1. Introduction
The use of cell culture to characterize bone and tooth mineralization has blossomed thanks to the development of genetically engineered animals with altered bone and tooth phenotypes 1,2, the tissue engineering of bones, teeth, and cartilage 3,4, and genetic profiling of mineralized tissue development 5-8. There is little uniformity in the conditions used in these cultures, and where there is uniformity the chemical basis for the reagents used is sometimes questionable. Moreover, while it is well established that histochemical staining is a poor substitute for physicochemical assays of the nature of the mineral formed 9, the majority of both recent and classical papers in the literature rely on histochemistry to report the presence of bone- or tooth-like mineral. The goal of this review is to place cell culture methodologies in a chemical context, to review the different types of cell culture systems that have been used to study the deposition of mineral in bones and teeth, discuss their limitations, and suggest some guidelines for future studies.
1.1. The Cells and Tissues of Bones and Teeth
Cell culture systems generally seek to recapitulate the events in the development of the tissue in situ. While some culture systems start with preformed substrates and others rely on the differentiation, proliferation, and maturation of cells, the ultimate goal is the formation of an analogue of the naturally occurring tissue. Questions that arise during these studies may include whether the cells in question have an altered ability to form this tissue, what the role of the genetic modification might be, or whether the material formed can be used to repair and replace native tissue. To understand the cell culture methodologies, it is necessary to first review the composition of these tissues and the cells involved in their formation.
1.1.1. The Mineral and Extracellular Matrix of Bones and Teeth
The mineral phase that is found in bones and teeth as a highly substituted analogue of the geologic mineral, hydroxyapatite (Ca10(PO4)6(OH)210. In bone, cementum, and dentin, the plate-like OH-deficient, CO3-substituted apatite nano-crystals are oriented with their long axis parallel to the axis of the collagen fibrils. The insoluble fibrillar protein, collagen, is the major component of the organic matrix. Other noncollagenous proteins are important for the maintenance of the cell-matrix interactions, cell-signaling, regulation of cell metabolism, and control of the mineralization process11,12.
In mature enamel, the hydroxyapatite crystals are also carbonate-substituted, but they are larger, and there is little (<3%) organic matrix. There is no collagen in enamel; the major protein in mature enamel is amelogenin, but proteins account for a very small amount of the mature enamel’s composition. During early enamel formation there is a greater proportion of proteins, and these include enamel specific proteins such as amelogenin, enamelin, tuftelins, and amelin, and some non-specific proteins such as phosphophoryn 12,13.
1.1.2. Cells in Mineralized Tissues
Bone, dentin, cementum, and calcified cartilage contain cells that deposit the non-mineralized tissue, initiate and control tissue mineralization, and regulate tissue metabolism. In the case of bone there are also cells that remove and remodel the tissue. In teeth the remodeling process does not generally occur. Within the mineralized tissues there are also neuro- and vascular elements but those cells are not of concern for this review.
The bone, calcified cartilage, and dentin forming cells are osteoblasts 14, hypertrophic chondrocytes 15, and odontoblasts 16, respectively. These are the cells that produce the extracellular matrix and control the initial mineralization process. In bone, as osteoblasts become engulfed in mineral, they extend long processes to connect to one another. These cells, now called osteocytes, 17 are connected by this long canalicular (dendritic) network. The cells have a different phenotype than osteoblasts, expressing different amount of phenotypic markers 18-21 some of which are osteocyte specific 22.
In teeth, there are three major cell types, the odontoblasts that form dentin, the cementoblasts in cementum (a tissue intermediate between bone and dentin) 23, and the ameloblasts that form enamel 24. Table 1 lists protein markers that are commonly used in immunohistochemistry, cell sorting, or related assays, to distinguish these different mineralized tissue-forming cell types. The mineralized tissue resorbing cells, osteoclasts and chondroclasts, whose functions are coupled with those of the osteoblasts and osteocytes, while often studied in culture, will not be discussed here, and the reader is referred to recent reviews for more information 25-27.
Table 1.
Mineralized Tissue Cell Types and their Cell Specifica Protein Markers
| Cell | Marker | Also Used as Markersb |
|---|---|---|
| Hypertrophic Chondrocyte | Type X collagen 257 | Types II and IX collagen |
| MMP-9 258 | DMP1 | |
| Osteoblast | Osteocalcin259 | Type I collagen |
| Periostin 260 | Alkaline phosphatase | |
| Bone sialoprotein (BSP) 261 | Runx2 264,265 | |
| MEPE/OF45 262 | Osterix 266 | |
| PHEX/Pex 263 | Response to PTH 267 | |
| Osteocyte | Sclerostin (SOST) 268,269 | Osteocalcin |
| DMP1 19,270 | Type I collagen | |
| Actin-binding proteins 271 | Alkaline phosphatase | |
| Fimbrin 271 | ||
| Podoplanin/E11 21 | ||
| MEPE/OF45 262 | ||
| PHEX/Pex 272 | ||
| Odontoblast | Dspp (gene) 156,273,274 | Type I collagen |
| -Phosphophoryn 156 | Alkaline Phosphotase 276 | |
| -Dentin sialoprotein 156,274 | MEPE 276 | |
| DMP4 275 | ||
| Cementoblast | Cementum protein 23 277 | Bone matrix proteins |
| Ameloblast | Amelogenin 207 | |
| Ameloblastin 207,278 | ||
| Tuftelin 279 | ||
| Enamelin 280 | ||
| Amelotin 281 | ||
| Cytokeratin 14 282 |
Some of these proteins are found at low levels in other tissues
Many of the protein markers listed here are expressed at high levels by two or more of these cell types but are frequently designated as markers
2. Is the Mineral formed in culture similar to “Physiologic Mineral”?
Before discussing how cell culture is performed in the different systems, it is important to describe methods that have been used to demonstrate that the mineral formed in culture is similar to that which occurs in nature. A concern in the analysis of mineral is the chemical treatments done prior to analysis, as some of these treatments can change the properties of the mineral. Often, the question of whether the mineral formed is at all like that present in the body is ignored, and authors show the presence of calcium and phosphate ions without showing whether the mineral is hydroxyapatite-like, that the mineral is deposited with the correct organization on the appropriate matrix (i.e., aligned with collagen for bone, dentin, and cementum; and associated with amelogenin nano-spheres for initial enamel deposition), and that the crystals are of the size of physiologic crystals.
2.1. X-ray diffraction
The gold-standard method for identifying the mineral phase present in any material is x-ray diffraction 28. X-ray diffraction can also be used to measure and calculate the average crystal size and orientation of the mineral crystals in the sample. In the case of calcified tissue analysis, this requires homogenization of the tissue (or culture), which in turn mandates dehydration (lyophilization) and grinding; procedures that may prevent the observation of precursor phases 29,30. Further, in the case of cultures where the yield of mineral is small, it is usually necessary to perform some type of micro-diffraction, or to pool material from multiple cultures to determine if the mineral phase present is hydroxyapatite or some other calcium phosphate. Although x-ray diffraction is the most definitive way to characterize the mineral formed in culture there are few reports where x-ray or the related electron-diffraction has been used to identify mineral in culture. Some examples of are listed in Table 2.
Table 2.
Applications of X-ray and Electron Diffraction and Vibrational Spectroscopy, to Identify Mineral Formed in Culture
| Culture Type | Diffraction Used | Vibrational Spectroscopy Used | Notes | Reference |
|---|---|---|---|---|
| Primary Osteoblast | X | No BGP | 283 | |
| X | ±BGP | 65,284 | ||
| Osteoblast cell lines | ||||
| MC3T3-E1 | X | X | +BGP | 137,138 |
| UMR106 | X | X | +BGP | 79 |
| Marrow Stromal Cells | X | X | ±BGP | 47,267,285-287 |
| Osteoblasts on Scaffolds | ||||
| Chitosan | X | 288 | ||
| Collagen fibrils | X | 289 | ||
| Hydrogels | X | 289 | ||
| Collagen honeycombs | X | 290 | ||
| Other Cell Types | ||||
| Odontoblasts | X | X | Pulp cells & organ culture | 291-293 |
| Chondrocytes | X | X | 4mM P | 71,191 |
| Ameloblasts | X | Organ culture without serum | 291,292 | |
| ATDC5 cells | X | No BGP | 167 | |
2.2. SEM and TEM and related techniques
Selected area diffraction, performed under the transmission electron microscope (TEM), and/or dark field evaluation of mineral crystallites, enables evaluation of the individual crystals formed in a tissue or in culture. To perform these analyses the cultures are often fixed, embedded, and sectioned by methods described elsewhere 31; some of these methods can cause dissolution of the mineral crystals yeilding crystals that are different in size or composition than those initially present. Alternatively, use of nonaqueous fixation in ethylene glycol, has long been known to prevent these changes 32, however non aqueous fixation rarely has been used to prepare cultures for mineral analyses 33,34.
Selected area electron diffraction is a more difficult procedure than x-ray diffraction requiring alignment of the electron microscope aperture with the crystals in question. Additionally, calibration of the diffraction pattern obtained with a standard run under the same experimental conditions is necessary. There are however a few culture studies where electron diffraction has been used to identify the presence of hydroxyapatite; for example, studies showing the need for 1-2 mM inorganic phosphate for chondrocyte mediated mineralization in both monolayer and agarose-suspension cultures 35, and a study of high density suspension cultures of chondrocytes in which TEM was used to identify matrix vesicles, and then selected area diffraction used to confirm the presence of hydroxyapatite 36. While there are few studies that use selected area diffraction to veify the presence of hydroxyapatite, TEM and SEM are widely used to show that the mineral crystals are aligned with respect to the collagen axis, as is typical of in situ calcification (Figure 1). Sizes of mineral aggregates, or in the case of dark field analyses of individual crystals, can be meaasured by TEM. Most importantly, using TEM, the orientation of the mineral on the collagen or amelogenin substrates can readily be demonstrated 37-43.
Figure 1.
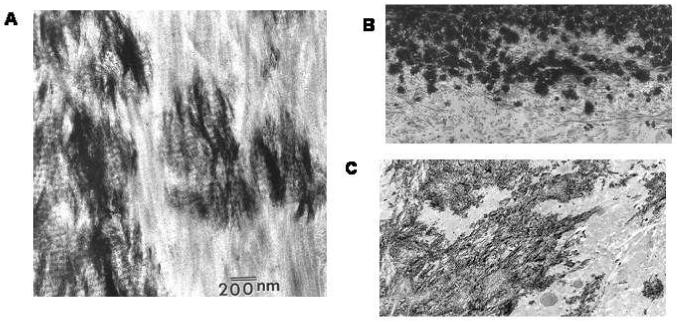
Transmission electron microscopy (TEM) reveals the orientation of the mineral crystals relative to collagen fibrils. A) High resolution image of a bone specimen. Note the electron dense mineral is aligned parallel to the collagen fibrils. B) Lower resolution image of an osteoblast culture, mineralized in the presence of 5 mM BGP, clumps of mineral crystals are associated with the collagen fibrils, but the crystals do not appear to be aligned. C) Low resolution image of a differentiating mesenchymal cell micro-mass culture at 23 days shows the electron dense mineral associated with collagen fibrils; because the cells are chondrocytes, they make type II collagen, yet the mineral is associated with the collagen fibrils. (Photomicrographs were provided from Dr. S. B. Doty, Hospital for Special Surgery, New York, NY).
