logarithm to base 10 of the molar concentration of an agonist that produces 50% of the maximal possible effect.
CL95%: 95% confidence limits.
Emax: the maximal effect elicited by the agonist expressed as % over the baseline.
sem: standard error of the mean.
crc: concentration response curve.
p > 0.05 vs NPS Emax according to one-way ANOVA followed by the Dunnett test. Data are means of at least 4 separate experiments.
To further investigate the possible role of chirality in this position, the D enantiomers of the same amino acids were used to generate compounds 7-12. The substitution of Gly5 with D amino acids with hydrophobic aromatic side chains (compound 7 and 8) produced a complete elimination of efficacy and, as in the case of their L enantiomers, an important reduction (approx. 100-fold) of potency. The replacement of Gly5 with D amino acids with hydrophobic aliphatic side chains (compound 9-12) generated NPSR partial agonists ([D-Leu5]NPS and [D-Cys5]NPS) or pure antagonists ([D-Val5]NPS and [D-Met5]NPS) with moderate to high potency. These results clearly indicate that the insertion of a Cα chiral carbon with relative D-configuration in NPS position 5 produces, depending on the chemical features of the side chain, an important reduction of efficacy or its total elimination. Interestingly and corroborating this evidence, [D-Ala5]NPS behaves as an NPSR partial agonist while, as mentioned before, [Ala5]NPS is a full agonist.15 As far as peptide potency is concerned, the rank order of agonist potency obtained with L amino acid substitutions (Cys > Val ≥ Met > Leu > Phe > Trp) is very similar to the rank order of antagonist potency obtained with the D enantiomers (Cys ≥ Val ≥ Leu > Met > Trp > Phe). On this basis it can be proposed for position 5 that the amino acid side chain size is very important for NPSR binding and inversely related to peptide potency, while the amino acid chirality has a crucial impact on the ability of the peptide to activate the receptor with L residues acting as partial/full agonists and D residues acting as low efficacy partial agonists or pure antagonists.
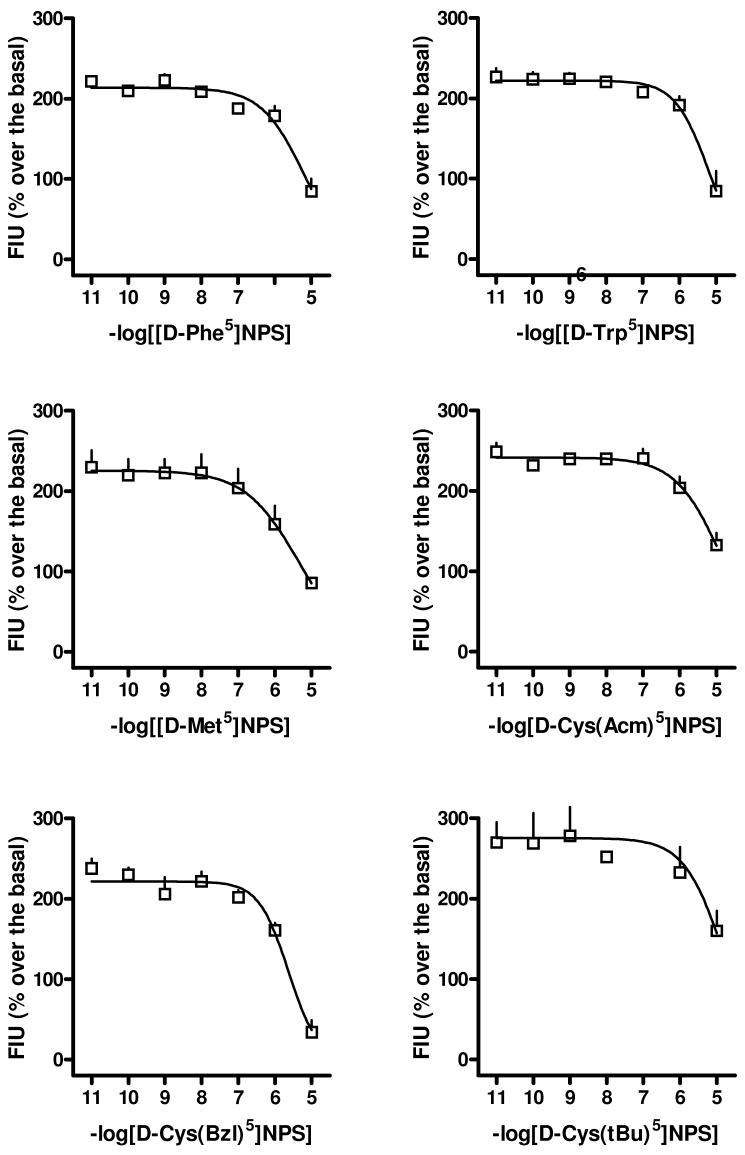
In order to increase peptide potency and/or reducing its efficacy some D-Cys derivatives were used to substitute NPS Gly5 (compounds 13-15). These peptides behaved as pure antagonists, however their potency was always lower than that of the partial agonist [D-Cys5]NPS. Inhibition response curves to D-Xaa5 substituted NPS analogs vs NPS 100 nM are displayed in Figure 1.