At the same resolution, using an electron microscope, backscatter electron imaging and energy dispersive x-ray analysis (EDX) can be used to provide insight into the size distribution of the crystals and the chemical composition of the mineral deposited in culture. There are but a few examples, however, where these techniques have been used to analyze mineral formed in culture 43-46. One interesting illustration of the combined power of these quantitative techniques is a report of a chick bone marrow stromal cell culture system. In these cultures, mineralization occurred in the presence of 10 mM BGP (beta-glycerophosphate) supplemented DMEM, with vitamin D and BMP-2 to stimulate differentiation. While no mineralization occurred in BMP-free cultures under the same conditions, in this study TEM and SEM as well as EDX were used to quantify the distribution of mineral ions as well as crystal shape, x-ray diffraction and quantitative infrared to show the formation of small crystals in vacuoles which spread to the collagen matrix and matured into aligned hydroxyapatite crystals 47.
2.3. AFM
The atomic force microscope (AFM) also called the scanning force microscope 48 has been used to visualize living cells (osteoblasts, osteocytes, odontoblasts, ameloblasts) and protein surfaces, as well as the properties of the crystals formed on the matrix produced by these cells. Certain cautions apply as the force used to “tap” the cells must be limited to prevent compression of the cell or induction of cell death, none-the-less 50nm resolution has been reported for osteoblasts 49. At this high resolution, AFM has been used to characterize the adherence of osteoblast-like cells to different matrices 50 including different size hydroxyapatite crystals 51; to characterize the interaction of both fibroblasts and osteoblasts with amelogenin 52; to characterize the growth of hydroxyapatite crystals into enamel prisms in the absence of cells 53-55, but to the best of this reviewer’s knowledge has only been used twice to study mineralization in culture. AFM was used to characterize mineral mechanical properties in an orthotopic transplantation model where pre-cultured human bone marrow stromal cells were implanted in a mouse calvaria 56 and recently to characterize the spherical bodies with which mineral is associated in the MLO-A5 late osteoblast/early osteocyte cell line (Figure 2) 57.
Figure 2.
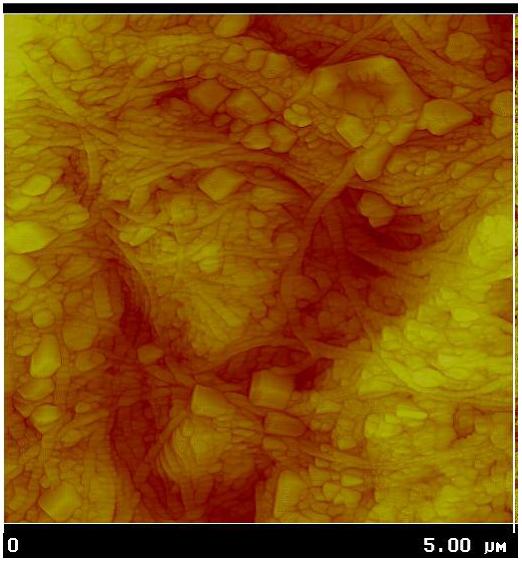
Atomic Force Microscopy (AFM) of mineral in culture. AFM height map of MLO-A5 osteocytes at 12 days in culture showing multiple spherical structures intercalated between collagen fibers. MLO-A5 cell culture figure provided by Dr. Cielo Barragan-Adjemian and Dr. Lynda Bonewald UMKC, Kansas City, MO and analyzed with AFM by Dr. Dan Nicollela Southwest Institute, San Antonio, TX. For detailed of the mineral-containing spherical structures see reference 57.
2.4. Light microscopy
Observation of tissues or cultures under the microscope usually requires fixation and application of chemical stains. Cultures, of course, can simply be examined under a microscope and the types of cells present and their arrangement identified. Dallas’s group 58 has coupled a time-lapse imaging technique to monitor how the cells move showing that osteoblasts and osteocytes are motile cells, even when engulfed in the mineralized matrix they form. That sort of analysis is more difficult to perform in tissues, and thus comparisons to what happens in the body are not yet available. In other cases, the tissue is often dehydrated to allow staining, a process that could cause the artifactual/spontaneous deposition of mineral, or fixed with a number of different materials that are known to change the solubility of apatite, to change the cross-links within the matrix, and/or to cause solubilization and redeposition of mineral crystals 31. Keeping the pH of the fixative and staining solution physiologic (>7.4) minimizes dissolution and reprecipitation of mineral phases.
At the light microscopic level the stains used to identify the hydroxyapatite mineral in culture are alizarin red (which chelates calcium) and von Kossa (which is a silver stain that causes silver phosphate to precipitate. The silver is then oxidized leaving a black precipitate (Figure 3)). The alizarin red stain is often solubilizied and quantified spectrophotometrically. Newer fluorescent dyes such as xylenol orange or calcein blue can also be used to illustrate the distribution of calcium in the culture matrix without affecting cell viability, and to associate the mineral with the presence of matrix proteins 59,60.
Figure 3.
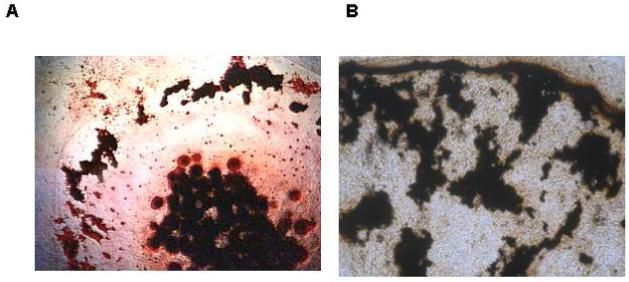
Histochemical analysis of mineral formed in chondrocyte micro-mass cultures using the silver-stain (von Kossa) technique. A) Primary chick limb-bud mesenchymal cells that differentiated into chondrocytes shown at day 24. Micro-mass cultures were maintained in DMEM containing 10% fetal bovine serum with 4mM inorganic phosphate, 50 ug/ml ascorbate, and antibiotics. B) ATDC5 cells maintained in culture for 35 days in the presence of the same additives plus 100 ng/ml BMP-2. Cultures counter stained with neutral Red. Black deposits in the center of the culture dish and around the periphery are the von Kossa positive material.
2.5. Vibrational Spectroscopy and Vibrational Spectroscopic Imaging
Infrared and Raman spectroscopy provide information on the local environment of ions with asymmetric and symmetric vibrations, respectively 61. Several investigators have used these techniques to analyze the mineral phase formed in homogenized cultures, and the relative amounts of carbonate substitution in precipitated apatites 44,62-68. Of interest, in light of the concerns expressed below concerning the use of BGP, a study of marrow stromal cells treated with basic Fibroblast Growth Factor (bFGF) was exposed to 1 or 3 mM BGP for up to four weeks, and the mineral content (measured as the ratio of the area of the phosphate peak to the amide I peak as determined by FTIR) increased with increasing BGP concentration, along with the mechanical properties of the mineralized matrix formed in the cultures, but crystallinity and carbonate/phosphate ratio were not altered by BGP treatment62.
More recently, the coupling of an array detector to the Infrared or Raman spectrometer has enabled localized changes to be displayed with 10∞m or 1 ∞m spatial resolution, respectively 69. Infrared spectroscopic imaging has been applied to characterize mineralization in differentiating chick limb-bud mesenchymal cell cultures 70-73, osteoblast cultures 9,74,75, and in one case to odontoblast cultures 76 (Figure 4A). Typical FTIR images of calcifying cultures are seen in figure 4B-D. Unlike for infrared imaging, for Raman microspectroscopy and imaging, the tissue does not need to be dehydrated, and can be examined directly in the cell culture dish or on a slide. Infrared spectroscopic imaging requires thinner sections, and there is interference from water, thus most commonly the culture is removed from the dish and either air dried, or embedded and sectioned. Raman spectroscopic imaging has been used to study the mineral formed in calvarial organ cultures 77 marrow stromal cell cultures 44, and dental pulp cells 68. Additional examples of the use of vibrational spectroscopy for the analysis of mineral formed in culture can be found in Table 2.
Figure 4.
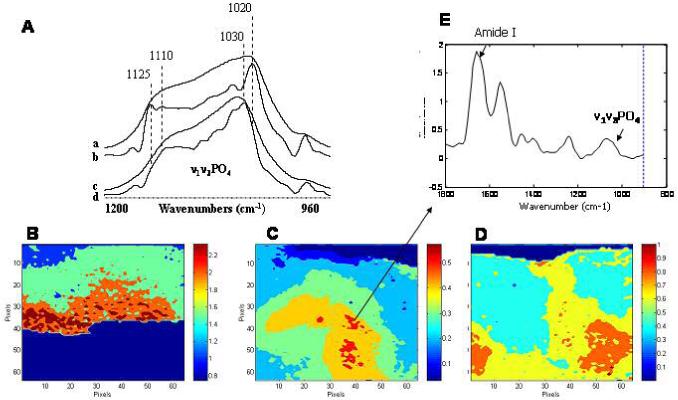
Fourier Transform Infrared Analysis of mineral formed in culture. Infrared spectroscopy can be used to characterize the mineral formed in culture. A) The phosphate absorption band observed in the odontoblast M2H4 cell line maintained in culture for days 8-21 with 10 ng TGFbeta 1, 100 ng/ml BMP-4, and 3 mM inorganic phosphate (a), when deconvoluted to reveal underlying peaks (b), resembles that obtained from a dentin slice (c) and its deconvoluted spectrum (d). The subbands at 1125 and 1020 cm-1 are characteristic of an immature hydroxyapatite rich in acid phosphate and carbonate substituents. Generously provided by Professor J. Guicheux and D. Magne. Details of the culture system are in reference 76. B) By attaching an array detector to the infrared microscope, images corresponding to each of the parameters of interest can be obtained. FTIR spectroscopic imaging of the mineral/matrix ratio in a mineralizing chick limb-bud micromass culture at day 21 showing the distribution of mineral. These cells were maintained in DMEM with 1.4 mM calcium and 4 mM inorganic phosphate plus antibiotics and 40 uM ascorbate. C) FTIR spectroscopic image of the mineral/matrix ratio in a “bone nodule” formed in an osteoblast culture at day 14. The cells were cultured with alpha -MEM containing ascorbate, vitamin D, and a total of 3 mM inorganic phosphate. Note the mineral/matrix ratio in the day 21 chondrocytes is higher than thatin the 14 D osteoblast culture. D) Image showing the distribution of crystal size (and perfection (in the culture illustrated in figure C. E) Spectrum corresponding to the pixel indicate in C is shown in figure 3. The amide I and phosphate bands are noted.