Figure 1.
Inhibition response curves to [D-Phe5]NPS, [D-Trp5]NPS, [D-Met5]NPS, [DCys(Acm)5]NPS, [D-Cys(Bzl)5]NPS, and [D-Cys(tBu)5]NPS vs NPS 100 nM in HEK293 cells stably expressing the mouse NPSR. All peptides were inactive as agonists up to 10 μM Data are mean ± sem of at least 4 experiments made in duplicate
Based on Ala- and D-scan studies we previously proposed an NPS pharmacophoric model (see Figure 3 in reference6) in which the Phe2-Arg3-Asn4 sequence represents the message domain of this peptide, the sequence Thr8-Gly9-Met10 is also important for receptor binding, although with non-stringent chemical requirements, and the sequence Val6-Gly7 behaves as hinge region, determining the correct spatial arrangement of the two above mentioned peptide portions. Based on the present findings we propose to include Gly5 into the above mentioned hinge region. In fact, structural changes induced by substituting the achiral residue Gly with amino acids of the L and D series produced similar changes in peptide potency while determining their efficacy. Peptide efficacy was not modified by L amino acid residues (with the only exception of Phe) while being strongly reduced or even abolished by D residues. This clearly suggests that modifications of the relative spatial disposition of the N (message) and C terminal domains of NPS induced by chirality changes in position 5 have little effect on peptide potency while having a profound impact on peptide efficacy with the L amino acid favoring agonist and D amino acids inducing antagonist bioactive conformations.
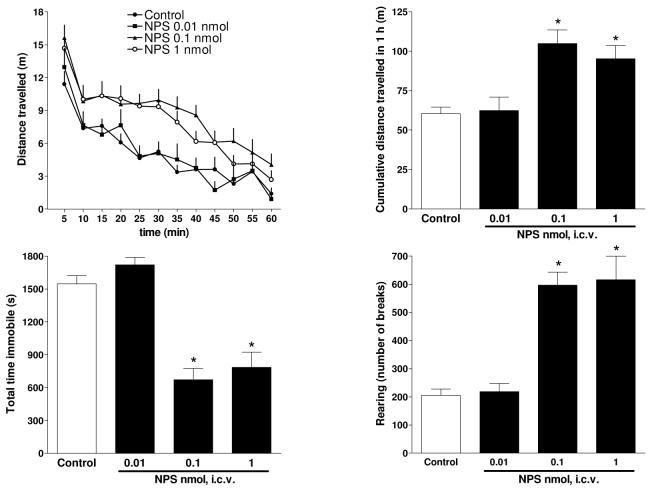
Figure 3.
Dose response curve to NPS (0.01 – 1 nmole) on mouse locomotor activity. The time course of NPS effects is shown in the top left panel while the other panels display the cumulative effects exerted by the peptide over the 60 min observation period. Data are mean ± sem of 4 separate experiments (4 mice/group/experiment). *p < 0.05 vs control, according to one-way ANOVA followed by the Dunnett test.