2.6. Radiographic and Related Methods
Radiographic methods detect changes in scattering elements, and thus can distinguish the presence of calcium, usually as an increase in density. X-ray microcomputed tomography (XTM or micro-CT) were only recently introduced for the study of mineralization in cultures. Other techniques are related to nuclear magnetic resonance (NMR) and show the difference in the environments of elements with spin dipoles (1H, 31P,) thereby giving insight into the changes in the phosphate distribution.
2.6.1. Magnetic Resonance Methods
Potter has pioneered the technique of magnetic resonance microscopy with or without manganese to characterize mineral formation in culture. She used this technique to monitor and quantify bone formation on scaffolds 78, in bioreactors 74, and in calcifying cartilage cultures 39, monitoring relaxation times to obtain maps of mineral deposition 74. Magnetic resonance microscopy has also been used to monitor the mineralization of tissue engineered constructs in vitro 79. The lack of general availability of the equipment to do such studies has limited its broad applicability, but it provides new information not otherwise accessible on mineral and matrix without having to dehydrate the tissue.
2.6.2. Micro-computed tomography (microCT) or X-ray Micro tomography (XMT)
Changes in material density are routinely assessed by microCT for tissue samples. The same technique can be used with cell culture systems (Figure 5) providing insight into porosity and mineral deposition. Combining micro-MRI with microCT provides good visualization of water and mineral, although each technique has limitations 80. The limitation of this method is that while the density of the matrix formed can be easily determined, along with its porosity, no information is provided on the chemical composition of the mineral or its crystalline phase.
Figure 5.
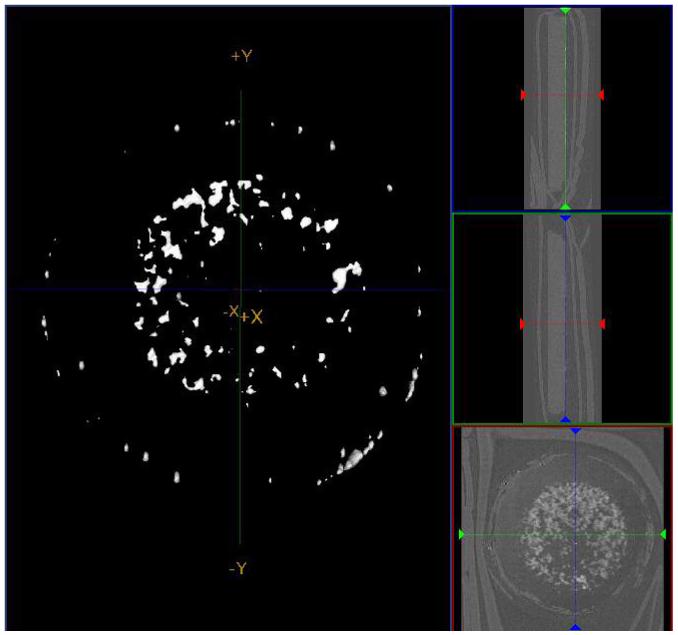
Micro-computed tomography (microCT) shows the increased density due to the mineral deposited in C3H10T1/2 cells grown in micro-mass culture at 35 days. These cultures were supplemented with ITS, 1% fetal bovine serum, ascorbate, and 4 mM phosphate. Figures on the right show lateral and bottom views of the dish and culture.
In a recent study, Cowan et al. 81 compared the use of microCT with alizarin red staining and scanning electron microscopy to assess BMP-2 induced mineralization in MC3T3-E1 osteoblast-like cell loaded scaffolds. MicroCT has also been used to monitor scaffolds cultured in vitro and implanted in vivo 82. The obvious advantage of microCT is that it provides a three dimensional view of the culture, in contrast to the two-dimensional information provided by most other methodologies. Thus it is likely that as microCT becomes more routinely available, there will be an increase in the applications to characterization of mineralization in culture, however it must be noted that this technique provides no information about the nature of the mineral present.
2.7. Chemical analyses
Chemical analysis of the calcium and phosphate content of cultures, or monitoring of the uptake of labeled calcium or phosphate during culture development is frequently used to measure rates of mineral accretion. Measurement of Ca/P ratios for mineral identification requires other techniques to verify that the mineral that forms is comparable to that in the tissue whose composition is being mimicked. Similarly, just showing that calcium or phosphate contents of the culture are increased with time does not suffice as many anionic matrix molecules can bind calcium, and the development of the culture generally involves changes in matrix protein phosphorylation 9.
3. Systems for studying mineralization in culture
While the conditions for forming bone, calcified cartilage, dentin and cementum in culture are variable, they all share common features. First, there must be a source of cells, second, media that will support the growth of cells and allow mineralization to occur is required; finally, a substrate is needed upon which those cells will proliferate and grow. From a chemical point of view, the solution must be saturated with respect to hydroxyapatite (HA); hence media is usually supplemented with calcium and phosphate, and kept buffered at physiologic or slightly more basic pH. The cells must synthesize a matrix (collagen or the initial enamel matrix) upon which the mineral will deposit, and that matrix should include those proteins and peptides that support mineralization 83. Factors that stimulate cell proliferation, differentiation, and maturation are generally added in the form of serum, although individual growth factors, hormones, and other exogenous regulators may be added.
3.1. Cell sources
The cells used in cultures may be provided directly in the form of an immature intact matrix (organ culture), they may be released from tissues (primary cultures) or they may have been immortalized or derived from tumor cells (cell lines) enabling standardized studies. There are also a variety of ways of distributing the cells in culture (plating the cells) and these will be discussed after the different types of cultures. The methodologies for releasing osteoblastic cells from different types of bone have recently been reviewed 84, and essentially consist of treatments that release the cells from both the mineral and matrix without disturbing the cellular membranes. Similar methods are used to release the other cell types from their matrices.
3.1.1. Organ culture
Historically, the earliest bone and tooth cultures were performed with limb rudiments or tooth buds 85-87. In general, the non-mineralized embryonic limbs, calvaria, or tooth organs are placed on grids and are maintained in tissue culture media for relatively short periods of time. The presence of mineral is usually determined histochemically or radiographically. These systems may also be used to study gene and protein expression during development and mineralization 88. The rudiment system has been recapitulated recently by Price’s group in a study of the effects of serum proteins on calcification 89-91.
The advantage of the organ culture systems is that the tissue shape is maintained, formation and remodeling can be evaluated in the sample and the system is less sensitive to media changes. On the other hand, the presence of small amounts of mineral already in the rudiments (which may be non-detectable) can easily serve as a nidus for further crystal proliferation, potentially invalidating the study. Cultured limb rudiments can be used for evaluation of both bone89-92 and cartilage calcification 93-95.
Fetal parietal (skull) bones will also mineralize in culture in the presence of 3 mM phosphate without any additional phosphate source 96. In fact, when these bones were cultured in the presence of 6 mM inorganic phosphate, or 1-10 mM BGP there was ectopic (unwanted/out of place) mineral deposition in areas of cell death and debris 96.
Tooth bud organ cultures are frequently used to study both dentin and enamel development. Embryonic tooth buds, or early post-natal tooth buds have been used. They are generally cultured in BGJb media (see Table 3) and mineralization monitored by radioactive nuclide uptake (45CaCl2 and 32PO4) and by light and electron microscopy 97. Pulp organ cultures can also be used to generate both dentin and enamel 98.
Table 3.
Ca x P Concentrations in Media used for Mineralization Studies
| Media | Culture System | Ca | P | CaxP (mM2) | Other factors that could affect mineralization |
|---|---|---|---|---|---|
| Alpha-MEM | All | CaCl2 1.8mM | NaH2PO4.H2O 1.0 mM | 1.80 | |
| DMEM | All | CaCl2 1.8mM | NaH2PO4.H2O 0.90mM | 1.62 | MgSO4 0.8mM |
| DMEM-F12 | Osteoblast | CaCl2 1.05 mM | NaH2PO4.H2O 0.45mM | 0.995 | MgSO4 0.4mM |
| Na2HPO4 0.5mM | MgCl2 0.3mM | ||||
| BGJb-Fitton Jackson modification 294 | Osteoblast | Ca -lactate 2.54 | NaH2PO4.H2O 4.065mM | 4.1 | MgSO4 0.8mM |
| CaCl2 1.25 mM | KH2PO4 1.0 mM | 5.0 | |||
| BGJb-Fitton Jackson modification 294 | Osteoblast | Ca -lactate | NaH2PO4.H2O 0.65mM | 4.1 | MgSO4 0.8mM |
| 2.54mM | KH2PO4 1.0mM | ||||
| CMRL | Osteoblast | CaCl2 1.8mM | NaH2PO4.H2O 1.0mM | 1.8 | MgSO4 0.8 mM |
| HAMS F-12 | Osteoblast | CaCl2 0.30 mM | NaH2PO4.H2O 0.86mM | 0.26 | MgCl2 0.60 mM |
| FeSO4.7H2O 0.003mM | |||||
| OptiMem | All | CaCl2 0.91mM | NaH2PO4.H2O 1.0 mM | 0.91 | Insulin & transferin supplements |
| Osteogenic Media | Osteoblast | CaCl2 1.8mM | 10mM BGP | 12.6a | nM dexamethasone |
| RPMI 1640 295 | Odontoblast | CaCl2 0.8mM | 5 mM Pi | 4.0 | |
| Low Ca High Pi Media | Chondrocyte | CaCl2 1.0 mM | 4 mM Pi | 4.0 | |
| Chondrocyte calcification media 295 | Chondrocyte | CaCl2 1.8mM | 5 mM BGP | 7.2a | 1,25 (OH)2D3 and 24,25(OH)2D3 |
| LHC-9 Lechner and LaVeck medium-9 296 | Ameloblast | ||||
| KGM-2 (low Ca) | Ameloblast | CaCl2 0.15 mM | Not specified | ? | bovine pituitary extract, human epidermal growth factor, insulin, hydrocortisone, epinephrine, transferrin, 10% FBS and 10 ng/ml |
| High Ca | CaCl2 2 mM | ||||
| High glucose DMEM | Mineralizing ameloblasts | 2.5 mM | NaH2PO4.H2O 1.0 mM | 2.5 | EGF 3 ng/ml TGF-β |
assumes 60% of BGP is hydrolyzed in media with 1.0 mM Pi.