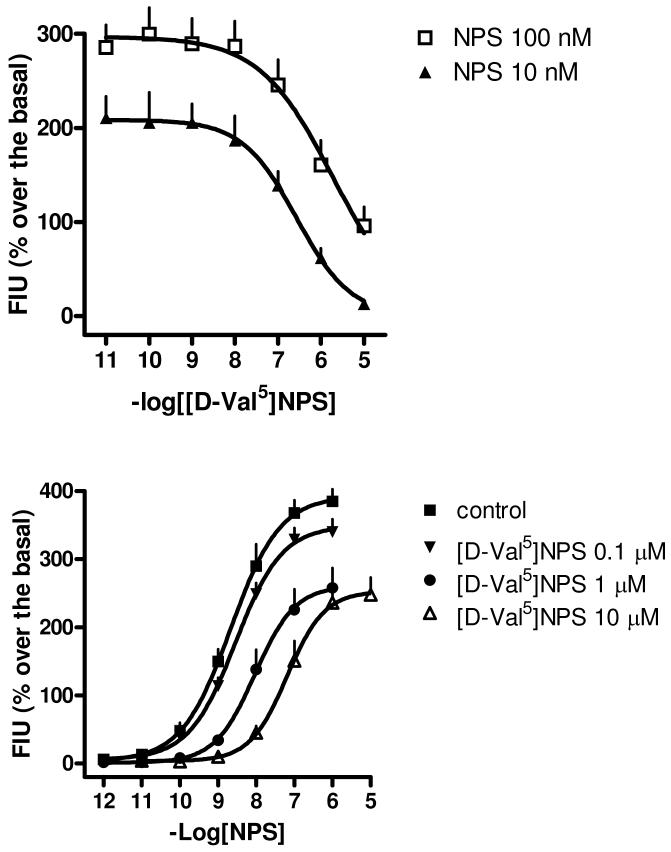
In the frame of the present SAR study focused on NPS position 5 we identified a series of NPS analogues devoid of efficacy and able to block agonist activity at NPSR with moderate to high potency: thus the first series of NPSR peptide antagonists has been discovered. Among these, [D-Val5]NPS was selected, based on its high potency, for further in vitro and in vivo characterization. In vitro, the antagonistic properties of [D-Val5]NPS were further investigated in HEK239mNPSR cells by performing inhibition response curves against the effect elicited by 10 and 100 nM NPS as well as by testing the peptide with the classical Schild protocol. In order to asses the specificity of this peptide analog, [D-Val5]NPS was tested in HEK293 cells against the stimulatory effect evoked by carbachol via stimulation of endogenously expressed muscarinic receptors. In addition, the in vivo effects of [D-Val5]NPS on the stimulatory effect of i.c.v. injected NPS on mouse locomotor activity was investigated. Figure 2 top panel displays the inhibition response curve of [D-Val5]NPS (0.01 nM - 10 μM) against the stimulatory effect of NPS 10 and 100 nM corresponding to agonist submaximal and maximal concentrations, respectively. As mentioned before, [D-Val5]NPS was completely inactive per se up to 10 μM, however it produced a concentration dependent inhibition of NPS effects. Thus [D-Val5]NPS behaves in these experiments as a pure NPSR antagonist devoid of residual agonistic activity. Regarding the type of antagonism exerted by [D-Val5]NPS vs NPS, it should be kept in mind that in inhibition curve experiments competitive antagonists are expected to display different pIC50 values depending on the concentration of agonist used (and the corresponding pKB can be derived from equation #1 described in the methods section) while for non-competitive antagonists similar pIC50 values are expected against different concentrations of agonist and pIC50 ≈ pKB.16 In the present experiments [D-Val5]NPS displayed different pIC50 values dependent on agonist concentrations, i.e. 6.59 vs NPS 10 nM and 5.96 (assuming full inhibition at concentrations higher than 10 μM) vs NPS 100 nM. However, the increase of 1 log unit of agonist concentration produced a decrease of only 0.63 log unit in antagonist pIC50. Therefore, inhibition response data are not compatible with a simple competitve type of interaction between [D-Val5]NPS and NPS. A [D-Val5]NPS pKB value of 7.50 (CL95% 7.18– 7.82) was derived from these experiments using equation # 1 (see experimental section). In order to obtain further information on the nature of the antagonist action exerted by [D-Val5]NPS the classical Schild analysis was also performed. As depicted in Figure 2 bottom panel, [D-Val5]NPS in the range 0.1 – 10 μM did not produce any effect per se but displaced the concentration response curve to NPS to the right in a concentration dependent manner; however this was associated with a slight but statistically significant decrease of the maximal effect elicited by NPS at the higher concentrations of antagonist (i.e. 1 and 10 μM). The pattern of antagonist effect of [D-Val5]NPS vs NPS can be described as dextral displacement of the agonist concentration response curve with depression of maximum at high concentrations. Thus, in line with inhibition response data, the results obtained by Schild analysis are not compatible with a competitive type of antagonism. Data displayed in Figure 2 bottom panel and the equation # 2 described in the experimental section were used for deriving a pKB value of 7.02 for [D-Val5]NPS, which is not too far from that obtained in inhibition experiments (7.50).