If no reference provided data is available in the Invitrogen catalouge
3.1.2. Primary cells and undifferentiated (stem) cells
3.1.2.1. Stem Cells
Osteoblasts are derived from osteoprogenitor cells that exist in the bone marrow stroma and are referred to interchangeably as mesenchymal stem cells or marrow stromal cells (MSCs). Marrow suspensions were first reported to differentiate into osteoblast like cells when implanted in diffusion chambers 99. Based on histochemistry, both calcified cartilage and bone were formed. Extension of this work 100 demonstrated that the mineral formed in confluent cultures of MSCs was hydroxyapatite. The marrow suspensions can also differentiate into osteoclasts 101, cells of the macrophage lineage. Following the early reports, marrow stromal cells then became a standard for studying the effects of different agents on mineralization. However marrow stromal cells and mesenchymal stem cells (same abbreviation) are not the same thing. Embryonic MSCs are distinct from embryonic stem cells, which by definition, have the ability to differentiate into any cell type, but MSCs only make cells of the mesenchymal lineage. MSCs have been shown to form osteoblasts and make a mineralized matrix (based on histochemistry), and express early bone cell markers in culture with BGP, ascorbic acid, and 1,25-(OH)2 vitamin D3 102. The MSCs form fibroblasts, chondrocytes, adipocytes, hematopoetic cells, and osteoblasts 103, in the presence of appropriate growth factors 62,104,105.
Mesenchymal stem cells from limb buds as well as MSCs from marrow have been used as a source of cells for studying mineralization. Isolated from embryonic limb buds, they will differentiate into chondrocytes, osteoblasts, and adipocytes depending on the local environment and the nature of the growth factors present 106.
Dental pulp stem cells differentiate into odontoblasts and deposit a mineralized matrix in the presence of 10% fetal bovine serum, Dulbecco’s modified Eagle’s medium (DMEM), dentin extract, and the mineralization supplements ascorbic acid and 10 mM BGP 68. Raman microscopy was used to identify that these cells formed a hydroxyapatite containing mineralized matrix in culture.
3.1.2.2. Primary Cells
The most frequently used source of osteoblasts for studies of mineralization in culture are fetal calvarial cells. Primary osteoblasts can also be obtained from long bone and periosteum107. These cells are generally released by enzymatic digestion of the poorly mineralized calvaria but may be derived from explant cultures 108. Cultures of primary osteoblasts were described as undergoing two stages of mineralization; a proliferation phase which was independent of BGP and a mineral deposition phase which required BGP and responded in a dose-dependent manner to 1-14mM BGP. BGP in these cultures was almost completely hydrolyzed in 8 hrs 109, and mineralization could be blocked by inhibition of alkaline phosphatase during the first, but not the second phase110. Mineralization could equally be initiated and maintained by 2-5 mM inorganic phosphate 109.
The initial odontoblast primary cultures used methods derived from bone biology 111 to obtain cells from dental pulp. Pulp tissue from incisors of adult male rats were shown to mineralize in the presence of “osteogenic media” with a minimum of 5 mM BGP 112 although more recently Balic and Mina produced mineralization in similar cultures with 4 mM BGP 113. There are no reported studies of primary odontoblasts-mediated mineral deposition without dexamethasone and BGP.
For calcified cartilage formation chondrocytes are frequently isolated from the growth plates of young animals 114 or non-calcified regions of the ribs 115. In some cases, animals are made vitamin D deficient to inhibit cartilage calcification, and these rachitic animals provide the source of chondrocytes 116. Culturing of these cells in medium with BGP or inorganic phosphate, and either retinoic acid (to induce alkaline phosphatase expression) or ascorbic acid (to stimulate matrix formation), results in the deposition of mineral 117.
Primary cultured ameloblasts are reported to keep differentiated phenotype in vitro, including the expressions of ameloblast specific genes and the potential to form calcified nodules, but have restricted proliferation potential 118-120.
3.1.3. Cell Lines
Cell lines offer the advantage of reproducing the same phenotype every time they are grown under the same conditions, thus they are very useful for probing the effects of different genetic or chemical modifications on mineral deposition. However because they are cell lines they do not recapitulate the situation in vivo as closely as organ cultures, or even primary cultures. These cell lines are generally produced from tumor cells or by immortalization by a virus, and hence provide a more homogeneous population, although they are not regulated in the same way as primary cells. Techniques for generation cell lines are reviewed elsewhere 121,122.
There are numerous mesenchymal-cell lines that can be differentiated into osteoblasts and chondrocytes in different media. These include: ATDC5 123, C3H10T1/2124, HEPM (human embryonic palatal mesenchyme)125, HFOB1.1.9 (human fetal osteoblasts)126, MC3T3-E1127, 2T3128, Oct-1129, ROS 17/2.8130, SAOS-2131-133, TE-85134, and UMR 106135. Since mineralization has only been reported in some of these, this discussion will be restricted to those cell lines. The MCT3T-E1 cells isolated and cloned from newborn mouse calvarial cells 127,136 express all the bone phenotypic markers, and represent mature osteoblasts, but require additives (osteogenic media or BMP2) to reproducibly form hydroxyapatite mineral 137,138. BGP, ascorbic acid and dexamethasone are not mandatory additives to get mineralization in these cultures 139. The OCT-1 cells, well-differentiated secretory osteoblast-like cells isolated from rat calvaria, when cultured on a scaffold, deposit hydroxyapatite mineral 140. The rat osteosarcoma cell line (ROS 17/2.8) differentiates in the presence of mineral ions 141 but there are no reports of the analysis of the mineral formed in this system, although these cells form osteocalcin (which usually is a sign of mineral deposition 142) when treated with TGFbeta 143 and accumulate calcium in the presence of BGP 144. When implanted in diffusion chambers in vivo the ROS 17/2.8 cells form nodules identified as mineral by electron microscopy 145. SAOS-2 (human) and UMR-106 (rat) cells are both derived from osteosarcomas, and both deposit mineral in the presence of either 4 mM inorganic phosphate or 5-10mM BGP as demonstrated by calcein labeling 146, in response to 17-beta estradiol (SAOS-2) as evidenced by electron microscopy 147, or for the UMR106 cells by X-ray diffraction, TEM, and SEM 148.
In an interesting paper, 108,149 Cornellissen compared primary osteoblasts from both adult fetal rat calvaria and long bones and UMR 106 cells. While all cultures contained mineral based on histochemistry and infrared spectroscopy, alkaline phosphatase activity was reported to be essentially absent in the cells from the fetal calvaria. Calvarial cell also maintained lower alkaline phosphatase activities than the UMR106 cells, but collagen fibril formation (and mineralization of these fibrils) was not detected in the UMR106 cells and in the long bone derived cultures. The poor mineralization of the UMR106 cells most likely was due to the impaired collagen production. These findings are very important to note because they indicate that many of these cell lines, and even primary cultures from long bones, may not produce physiologic mineral, thus care must be taken in evaluating papers that report the presence of alkaline phosphatase as evidence of physiologic mineralization.
The osteocyte cell line MLO-A5 was established by Bonewald’s group 150,151 by cloning from cultures of long bone cells of transgenic mice expressing the SV40 Large T antigen oncogene under the control of an osteocalcin promoter. Osteocalcin mRNA is expressed just prior to mineralization, and the protein is expressed during mineralization 142. These cells express long processes comparable to dendrites, have low levels of alkaline phosphatase but high levels of the osteocyte phenotypic markers DMP-1, E11, and connexin 43 21, and mineralize spontaneously in culture 152. The mineral properties in these cultures were established by FTIR 152.
There are fewer detailed studies of mineralization in odontoblast cell lines. Magne et al 76 characterized mineralization in the M2H4 rat odontoblast cell line by FTIR microspectroscopy. The cells, cultured in alpha-MEM with additional 3mM inorganic phosphate at pH 7.3, in the presence of 10 ng/ml TGFbeta and 100 ng/ml BMP4, formed a mineralized matrix as shown by FTIR to be equivalent to that in rat dentin.
Odontoblast cell lines have also been established by a number of investigators. One of the first was derived by Panagakos 153 by transfecting primary cultures of human pulp cells with an SV40-adenovirus construct; the development of the transformed pulp cell (HPC-T) was followed by the development of the mouse MO6-G3 line produced by MacDougall for evaluation of gene expression 154. MacDougall also produced the M2H4 cell line 155 that mineralize in the presence of 3mM inorganic phosphate 76,156. George’s group developed a cell line immortalized by telomerase with a high proliferation potential, and the ability to make a mineralized matrix (based on von Kossa staining) in vitro 157. Immortalized odontoblasts were more recently established from porcine pulp 158. This cell line makes mineralized nodules, and expresses dentin specific markers dentin sialoprotein (DSP) and dentin matrix protein 1 (DMP1).
The pluripotent C3H10T1/2 cells can differentiate into osteoblasts 159, chondrocytes 160, adipocytes161, and odontoblasts 162. The requirements for inducing the expression of each of these cell types are quite variable. Where mineralization has been noted in these cultures, for osteoblasts both exogenous BMP2 and adenovirus expression of Runx2 or BMP2 have been used 159,163; for chondrocytes, BMP2 160,164, and for odontoblasts Wnt10 165. There is a long list of chondrocyte cell lines, reviewed elsewhere 166, but only a few of these, ATDC5 167, RCJ3.IC.18 168, and N1511 169 formed mineral deposits in the presence of dexamethasone and 10mM BGP as evidenced by histochemical stains and electron microscopy. The ADTC5 cells mineralize, as shown by FTIR, in long term insulin-treated culture (5 wks) both in the presence and absence of BGP 167.
Cementoblast cell lines 170, for example OCCM-30 171, a tumor derived cell line 172, and BCPb8 173, all have been shown to differentiate into cementoblasts and produce a mineralized matrix as identified by histochemical stains. The mineralization in the tumor cell line was shown to be apatitic by electron diffraction 172.
There are also several ameloblast cell-lines that have been used to study mineralization. There are reports on immortalization of ameloblast-lineage cells using T-antigen of SV40 or polyoma viruses 118,174, however while these cultures expressed phenotypic markers, their ability to support mineralization was not established. In contrast, Nakata et al 175 used low Ca (0.2 mM) media to develop a spontaneously immortalized cell line that expressed amelogenin, tuftelin, and enamelin and deposited mineral when 2.5 mM calcium was added to the culture in the absence of BGP.
3.2. Culture conditions
3.2.1. Confluent Cultures
There are a variety of ways in which single cells can be cultured resulting in a matrix that will mineralize. Most cultures are developed to confluence (i.e., 100% of the culture dish is covered). Cells generally have been shown to stop proliferating when matrix production begins. Some are plated in high density or left in suspension, sometimes within carriers, while others are placed upon a feeder culture. Each of these, under the proper conditions, can produce a mineralized matrix.
Some cultures require confluency to mineralize in a monolayer. This is true for both osteoblast cultures as well as chondrogenic cultures such as the ATDC5 cells 167. The cultured cells often form matrix vesicles (membrane bound bodies which bleb out from the cell bodies and provide a protected environment for initial nucleation) as well as an extracellular matrix and the cultures generally mineralize over a 3-5 week period depending on cell type.
3.2.2. Suspension Cultures
In suspension cultures, it has been shown that chondrocytes will form aggregates that can act more “tissue-like.” These chondrocytes will produce matrix and in turn mineralize that matrix. Embryonic stem cells have also been grown in suspension cultures which will then form embryonic bodies that can go on to differentiate into an ostegenic lineage and form mineral 176-179. In some instances groups have allowed embryonic bodies to remain in culture and then disrupted them in order to drive them down the ostegenic line 177. These have been shown to have mineralizing potential when they differentiate into hypertrophic chondrocytes or osteoblast-like cells, however this appears to require an anchorage dependent step 180,181.
3.2.3. High density cultures
The most common type of high density culture are micromass cultures 182. In this system a high density of cells is plated in a low volume of media and allowed to attach to the dish and to each other for a few hours before media is added. This method has been used extensively for primary chick 3-D cultures and for some mouse cultures 183,184. Each have been shown to mineralize 71,185,186 in the presence of inorganic phosphate or 2.5mM BGP using x-ray diffraction, TEM, and FTIR.
3.2.4. Encapsulation Culture
In many culture systems it has been shown that the morphology of the cell is important to gene expression and matrix production187. To provide both mechanical support and to deliver cells to an in vivo defect site, it can be beneficial to use “encapsulation” techniques. In these methods, cells are included in beads made from agarose, alginate, or other bio-compatible hydrogels. Beads are often small enough to be injectable, while maintaining the ability to support the growth and differentiation of cells and matrix. With these methods a large number of cells can be delivered or grown at once.
To promote a differentiated phenotype, alginate 188 and collagen-agarose beads have been used 35,189. These systems are based on the concept that cells maintained with a 3-D matrix will differentiate into mature chondrocytes or osteoblasts. It has been shown that this is a valuable way to encourage differentiation while preventing dedifferentiation of these cells into fibroblasts 187. It is also useful for driving them down an ostegenic lineage. In one recent study, murine embryonic stem cells encapsulated in alginate beads over one month in a rotating bioreactor were shown to mineralize in the presence of 10 mM BGP based on histochemistry, microCT and FTIR 190.
3.3. Matrices for cell culture
Matrices are primarily used to lend mechanical support to in vitro culture systems. They can be used to provide a three dimensional shape to a construct, aid in delivery of growth factors and other additives, as well as promote growth and differention of cells and matrix depending on the chemical and physical properties of the scaffold. Scaffolds are also very important because morphology and adhesion and the organization of cytoskeletal elements are very important to gene expression and matrix mineralization.
3.3.1. Plastic
Many studies of mineralization are carried out in dishes made from polystyrene (tissue culture plastic). This is very common when cells are grown in monolayer cultures for mineralization. There are commercially available coated surfaces which can aid in attachment and mineralization of cells. Such coatings include laminin, type I collagen, fibronectin, and matrigel, a basement membrane matrix containing growth factors. The proteins in these coatings have domains with cell binding (RGD) sequences that facilitate cell adherence. There are several examples where using tissue culture plastic coated with type I collagen191-193, fibronectin194,195, and matrigel165 promoted mineralization relative to uncoated dishes.
3.3.2. Polymers
There are various polymer scaffolds that have been used as scaffolds for in vitro growth and differentiation of cells. Many of these are resorbable scaffolds that are biocompatible and support cell growth and matrix accumulation. Poly-lactic acid (PLA) or poly-l-lactic acid and PLGA (poly-lactic glycolic acid) are commonly used polymers in tissue engineering techniques. In bone tissue engineering, PLA and PLGA scaffolds have been shown to support adhesion and proliferation of osteogenic cells.
3.3.3. Surface topography
There have been many studies that show that the topography of a substrate can affect mineralization. It has been shown that grooved polystyrene influences collagen alignment by osteoblast-like cells 196 and a number of studies show increases in mineralization with micropatterning or roughened growth surfaces197-200. The reports vary with the types of micro or nano patterning used; groove size can vary from millimeter all the way to nanometer scales resulting in differences in matrix alignment and bone nodule formation depending on the size and type of grooves199. Boyan’s group has shown that MG63 cells have a more differentiated osteoblast phenotype with greater alkaline phosphatase activity and osteocalcin production on roughened titanium surfaces than on smooth surfaces201. Boyan et al also correlated mineral:matrix ratio with FTIR imaging of titanium surfaces with microtopographies (grit blasted/acid etched or plasma sprayed) seeded with fetal rat calvarial cells and showed increased bone-like apatite deposition on these surfaces75. In another study that looked at surface topography, they took into account the hydrophobicity and hydrophilicity of pyramid-like structured polydimethylsilozane showing surface-dependent differences in mineral production by osteoblasts198. Because surface topography may influence differentiation, gene expression197,202, and matrix formation, it may be important in bone tissue engineering techniques as well as in osteointegration studies. Because there is not a great amount of uniformity in the studies it is still unclear as to exactly what type of patterning or grooving is preferred or necessary for mineralization.
3.3.4. Demineralized Bone and Ceramics
Although both demineralized bone and ceramics have been used as scaffolds for in vitro cell growth techniques, it is very difficult to determine the extent of mineralization on these scaffolds in part because the ceramics already consist of mineral or silicate glasses doped with mineral, thus it is difficult to distinguish new from pre-existing mineral. One example in which new mineral deposition (as opposed to the existing mineral in the ceramic) was characterized used SEM and EDAX to study rat osteoblast cells cultured with 10 mM BGP, ascorbate, and fetal calf serum. An electron dense layer formed on the surface of the ceramic consisted of a layer of collagen and then a layer of mineral crystals 203. Other studies use alkaline phosphatase activity and osteocalcin expression as a measure of mineralization, but these measures, especially in cultures with BGP, do not show physiologic mineral has formed. On the other hand, a phosphate-free ceramic was used to culture human osteoblasts without any additives (ascorbate, BGP, dexamethasone) and type I collagen was deposited prior to the formation of bone nodules 204. Since ascorbate is required for collagen hydroxylation, one wonders whether the inclusion of ascorbic acid might have enhanced the bone formation in these cultures. Unfortunately, the detailed properties of the mineral were not described.
3.3.5. Cultures with feeder layers
One of the ways to maintain cells before development is to maintain them on a feeder layer. This has been done with embryonic marrow stromal cells which differentiate in to osteoblasts 205 and with epithelial cells that differentiate into ameloblasts 206, or cells that produce ameloblast markers 207, and become mineralized.
3.4. Media for cell cultures
The chemical composition of the media used to induce and monitor mineralization of these different cell types is variable. Classically the liquid substance used to nurture the cells and to study mineralization was BJGb media with and without the Fitton-Jackson modification, or Dulbecco’s Modified Eagle medium (DMEM) and variations thereof (Table 3). Low phosphate media is often used for chondrocyte culture, and “osteogenic media” for osteoblast and odontoblast cultures. Several different such osteogenic media have been described. The first called “osteogenic media” contained 10% fetal calf serum (FCS) and 10nM vitamin D along with DMEM 208. In this media there was no added phosphate source, yet mesenchymal cells expressed osteoblast markers. Similarly chondrocyte cultures will mineralize without BGP present 209. In later studies “osteogenic media” consisted of 1-100nM dexamethasone or retinoic acid, 10mM BGP, and 50∞M ascorbate added to one of the basal media. Most recently, osteogenic media is defined as 100nM dexamethasone, 10mM BGP, and 50∞M ascorbate. The dexamethasone accelerates cell proliferation and expression of “osteogenic genes” 210.
The variation in the Ca x P products and other major ingredients of this media determine whether mineralization will occur spontaneously without cells or matrix (i.e. it is not physiologic). As is apparent from the Table 3, the major variables in these media are the Ca x P mM product, and the Ca/Mg ratio. Since at pH 7.4, solutions in which the Ca x P product exceeds 5.5 mM2 will precipitate unless mineralization inhibitors are present 211, it is obvious that many of the media used in cell culture are supersaturated with respect to hydroxyapatite. The addition of serum (which increases calcium concentration) but provides both mineralization inhibitors and promoters 91,212 as well as beneficial growth factors, can be avoided using a defined media, such as ITS 76 (insulin, transferrin, selenium) or other serum supplements. Many investigators add calcium or inorganic phosphate (or phosphate sources) to the media in addition to what is in the basal media. To insure that these media are not supersaturated with respect to hydroxyapatite, and that any mineral deposition observed is not artifact, control cultures without cells should be shown not to form mineral. However BGP supplements can deposit mineral if the enzyme alkaline phosphatase is present, even if cells are not 213.
It is important to comment on the use of BGP as a phosphate source, especially in terms of its concentration. Assuming 80% hydrolysis (higher proportions have been measured 109), if alkaline phosphatase is made by the cells, the solution will contain higher than 8mM phosphate concentrations, and most basal media have more than 1mM calcium making the deposition of hydroxyapatite inevitable and non-physiologic if the deposition does not occur on a proper matrix. Inorganic phosphate and other phosphate sources, ATP, phosphoenthanolamine, etc., have all been used in lower concentrations to elevate phosphate concentrations, but care must be taken that the media does not cause apatite precipitation. BGP has been used as a phosphate source at 2-10mM concentrations. The Canadian group found increased mineral deposition with 10mM BGP, and this soon became the classic “osteogenic media”. It was known early on, however, that if alkaline phosphatase was added to this media, without cells or matrix present, mineral deposition would occur 213. Thus many of the studies of osteogenic media provide proof that alkaline phosphatase activity is present in the cultures, not that physiologic mineral is being deposited, a feature that must be identified by one of the physicochemical approaches described previously. An alternate control would be media with phosphatase inhibitors, which would prevent hydrolysis of BGP 109,110.
The observation that increasing BGP concentration from 1-3mM 62 in marrow stromal cell cultures did not alter mineral properties suggests that where BGP levels are low (and where the CaxP mM product is below supersaturation), that BGP may be an acceptable source of inorganic phosphate for nucleation and growth of hydroxyapatite crystals. However high concentrations of BGP are problematic.
Cultures of ameloblasts and odontoblasts also often use “osteogenic media” yet the reason for this is not known. Interestingly, in all these cases there is mineral deposition, suggesting that there is some alkaline phosphatase activity. Den Besten has established media for growth of pre-ameloblasts and ameloblasts. LHC-9 media, which is selective for epithelial cells, maintains them in a confluent state, and exhibits strong expression of secreted amelogenin and ameloblastin proteins 119. More recently for cultures of epithelial cells released from embryonic tooth buds, she has used KGM-2 media with or without serum supplemented with 0.05mM calcium, and observed production of all markers of amelogenesis and no evidence of mesenchymal cell phenotypic markers 214. It is of interest to note that alkaline phosphatase is expressed during enamel/ameloblast maturation 215, indicating why users of BGP have observed mineralization in their cultures 216. However, the initial studies of ameloblast mediated mineralization found that mineral deposition occurred in alpha-MEM without supplemented phosphate 119.