Figure 2.
Top panel: Inhibition response curves to [D-Val5]NPS vs NPS 10 and 100 nM. Bottom panel: concentration response curves to NPS in the absence (control) and presence of increasing concentrations of [D-Val5]NPS. Experiments were performed on HEK293 cells stably expressing the mouse NPSR. Data are mean ± sem of 5 experiments made in duplicate.
To investigate [D-Val5]NPS selectivity of action, this peptide was tested against the effect produced by carbachol via stimulation of endogenously expressed muscarinic receptors in HEK293mNPSR cells. Carbachol produced a concentration dependent stimulation of calcium levels with Emax and pEC50 values of 305 ± 31% over basal, and 5.58, respectively. The concentration response curve to carbachol was not significantly modified in the presence of [D-Val5]NPS 10 μM both in terms of efficacy (Emax 309 ± 33% over basal) and potency (pEC50 5.69). These results demonstrate that the antagonist effect of [D-Val5]NPS is selective for NPSR over muscarinic receptors and certainly does not derive from a non-specific inhibitory effect of the peptide on calcium signaling.
Collectively these results demonstrate that [D-Val5]NPS behaves as a pure and potent antagonist at NPSR. Therefore the peptide was further investigated in vivo against the stimulatory effect on locomotor activity exerted by supraspinal administration of NPS in mice. In the first series of experiments the dose response curve (0.01 – 1 nmole/mouse) to i.c.v. injected NPS was assessed. As shown in Figure 3, NPS evoked a stimulatory effect on mouse locomotor activity by increasing cumulative distance traveled by the animals, the number of rearings, and reducing the total immobility time in a statistically significant manner at doses of 0.1 and 1 nmole. These results are superimposable to those reported in the literature in terms of amount and time course of the effect as well as peptide potency and corroborate the proposal that the NPS stimulatory effect on locomotor activity is a very robust phenomenon consistent among experimental conditions, different laboratories and animal species (rats and mice) 1, 5-7, 9. From these experiments the dose of NPS 0.1 nmole was selected, as the lower dose producing statistically singnificant effects, to be challenged with the NPSR antagonist [D-Val5]NPS. Since the in vitro ratio of potency NPS / [D-Val5]NPS is approximately 1 / 30, [D-Val5]NPS was tested in vivo at 10 nmoles (agonist / antagonist ratio = 1 / 100).
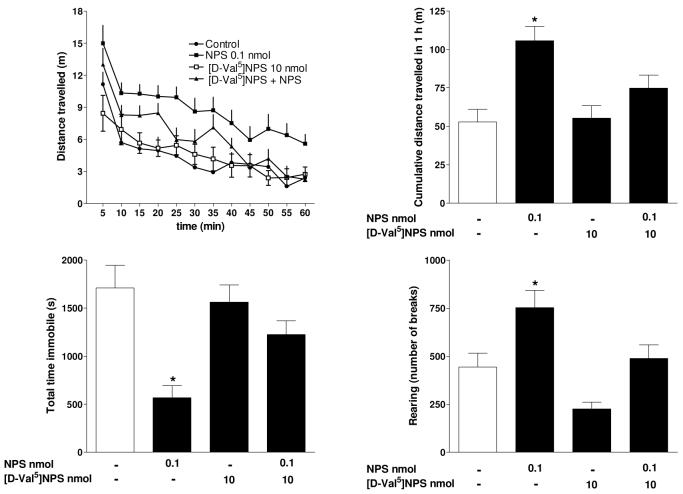
[D-Val5]NPS injected i.c.v at 10 nmoles/mouse did not statistically modify the animal locomotor behaviour (Figure 4). However it is worthy of mention (although this effect did not reach the statistical level of significance) that there was a clear tentency of animals treated with 10 nmoles [D-Val5]NPS to reduce their rearing behaviour. Interestingly, this effect is opposite to that of NPS which produces a statistically significant increase in number of rearings. More importantly, [D-Val5]NPS completely prevented the locomotor stimulatory effect of 0.1 nmole NPS on cumulative distance traveled, number of rearings, and total immobility time. These in vivo results further confirm the NPSR antagonist properties of [D-Val5]NPS. In addition, these findings allow to attribute to the selective activation of the NPSR protein the stimulatory effect of NPS on locomotor activity. The present results are also in line with previous findings obtained with the peptide NPSR partial agonist [Ala3]NPS 17 and, more importantly, with the non-peptide pure antagonist SHA 68 18. Interestingly SHA 68 at doses able to counteract the stimulatory effect of exogenously applied NPS produced per se a selective reduction of mouse vertical activity 18. These results together with the present findings obtained with [D-Val5]NPS might implicate a tonic control of the endogenous NPS / NPSR system on this particular animal behaviour.
Figure 4.
Effects of 0.1 nmole NPS, 10 nmoles [D-Val5]NPS, and their coapplication on mouse locomotor activity. The time course of peptide effects is shown in the top left panel while the other panels display the cumulative effects exerted by the peptides over the 60 min observation period. Data are mean ± sem of 4 separate experiments (4 mice/group/experiment). *p < 0.05 vs control, according to one-way ANOVA followed by the Dunnett test.
In conclusion, the present study demonstrated a crucial role of the chirality of amino acid residues at position 5 of NPS for peptide efficacy with L amino acids favoring agonist and D amino acids promoting antagonist bioactive conformations. Moreover, in the frame of the present study the first generation of NPSR peptide pure antagonists was identified and [D-Val5]NPS was pharmacologically characterized in vitro and in vivo. These peptide molecules together with the recently discovered NPSR non-peptide antagonist SHA 68 represent very important tools needed for understanding which and how biological functions are controlled by the NPS / NPSR system; in addition these NPSR ligands might be instrumental for identifying innovative strategies for treating neurological as well as psychiatric diseases.
Experimental Section
Materials
Amino acids, protected amino acids, resins for solid phase synthesis and chemicals were purchased from Bachem, Novabiochem, or Fluka. Stock solutions (1 mM) of peptides were made in distilled water and kept at −20 °C until use. All other reagents were from Sigma (Poole, U.K.) or Merck (Darmstadt, Germany) and were of the highest purity available.
General procedures for the solid phase peptide synthesis
All peptides were synthesised following the procedures previously reported in details13 using Fmoc/tBu chemistry. Purification of the crude peptides were achieved by preparative HPLC and the purity grade was checked by analytical HPLC and mass spectrometry.
Calcium mobilization experiments
HEK293 cells stably expressing the mouse recombinant NPSR (HEK293mNPSR) were generated as previously described4 and maintained in DMEM medium supplemented with 10% fetal bovine serum, 2 mM L-Glutamine, hygromycin (100 mg/l), and cultured at 37 °C in 5% CO2 humidified air.