3.5. Other Additives
One of the advantages of studying mineralizing in culture is the ability to modify the environment in which mineralization is occurring to test hypotheses about the factors affecting mineral deposition. Culture media is usually supplemented with serum (to provide needed growth factors) or cocktails containing specific growth factors. Some of these, along with proteins in the serum can affect the mineralization process and it is important to verify that observed effects are not simply due to the interaction of the additive with mineral crystals. More importantly, in culture one can vary oxygen tension, pH, or block the expression of specific genes hypothesized to be crucial in inducing the formation of mineral or regulating the proliferation of mineral crystals.
3.5.1. Serum
Most culture systems include 1-20 percent of bovine or fetal calf serum, while the majority of studies use 10 percent fetal bovine serum. The reason for including serum is to enhance exposure of the cell to cytokines and growth factors that will aid in growth and differentiation. The caveat is the tremendous variability in the composition of fetal calf serum 217, and that serum contains detectable amounts of calcium making it essential to measure total CaxP of the “full media” after addition of all materials to ensure that the solution is not supersaturated.
3.5.2. Antibiotics
To prevent bacterial and fungi growth antibiotics (penicillin and streptomycin) and antimycotics (fungizone) are often added to culture media. These reagents do not generally affect mineral ion concentrations in the media and will not be further discussed. Some metabolites such as glutamine (low in most media) or glucose are added to mineralizing cultures to maximize metabolism; these are reviewed in detail elsewhere 218.
3.5.3. Other additives
To address questions about effects of growth factors, cell signaling, or specific proteins on mineralization, such factors may be added exogenously, antagonists to their receptors added to alter signaling, silencing RNA or viral transcripts included to alter expression, or blocking antibodies added to test the effect of specific proteins. While these generally do not alter the supersaturation of the solution, they can and do alter the rate of mineral deposition, and the physicochemical properties of the matrix. Despite the fact that these additives may not generally alter supersaturation, it is urged that control, non-mineralizing cultures, always be included. Table 4 provides examples of factors that have been included in mineralizing cultures resulting in alteration of the mineral formed in culture.
Table 4.
Growth factors and CytokinesExogenous Agents that Affect Mineralization in Culturea
| Factor | System | Effect on Mineralization | Reference |
|---|---|---|---|
| TGF beta | Primary Osteoblast | Decrease | 297 |
| FGF | Primary Osteoblast | Decrease | 297 |
| FGF-2 | Tooth Buds | Decrease | 298 |
| 1,25D3 & 24,25D3 | Primary Osteoblast | Increase | 297,299 |
| BMP2 | MC3T3; Primary osteoblast | Increase | 81,137,300 |
| BMP6 | Chondrocyte | Increase | 72 |
| BMP7 | Chondrocyte | Decrease | 301 |
| Dexamethasone | Osteoblasts, Odontoblasts, Chondrocyte cell lines, MSCs | Increase or Decrease (depends on cell maturity) |
302 265 |
| Estrogen | Bone organ culture | Increase | 303 |
| FGF | Primary Osteoblast | Decrease | 297 |
| FGF-2 | Tooth Buds | Decrease | 298 |
| IL-1 b | Periodontal ligament cells | Decrease | 304 |
| IL-6 | Osteoblasts | Decrease | 253,305 |
| LMP | Mesenchymal stem cells | Increase | 306 |
| Leptin | Stem Cells | Increase | 307 |
| Osteoprotegrin | Tooth Buds | Decrease | 308 |
| IL-1 b | Periodontal ligament cells | Decrease | 304 |
| IL-6 | Osteoblasts | Decrease | 253,305 |
| Dexamethasone | Osteoblasts, Odontoblasts | Increase | 302 |
| PTH | MCT3TE1 cells | Increase | 309,310 |
| Primary chondrocytes | Increase | 311 | |
| Chondrocytes | Retard | ||
| PGE2 | Osteoblasts, Cementoblast cell line | Increase | 312 |
| Retinoic acid | Osteoblasts, Periodontal ligament cells | Decrease | 313,314 |
| Chondrocytes, | Increase | 117,315 | |
| Osteoblast cell line | Decrease | 316 | |
| Runx2 | Osteoblasts | Increase | 264 |
| Fibroblasts | No Effect | ||
| 1,25dihydroxy VitaminD3 & 24,25dihydroxy VitaminD3 | Primary Osteoblast | Increase | 297,299 |
see review by Declercq 84 for concentrations of many of these additives that are used in osteoblast cultures
3.6. Physical Factors
3.6.1. Oxygen tension and pH
While most culture studies are performed in incubators with 5% CO2 and media at pH 7.4, there have been some studies where the oxygen tension is reduced to mimic the conditions thought to exist in calcifying cartilage 219, or the hypoxia hypothesized to exist in osteocytes 220. Hypoxia in cultures of an osteoblast cell line (MC3T3) in the presence of 10mM BGP resulted in increased accumulation of alizarin red staining (for mineral), and expression of osteocalcin, MEPE, connexin 43, DMP1, and FGF23, all osteocyte markers, suggesting that hypoxia accelerated the formation of osteocytes 220. In contrast, hypoxia in cultures of chondrocyte cell lines 221 increases matrix synthesis but retards transformation of cells to hypertrophic chondrocytes and thereby retards mineralization. In certain cell lines such as the ATDC5 cells, which have been shown to undergo chondrogenic differentiation, 3% CO2 is regularly used to induce mineralization along with other additives to the media 167.
The supersaturation of the media is very dependent on pH. However, to mimic the pH in body fluids, the media pH may be increased to be closer to the 7.6 values found at mineralizing sites 222. Reducing the pH to mimic acidotic conditions, reduces mineral accumulation in osteoblast cultures 223.
3.6.2. Pressure, Loading, and Perfusion
Pressure increases mineralization during in vitro loading of cartilage organ cultures as it mimics in vivo loading models 224. These methods are not only useful in looking at the mechanisms and factors affecting mineralization in vitro, but also in creating tissue engineered bone constructs and understanding therapies for fracture healing. In tissue engineering techniques, one group has looked at both the effects of hydrodynamic compression as well as cell stretching on osteoblasts grown on titanium coated scaffolds. The effect of compression was dependent on cell type, and the effects of stretching were dependent on intermittent versus static loading techniques 225-227. These compression methods may be modulated to create different properties of in vitro grown tissues.
There have been a few systems that use low pressure and/or perfusion to seed cells into scaffolds 228,229. Such systems do not necessarily mimic in vivo loading, but enhance cell seeding or nutrient delivery to the scaffolds. These methods have also been shown to increase mineralization 230. In more traditional tissue engineering techniques, cyclic mechanical compression has been shown to increase mineralization in polymer seeded scaffolds 231,232.
3.6.3. Hypogravity
During space flight, life on the International Space Station, and travel on the space shuttle there is a reduction in gravity. This hypogravity has been shown to decrease mineralization and increase calcium release in mouse long bone organ cultures 233 and in chick osteoblasts 234. Mineral resorption is also increased in hypogravity 235. It was not clear, however from these studies if the harsh launch conditions, rather than the exposure to hypogravity caused the reduction in cell proliferation, matrix production, and mineralization. Culture studies planned for the International Space Station should address these issues, however to date this information is not available.
4. What sorts of questions can successfully be addressed by culture studies
The major limitations of cell culture studies is that unlike in the body dead cells are not removed, and other cells with which there are interactions in situ are not generally present. While organ culture avoids the second limitation, it too is subject to the first problem. The other drawback is that the selections of culture conditions determine the final observations. There are questions that have been answered in vitro that are difficult or almost impossible to answer in animals. For example, where knockout or transgenic animals with proposed mineral defects are nonviable, cultures of fetal cells have been useful in providing insight into the effects of such proteins (Table 5).
Table 5.
In vitro Effects of Modifying Proteins Linked to Mineralization
| Protein | Culture System | Evaluation Method | Observed effect | Reference |
|---|---|---|---|---|
| Alkaline phosphatase | Osteoblasts | KO | No mineralization | 240,317 |
| MC3T3-E1 cells | Antisense | Decreased mineral | 185 | |
| Chondrocytes | Levamisole | No effect | ||
| Alpha-2HS glycoprotein | Osteoblasts | Addition | Inhibition | 318 |
| Amelogenin | Organ culture | Antisense | Inhibition of growth | 239 |
| BAG-75 | UMR-106 cells | Laser capture | Decreased mineral | 319,320 |
| Osteoblasts | Proteolytic degradation | Decreased | ||
| Bone sialoprotein | Chondrocytes | Immunoblocking | Decreased | 191,252,321 |
| Osteoblasts | RNAi | Decreased | 321 | |
| MTC3T3-E1 cells | Overexpression | Increased | ||
| Collagen I | Chondrocyte | Immunoblocking | Inhibition | 191 |
| DSPP | Stromal cells | Overexpression | Enhanced | 274 |
| Matrix gla-protein | Chondrocyte | KO | Accelerated mineralization | 322 |
| Osteocalcin | Tooth buds | Study of KO; blockade of carboxylation with warfarin | None | 237 |
| Osteopontin | Osteoblast | Overexpression | Decreased | 323,324 |
| Vascular smooth muscle cells | Immunoblocking | Increased | ||
| Phosphophoryn | Fibroblasts | Overexpression | Increased | |
| Proteoglycans | Chondrocytes | Degradation | Increased | 186 |
| Thrombospondin 1 | MC3T3E1 cells | Antisense to TSP1 | Increased | 241 |
| Overexpression | Decreased | |||
| Exogenous addition | Decreased |
Similarly, use of antisense RNA, siRNA, and antibody blocking can be used to prevent the expression of specific proteins or exposure of those proteins to the environment. But transfection efficiency varies among cell types, and often siRNA and even when methodologies are optimized these techniques may not give complete inhibition 236. Addition of reagents that might have affects on other organ system can be used to demonstrate the importance of certain enzymes, matrix proteins, and cellular organelles in the mineralization process. Such studies avoid the need to sacrifice numerous animals, enables exogenous factors to be examined, and provide greater reproducibility because of the innate heterogeneity of even inbred animals.
Some of the earliest studies on the effect of matrix proteins in culture were performed by Bronckers et al 237 who used organ culture of hamster tooth germs to demonstrate that exogenous osteocalcin had no effect on dentinogenesis or dentin mineralization. Later studies, summarized in Table 5 used genetic manipulation (overexpression, gene ablation) or chemical treatment to modify the amount and structure of different proteins.