HEK293mNPSR cells were seeded at a density of 50,000 cells/well into poly-D-Lysine coated 96-well black, clear-bottom plates. The following day, the cells were incubated with medium supplemented with 2.5 mM probenecid, 3 μM of the calcium sensitive fluorescent dye Fluo-4 AM and 0.01% pluronic acid, for 30 min at 37 °C. After that time the loading solution was aspirated and 100 μl/well of assay buffer (Hank’s balanced salt solution; HBSS) supplemented with 20 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), 2.5 mM probenecid and 500 μM Brilliant Black (Aldrich) was added. Concentrated solutions (1 mM) of NPS and related peptides were made in bidistilled water and kept at −20 °C. Serial dilutions were carried out in HBSS/HEPES (20mM) buffer (containing 0.02% BSA fraction V) in order to prepare a master plate at 3X concentration. After placing both plates (cell culture and master plate) into the fluorometric imaging plate reader FlexStation II (Molecular Devices, Sunnyvale, CA), fluorescence changes were measured at room temperature (≈ 22 °C). On-line additions were carried out in a volume of 50 μl/well.
Mouse locomotor activity experiments
All experimental procedures for in vivo studies complied with the standards of the European Communities Council directives (86/609/EEC) and National regulations (DL 116/ 92). Male Swiss mice (2 months old, 25–30 g) were used. They were housed in 425 × 266 × 155 mm cages (Tecniplast, MN, Italy), eight animals per cage, under standard conditions (22 1C, 55% humidity, 12-h light—dark cycle, lights on at 7.00 AM) with food (MIL, standard diet Morini RE, Italy) and water ad libitum for at least 10 days before experiments began. Each animal was used only once. NPS and [D-Val5]NPS were given i.c.v.. The i.c.v. injections (2 μl per mouse) were given under light (just sufficient to produce loss of the righting reflex) ether anaesthesia into the left ventricle according to the procedure described by Laursen and Belknap19 and routinely adopted in our laboratory.5 Experiments were performed during the light cycle (between 09.00 and 13.00). I.c.v. injections were performed 5 min before the beginning of the test and locomotor activity was recorded for 60 min. For these experiments the ANY-maze video tracking system was used (Ugo Basile, application version 4.52c Beta). Mice were positioned in square plastic cages (40 × 40 cm), one mouse per cage. Four mice were monitored in parallel. Mouse horizontal activity was monitored by a camera while vertical activity was measured by an infrared beam array. The parameters measured were cumulative distance travelled (total distance in m that the animal travelled during the test), total time immobile (the amount of seconds the animal stays immobile during the test; the animal is considered immobile when > 90% of his image remains in the same place for at least 2.5 s), and the number of animal rearings (the number of beam breaks due to vertical movements). Each data point derives from 4 separate experiments made in quadruplicated, therefore it reflects the behaviour of 16 animals. All procedures were randomised across test groups and, in all the experiments, food was not available during testing.
Data analysis and terminology
The data were expressed as mean ± sem of at least four independent experiments made in duplicate. Maximum change in fluorescence, expressed in percent of baseline fluorescence, was used to determine agonist response. Non-linear regression analysis using GraphPad Prism software (v.4.0) allowed logistic iterative fitting of the resultant responses and the calculation of agonist potencies and maximal effects. Agonist potencies are given as pEC50 (the negative logarithm to base 10 of the molar concentration of an agonist that produces 50% of the maximal possible effect). Differences in maximal effects between ligands were statistically analyzed via one-way analysis of variance followed by the Dunnett test for multiple comparisons.
In inhibition response experiments antagonist potencies were calculated using equation #1:
where IC50 is the concentration of antagonist that produces 50% inhibition of the agonist response, [A] is the concentration of agonist, EC50 is the concentration of agonist producing a 50% maximal response and n is the Hill coefficient of the concentration response curve to the agonist. 16 In classical Schild protocol experiments the antagonist potency of [D-Val5]NPS was derived using equation # 2:
where slope is calculated from a double-reciprocal plot of equieffective concentrations of agonist in the absence and presence of antagonist (B).16
In vivo data were statistically analyzed via one-way analysis of variance followed by the Dunnett test for multiple comparisons.
Supplementary Material
Acknowledgments
This work was supported by funds from the University of Ferrara (FAR grants to GC and SS) and the Italian Ministry of the University (Cofin 2006 grant to GC and SS) and a grant from the National Institute of Mental Health (RKR).
Abbrevations
- BSA
bovine serum albumin
- cAMP
cyclic adenosine monophosphate
- DMEM
Dulbecco’s modified Eagles’s medium
- Fluo-4AM
4-(6-Acetoxymethoxy-2,7-difluoro-3-oxo-9-xanthenyl)-4′-methyl-2,2′-(ethylenedioxy)dianiline-N,N,N’,N’-tetraacetic acid tetrakis(acetoxymethyl) ester
- Fmoc
N-(9-fluorenyl)methoxycarbonyl
- GPCR
G-protein coupled receptor
- HBSS
Hank’s balanced salt solution
- HEPES
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid
- HEK
human embryonic kidney
- HPLC
high performance liquid chromatography
- i.c.v
intracerebroventriculary
- mRNA
messenger ribonucleic acid
- NPS
neuropeptide S
- NPSR
NPS receptor
- tBu
tert-butyl
- SAR
structure-activity relationship
- SHA 68
3-Oxo-1,1-diphenyl-tetrahydro-oxazolo[3,4-a]pyrazine-7-carboxylic acid 4-fluoro-benzylamide
Footnotes
Supporting Information Available: table and chromatograms reporting the retention time determined by analytical HPLC analyses using two different chromatographic systems and the calculated and found molecular weight. This material is available free of charge via the internet at http://pubs.acs.org
References
- (1).Xu YL, Reinscheid RK, Huitron-Resendiz S, Clark SD, Wang Z, Lin SH, Brucher FA, Zeng J, Ly NK, Henriksen SJ, de Lecea L, Civelli O. Neuropeptide S: a neuropeptide promoting arousal and anxiolytic-like effects. Neuron. 2004;43:487–497. doi: 10.1016/j.neuron.2004.08.005. [DOI] [PubMed] [Google Scholar]
- (2).Reinscheid RK. Phylogenetic appearance of neuropeptide S precursor proteins in tetrapods. Peptides. 2007;28:830–837. doi: 10.1016/j.peptides.2007.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Xu YL, Gall CM, Jackson VR, Civelli O, Reinscheid RK. Distribution of neuropeptide S receptor mRNA and neurochemical characteristics of neuropeptide S-expressing neurons in the rat brain. J. Comp. Neurol. 2007;500:84–102. doi: 10.1002/cne.21159. [DOI] [PubMed] [Google Scholar]
- (4).Reinscheid RK, Xu YL, Okamura N, Zeng J, Chung S, Pai R, Wang Z, Civelli O. Pharmacological Characterization of Human and Murine Neuropeptide S Receptor Variants. J. Pharmacol. Exp. Ther. 2005;315:1338–1345. doi: 10.1124/jpet.105.093427. [DOI] [PubMed] [Google Scholar]
- (5).Rizzi A, Vergura R, Marzola G, Ruzza C, Guerrini R, Salvadori S, Regoli D, Calo G. Neuropeptide S is a stimulatory anxiolytic agent: a behavioural study in mice. Br. J. Pharmacol. 2008;154:471–479. doi: 10.1038/bjp.2008.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Roth AL, Marzola E, Rizzi A, Arduin M, Trapella C, Corti C, Vergura R, Martinelli P, Salvadori S, Regoli D, Corsi M, Cavanni P, Calo G, Guerrini R. Structure-activity studies on neuropeptide S: identification of the amino acid residues crucial for receptor activation. J. Biol. Chem. 2006;281:20809–20816. doi: 10.1074/jbc.M601846200. [DOI] [PubMed] [Google Scholar]
- (7).Smith KL, Patterson M, Dhillo WS, Patel SR, Semjonous NM, Gardiner JV, Ghatei MA, Bloom SR. Neuropeptide S stimulates the hypothalamo-pituitary-adrenal axis and inhibits food intake. Endocrinology. 2006;147:3510–3518. doi: 10.1210/en.2005-1280. [DOI] [PubMed] [Google Scholar]
- (8).Beck B, Fernette B, Stricker-Krongrad A. Peptide S is a novel potent inhibitor of voluntary and fast-induced food intake in rats. Biochem. Biophys. Res. Commun. 2005;332:859–865. doi: 10.1016/j.bbrc.2005.05.029. [DOI] [PubMed] [Google Scholar]