There are a variety of techniques for blocking expression of specific proteins, either by binding an antisense chain to the proliferating RNA chain, or knocking down its expression with siRNA, shRNA, and iRNA 238. Antisense techniques for blocking RNA expression of a specific gene with small RNA segments (18-24 mers) was useful for showing that amelogenin could regulate hydroxyapatite crystal morphology 239, or for demonstrating that blocking alkaline phosphatase expression 240 and thrombospondin 1 expression 241, respectively in MC3T3-E1 cultures resulted in decreases and increases in mineral accretion. Interference with RNA expression by small-interfering (si)RNA knockdown 238,242 has been used to demonstrate the importance of the Runx proteins in chondrocyte maturation and mineralization 184 and of annexin V for matrix-vesicle induced mineralization by chondrocytes 243, while short interfering RNA (siRNA) was used to demonstrate that the calcium binding protein S100A4 when knocked down caused increased expression of osteoblastic markers suggesting that a function of S100A4 may be to inhibit mineralization by blocking expression of osteoblastic genes 244. Small hairpin RNA (shRNA) blocking the formation of the transcription factor Lef1 in osteoblasts accelerated matrix mineralization 245, while knockdown of bone sialoprotein in osteoblast cultures with shRNA inhibited matrix mineralization. In all cases, knockdown with short or hairpain RNA segments is rarely 100 % effective, thus mandating confirmation by other techniques. More importantly, sustained knockdown during long term cultures is difficult to achieve and may be confounded by “off target” knockdown (or knockdown of genes other than that designated for ablation), again requiring use of other validation techniques.
Transfection with viral or non-viral agents (such as calcium phosphate granules 246 and related strategies 247) is frequently used to over express regulatory factors in culture, and as reviewed elsewhere has been used in tissue engineering to over express certain growth factors in preparation for implantation into ectopic sites 248. These implanted constructs may not be mineralized, but it is hoped that they may be used in the regeneration of bone and other mineralized tissues. The caution is that some cells, such as hematopoietic cells, have low transfectability 249 and other cells, such as mesenchymal stem cells, may require unique methods to allow expression of specific gene products at physiologic levels 247. It is imperative that the level of expression not exceed physiologic values, as the effects of exposing cells to supra-physiologic levels of growth factors has not been well documented, although it has been shown that exogenous growth factor delivery alters the expression of other cytokines and their receptors and the cellular phenotype in 3D (alginate) cultures 250.
Antibody blocking has been used to study cell-based factors 251 and extracellular matrix proteins 252. For example, immunoblocking of type I collagen in differentiating mesenchymal cell micromass cultures retarded mineralization 191 suggesting a role for type I collagen in cartilage calcification; blocking Leukemia Inhibitor Factor (LIF) in osteoblast cultures increased the number of calcifying nodules 253. Other examples are summarized in Table 5. There are limits to these methods also, first because the antibody itself might interact with the mineral; second, because the antibody might not react with those epitopes of the protein that are involved in the mineralization process, thus a negative result might be refuted by an independent method, and finally, antibodies are not always available, and even when available may not penetrate the matrix.
5. Conclusions: Advice for cell culture studies of calcification
As stated throughout this chapter, the authors strongly believe that the mineralized tissue formed in culture should compare closely with that which exists in nature. This means that for bone, dentin, and cementum, hydroxyapatite mineral crystals should be found oriented with respect to the axes of the collagen fibrils. This in turn mandates that the cultures be examined in some way that will reveal this orientation (by scanning or transmission electron microscopy or AFM). Second, the hydroxyapatite mineral that is formed should be poorly crystalline, carbonate containing, with substitutions in its lattice. This can be demonstrated by a combination of a variety of techniques, including but not limited to chemical analysis of Ca/P ratio, x-ray diffraction, and/or vibrational spectroscopy.
In the case of enamel the mineralized matrix should contain few proteins when the cell mediated process is complete, and the hydroxyapatite crystals should be approximately 10x as long as those found in bone and dentin. Cell processes and membranes should not be included in the final matrix.
Finally, and perhaps most important, it is crucial that the process of mineralization being studied is cell-mediated. Thus control cultures without cells should not form mineral precipitates (as is the case in cell-free cultures with BGP and exogenous alkaline phosphatase 213 or cell-free cultures on collagen sponges that have not been appropriately washed 254). Cultures should not form mineral if matrix proteins are inactive, which may be demonstrated by blocking RNA and protein synthesis with agents such as actinomycin and cycloheximide, respectively.
This brings us to the final point of the review. BGP, in the authors’ opinion, is not a necessary component of “osteogenic media”. BGP can be replaced with physiologic levels of inorganic phosphate or other phosphate esters (ATP, pyridoxal phosphate, phosphoserine, etc. 255), and mineralization will occur in the absence of BGP 191,204. In addition, expression of some key genes relevant to mineralization has been shown to be comparable in mineralizing cultures with BGP or inorganic phosphate 72,185,256. Thus, authors and readers alike should remember that the presence of hydroxyapatite in cultures with BGP simply indicates the presence of alkaline phosphatase or other phosphatase activity, especially since mineralization does not occur in these systems when the phosphatases are inhibited.
6. Acknowledgements
Dr. Boskey and Dr. Roy’s data as presented in this review was supported by NIH grant AR037661. Imaging data was collected in the NIH sponsored Musculoskeletal Repair and Regeneration Core Center (AR046121). The authors appreciate the technical assistance of Lyudmila Spevak, Lyudmila Lukashova, and Hayat Taleb.
Biography
 Adele L. Boskey received her B.A. from Barnard College in 1964 and a Ph.D. in physical chemistry from Boston University in 1970. Dr. Boskey has been director of the Mineralized Tissue Laboratory at the Hospital for Special Surgery since 1999 where she is the Starr chair in mineralized tissue research and director of the Musculoskeletal Integrity Program. At Weill Medical College and Graduate School of Medical Sciences at Cornell University she is a Professor of Biochemistry and Professor in the graduate field of Physiology, Biophysics, and Systems Biology. She is also a Professor in the Biomedical Engineering Field at Cornell, Ithaca, and an adjunct Professor of Biomedical Engineering at City University of New York. She became a fellow of the AAAS in 2005, and serves on the scientific advisory board of Isotis, Skelescan, and RPI Bioengineering Department. A recipient of a Career Development Award from the National Institutes of Health-National Institute of Dental Research in 1975, an Award for Distinguished Research in Orthopedics from the Kappa Delta Sorority in 1979; and an NIH Merit Award as well as the Basic Research in Biological Mineralization Award, International Association for Dental Research in 1994, Dr. Boskey was the first woman president of the Orthopaedic Research Society, and is currently President of the International Conferences on the Chemistry and Biology of Mineralized Tissues. Dr. Boskey’s research is concerned with the mechanisms of biomineralization of bones and teeth. She was the first to apply the techniques of infrared microspectroscopy and infrared imaging to mineralized tissues, and is now using this technique to gain insights into changes in bone mineral and matrix properties in osteoporosis in the presence and absence of therapeutic interventions. The author of more than 200 peer reviewed publications, Dr. Boskey is the PI on three NIH R01 awards, and is the PI of a P30 Musculoskeletal Repair and Regeneration Core Center.
Adele L. Boskey received her B.A. from Barnard College in 1964 and a Ph.D. in physical chemistry from Boston University in 1970. Dr. Boskey has been director of the Mineralized Tissue Laboratory at the Hospital for Special Surgery since 1999 where she is the Starr chair in mineralized tissue research and director of the Musculoskeletal Integrity Program. At Weill Medical College and Graduate School of Medical Sciences at Cornell University she is a Professor of Biochemistry and Professor in the graduate field of Physiology, Biophysics, and Systems Biology. She is also a Professor in the Biomedical Engineering Field at Cornell, Ithaca, and an adjunct Professor of Biomedical Engineering at City University of New York. She became a fellow of the AAAS in 2005, and serves on the scientific advisory board of Isotis, Skelescan, and RPI Bioengineering Department. A recipient of a Career Development Award from the National Institutes of Health-National Institute of Dental Research in 1975, an Award for Distinguished Research in Orthopedics from the Kappa Delta Sorority in 1979; and an NIH Merit Award as well as the Basic Research in Biological Mineralization Award, International Association for Dental Research in 1994, Dr. Boskey was the first woman president of the Orthopaedic Research Society, and is currently President of the International Conferences on the Chemistry and Biology of Mineralized Tissues. Dr. Boskey’s research is concerned with the mechanisms of biomineralization of bones and teeth. She was the first to apply the techniques of infrared microspectroscopy and infrared imaging to mineralized tissues, and is now using this technique to gain insights into changes in bone mineral and matrix properties in osteoporosis in the presence and absence of therapeutic interventions. The author of more than 200 peer reviewed publications, Dr. Boskey is the PI on three NIH R01 awards, and is the PI of a P30 Musculoskeletal Repair and Regeneration Core Center.
 Rani Roy received her B.S. in Biomedical Engineering from Columbia University in 2000. She received her Ph.D. in Biomedical Engineering from Cornell University in 2006. Dr. Roy’s previous research interests focused on tissue engineering of cartilage using collagen gels. She developed a novel method of non-enzymatic glycation to stiffen type I collagen gels as scaffolds for cartilage repair models. Dr. Roy was awarded a NASA Graduate Student Researcher’s Fellowship (2003-2005) during her Ph.D. Her Ph.D. work naturally led to an interest in the musculoskeletal system and a post-doctoral fellowship in bone research at Hospital for Special Surgery in New York City. She currently works for Adele L. Boskey in the Mineralized Tissue Laboratory at HSS on a murine micromass tissue culture model of endochondral ossification. She resides in Manhattan with her husband and two-year old son.
Rani Roy received her B.S. in Biomedical Engineering from Columbia University in 2000. She received her Ph.D. in Biomedical Engineering from Cornell University in 2006. Dr. Roy’s previous research interests focused on tissue engineering of cartilage using collagen gels. She developed a novel method of non-enzymatic glycation to stiffen type I collagen gels as scaffolds for cartilage repair models. Dr. Roy was awarded a NASA Graduate Student Researcher’s Fellowship (2003-2005) during her Ph.D. Her Ph.D. work naturally led to an interest in the musculoskeletal system and a post-doctoral fellowship in bone research at Hospital for Special Surgery in New York City. She currently works for Adele L. Boskey in the Mineralized Tissue Laboratory at HSS on a murine micromass tissue culture model of endochondral ossification. She resides in Manhattan with her husband and two-year old son.