- (9).Leonard SK, Dwyer JM, Sukoff Rizzo SJ, Platt B, Logue SF, Neal SJ, Malberg JE, Beyer CE, Schechter LE, Rosenzweig-Lipson S, Ring RH. Pharmacology of neuropeptide S in mice: therapeutic relevance to anxiety disorders. Psychopharmacology (Berl) 2008;197:601–611. doi: 10.1007/s00213-008-1080-4. [DOI] [PubMed] [Google Scholar]
- (10).Jungling K, Seidenbecher T, Sosulina L, Lesting J, Sangha S, Clark SD, Okamura N, Duangdao DM, Xu YL, Reinscheid RK, Pape HC. Neuropeptide S-mediated control of fear expression and extinction: role of intercalated GABAergic neurons in the amygdala. Neuron. 2008;59:298–310. doi: 10.1016/j.neuron.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Meis S, Bergado-Acosta JR, Yanagawa Y, Obata K, Stork O, Munsch T. Identification of a neuropeptide S responsive circuitry shaping amygdala activity via the endopiriform nucleus. PLoS ONE. 2008;3:e2695. doi: 10.1371/journal.pone.0002695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Bernier V, Stocco R, Bogusky MJ, Joyce JG, Parachoniak C, Grenier K, Arget M, Mathieu MC, O’Neill GP, Slipetz D, Crackower MA, Tan CM, Therien AG. Structure-function relationships in the neuropeptide S receptor: molecular consequences of the asthma-associated mutation N107I. J. Biol. Chem. 2006;281:24704–24712. doi: 10.1074/jbc.M603691200. [DOI] [PubMed] [Google Scholar]
- (13).Camarda V, Trapella C, Calo G, Guerrini R, Rizzi A, Ruzza C, Fiorini S, Marzola E, Reinscheid RK, Regoli D, Salvadori S. Synthesis and biological activity of human neuropeptide S analogues modified in position 2. J. Med. Chem. 2008;51:655–658. doi: 10.1021/jm701204n. [DOI] [PubMed] [Google Scholar]
- (14).Camarda V, Trapella C, Calo G, Guerrini R, Rizzi A, Ruzza C, Fiorini S, Marzola E, Reinscheid RK, Regoli D, Salvadori S. Structure activity study at position 3 and 4 of human neuropeptide S. Bioorg. Med. Chem. 2008;16:8841–8845. doi: 10.1016/j.bmc.2008.08.073. [DOI] [PubMed] [Google Scholar]
- (15).Tancredi T, Guerrini R, Marzola E, Trapella C, Calo G, Regoli D, Reinscheid RK, Camarda V, Salvadori S, Temussi PA. Conformation-Activity Relationship of Neuropeptide S and Some Structural Mutants: Helicity Affects Their Interaction with the Receptor. J. Med. Chem. 2007;50:4501–4508. doi: 10.1021/jm0706822. [DOI] [PubMed] [Google Scholar]
- (16).Kenakin T. A Pharmacology Primer. Elsevier Academic Press; San Diego: 2004. [Google Scholar]
- (17).Calo G, Roth A, Marzola E, Rizzi A, Arduin M, Trapella C, Corti C, Vergura R, Martinelli P, Salvadori S, Regoli D, Corsi M, Cavanni P, Guerrini R. Structure activity studies on Neuropeptide S: identification of the amino acid residues crucial for receptor activation. Society for Neuroscience; Atlanta, GA, USA: 2006. p. 726.18/D56. [DOI] [PubMed] [Google Scholar]
- (18).Okamura N, Habay SA, Zeng J, Chamberlin AR, Reinscheid RK. Synthesis and pharmacological in vitro and in vivo profile of 3-oxo-1,1-diphenyl-tetrahydrooxazolo[3,4-a]pyrazine-7-carboxylic acid 4-fluoro-benzylamide (SHA 68), a selective antagonist of the neuropeptide S receptor. J. Pharmacol. Exp. Ther. 2008;325:893–901. doi: 10.1124/jpet.107.135103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Laursen SE, Belknap JK. Intracerebroventricular injections in mice. Some methodological refinements. J. Pharmacol. Methods. 1986;16:355–357. doi: 10.1016/0160-5402(86)90038-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.