References
- (1).McCauley LK. Curr. Opin. Rheumatol. 2001;13:316. doi: 10.1097/00002281-200107000-00014. [DOI] [PubMed] [Google Scholar]
- (2).Thyagarajan T, Totey S, Danton MJ, Kulkarni AB. Crit. Rev. Oral Biol. Med. 2003;14:154. doi: 10.1177/154411130301400302. [DOI] [PubMed] [Google Scholar]
- (3).Kimelman N, Pelled G, Helm GA, Huard J, Schwarz EM, Gazit D. Tissue Eng. 2007;13:1135. doi: 10.1089/ten.2007.0096. [DOI] [PubMed] [Google Scholar]
- (4).Langer R, Vacanti JP. Science. 1993;260:920. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- (5).Barkana I, Alexopoulou E, Ziv S, Jacob-Hirsch J, Amariglio N, Pitaru S, Vardimon AD, Nemcovsky CE. J. Clin. Periodontol. 2007;34:599. doi: 10.1111/j.1600-051X.2007.01076.x. [DOI] [PubMed] [Google Scholar]
- (6).Noth U, Osyczka AM, Tuli R, Hickok NJ, Danielson KG, Tuan RS. J. Orthop. Res. 2002;20:1060. doi: 10.1016/S0736-0266(02)00018-9. [DOI] [PubMed] [Google Scholar]
- (7).Qi H, Aguiar DJ, Williams SM, La Pean A, Pan W, Verfaillie CM. Proc. Natl. Acad. Sci. U. S. A. 2003;100:3305. doi: 10.1073/pnas.0532693100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Hanagata N, Takemura T, Monkawa A, Ikoma T, Tanaka J. J. Biomed. Mater. Res. A. 2007;83:362. doi: 10.1002/jbm.a.31240. [DOI] [PubMed] [Google Scholar]
- (9).Bonewald LF, Harris SE, Rosser J, Dallas MR, Dallas SL, Camacho NP, Boyan B, Boskey A. Calcif. Tissue Int. 2003;72:537. doi: 10.1007/s00223-002-1057-y. [DOI] [PubMed] [Google Scholar]
- (10).Boskey A. Elements. 2007;3:387. [Google Scholar]
- (11).Wiesmann HP, Meyer U, Plate U, Hohling HJ. Int. Rev. Cytol. 2005;242:121. doi: 10.1016/S0074-7696(04)42003-8. [DOI] [PubMed] [Google Scholar]
- (12).Margolis HC, Beniash E, Fowler CE. J. Dent. Res. 2006;85:775. doi: 10.1177/154405910608500902. [DOI] [PubMed] [Google Scholar]
- (13).Bartlett JD, Ganss B, Goldberg M, Moradian-Oldak J, Paine ML, Snead ML, Wen X, White SN, Zhou YL. Curr. Top. Dev. Biol. 2006;74:57. doi: 10.1016/S0070-2153(06)74003-0. [DOI] [PubMed] [Google Scholar]
- (14).Mackie EJ. Int. J. Biochem. Cell Biol. 2003;35:1301. doi: 10.1016/s1357-2725(03)00107-9. [DOI] [PubMed] [Google Scholar]
- (15).Gerstenfeld LC, Shapiro FD. J. Cell Biochem. 1996;62:1. doi: 10.1002/(SICI)1097-4644(199607)62:1%3C1::AID-JCB1%3E3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- (16).Linde A, Goldberg M. Crit. Rev. Oral Biol. Med. 1993;4:679. doi: 10.1177/10454411930040050301. [DOI] [PubMed] [Google Scholar]
- (17).Klein-Nulend J, Nijweide PJ, Burger EH. Curr. Osteoporos. Rep. 2003;1:5. doi: 10.1007/s11914-003-0002-y. [DOI] [PubMed] [Google Scholar]
- (18).Morinobu M, Ishijima M, Rittling SR, Tsuji K, Yamamoto H, Nifuji A, Denhardt DT, Noda M. J. Bone Miner. Res. 2003;18:1706. doi: 10.1359/jbmr.2003.18.9.1706. [DOI] [PubMed] [Google Scholar]
- (19).Toyosawa S, Shintani S, Fujiwara T, Ooshima T, Sato A, Ijuhin N, Komori T. J. Bone Miner. Res. 2001;16:2017. doi: 10.1359/jbmr.2001.16.11.2017. [DOI] [PubMed] [Google Scholar]
- (20).Yang W, Lu Y, Kalajzic I, Guo D, Harris MA, Gluhak-Heinrich J, Kotha S, Bonewald LF, Feng JQ, Rowe DW, Turner CH, Robling AG, Harris SE. J. Biol. Chem. 2005;280:20680. doi: 10.1074/jbc.M500104200. [DOI] [PubMed] [Google Scholar]
- (21).Zhang K, Barragan-Adjemian C, Ye L, Kotha S, Dallas M, Lu Y, Zhao S, Harris M, Harris SE, Feng JQ, Bonewald LF. Mol. Cell Biol. 2006;26:4539. doi: 10.1128/MCB.02120-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).van Bezooijen RL, Roelen BA, Visser A, van der Wee-Pals L, de Wilt E, Karperien M, Hamersma H, Papapoulos SE, ten Dijke P, Lowik CW. J. Exp. Med. 2004;199:805. doi: 10.1084/jem.20031454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Bosshardt DD. J. Dent. Res. 2005;84:390. doi: 10.1177/154405910508400501. [DOI] [PubMed] [Google Scholar]
- (24).Hubbard MJ. Connect. Tissue. Res. 1998;38:17. doi: 10.3109/03008209809017013. [DOI] [PubMed] [Google Scholar]
- (25).Michael Parfitt A. Bone. 2006;39:1170. doi: 10.1016/j.bone.2006.06.031. [DOI] [PubMed] [Google Scholar]
- (26).Teitelbaum SL. Am. J. Pathol. 2007;170:427. doi: 10.2353/ajpath.2007.060834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Mackie EJ, Ahmed YA, Tatarczuch L, Chen KS, Mirams M. Int. J. Biochem. Cell Biol. 2008;40:46. doi: 10.1016/j.biocel.2007.06.009. [DOI] [PubMed] [Google Scholar]
- (28).Wilson A. Elements of X-Ray Crystallography. Addison Wesley; Reading, MA: 1970. [Google Scholar]
- (29).Politi Y, Arad T, Klein E, Weiner S, Addadi L. Science. 2004;306:1161. doi: 10.1126/science.1102289. [DOI] [PubMed] [Google Scholar]
- (30).Weiner S. Bone. 2006;39:431. doi: 10.1016/j.bone.2006.02.058. [DOI] [PubMed] [Google Scholar]
- (31).Aparicio S, Doty SB, Camacho NP, Paschalis EP, Spevak L, Mendelsohn R, Boskey AL. Calcif. Tissue Int. 2002;70:422. doi: 10.1007/s00223-001-1016-z. [DOI] [PubMed] [Google Scholar]
- (32).Landis WJ, Burke GY, Neuringer JR, Paine MC, Nanci A, Bai P, Warshawsky H. Anat. Rec. 1988;220:233. doi: 10.1002/ar.1092200303. [DOI] [PubMed] [Google Scholar]
- (33).Landis WJ, Hodgens KJ. Anat. Rec. 1990;226:153. doi: 10.1002/ar.1092260205. [DOI] [PubMed] [Google Scholar]
- (34).Boskey AL, Stiner D, Doty SB, Binderman I, Leboy P. Bone Miner. 1992;16:11. doi: 10.1016/0169-6009(92)90819-y. [DOI] [PubMed] [Google Scholar]
- (35).Hunter GK, Holmyard DP, Pritzker KP. J. Cell Sci. 1993;104(Pt 4):1031. doi: 10.1242/jcs.104.4.1031. [DOI] [PubMed] [Google Scholar]
- (36).Nakagawa Y, Shimizu K, Hamamoto T, Kotani S, Yamamuro T. Calcif. Tissue Int. 1993;53:127. doi: 10.1007/BF01321891. [DOI] [PubMed] [Google Scholar]
- (37).Saruwatari L, Aita H, Butz F, Nakamura HK, Ouyang J, Yang Y, Chiou WA, Ogawa T. J. Bone Miner. Res. 2005;20:2002. doi: 10.1359/JBMR.050703. [DOI] [PubMed] [Google Scholar]
- (38).Akhouayri O, Lafage-Proust MH, Rattner A, Laroche N, Caillot-Augusseau A, Alexandre C, Vico L. J. Cell Biochem. 1999;76:217. doi: 10.1002/(sici)1097-4644(20000201)76:2<217::aid-jcb6>3.3.co;2-b. [DOI] [PubMed] [Google Scholar]
- (39).Potter K, Leapman RD, Basser PJ, Landis WJ. J. Bone Miner. Res. 2002;17:652. doi: 10.1359/jbmr.2002.17.4.652. [DOI] [PubMed] [Google Scholar]
- (40).Hao J, Shi S, Niu Z, Xun Z, Yue L, Xiao M. Eur. J. Oral Sci. 1997;105:318. doi: 10.1111/j.1600-0722.1997.tb00247.x. [DOI] [PubMed] [Google Scholar]
- (41).Fincham AG, Moradian-Oldak J, Simmer JP, Sarte P, Lau EC, Diekwisch T, Slavkin HC. J. Struct. Biol. 1994;112:103. doi: 10.1006/jsbi.1994.1011. [DOI] [PubMed] [Google Scholar]
- (42).Wen HB, Moradian-Oldak J, Fincham AG. Biomaterials. 1999;20:1717. doi: 10.1016/s0142-9612(99)00085-x. [DOI] [PubMed] [Google Scholar]
- (43).Beniash E, Simmer JP, Margolis HC. J. Struct. Biol. 2005;149:182. doi: 10.1016/j.jsb.2004.11.001. [DOI] [PubMed] [Google Scholar]
- (44).Bohic S, Pilet P, Heymann D. Biochem. Biophys. Res. Commun. 1998;253:506. doi: 10.1006/bbrc.1998.9781. [DOI] [PubMed] [Google Scholar]
- (45).Janssen FW, Oostra J, Oorschot A, van Blitterswijk CA. Biomaterials. 2006;27:315. doi: 10.1016/j.biomaterials.2005.07.044. [DOI] [PubMed] [Google Scholar]
- (46).Venugopal J, Low S, Choon AT, Kumar AB, Ramakrishna S. J. Biomed. Mater. Res. A. 2008;85:408. doi: 10.1002/jbm.a.31538. [DOI] [PubMed] [Google Scholar]
- (47).Rohde M, Mayer H. Calcif. Tissue Int. 2007;80:323. doi: 10.1007/s00223-007-9013-5. [DOI] [PubMed] [Google Scholar]
- (48).Santos NC, Castanho MA. Biophys. Chem. 2004;107:133. doi: 10.1016/j.bpc.2003.09.001. [DOI] [PubMed] [Google Scholar]
- (49).Domke J, Dannohl S, Parak WJ, Muller O, Aicher WK, Radmacher M. Colloids Surf. B. Biointerfaces. 2000;19:367. doi: 10.1016/s0927-7765(00)00145-4. [DOI] [PubMed] [Google Scholar]
- (50).Ismail FS, Rohanizadeh R, Atwa S, Mason RS, Ruys AJ, Martin PJ, Bendavid A. J. Mater. Sci. Mater. Med. 2007;18:705. doi: 10.1007/s10856-006-0012-2. [DOI] [PubMed] [Google Scholar]
- (51).Guo X, Gough JE, Xiao P, Liu J, Shen Z. J. Biomed. Mater. Res. A. 2007;82:1022. doi: 10.1002/jbm.a.31200. [DOI] [PubMed] [Google Scholar]
- (52).Kirkham J, Brookes SJ, Shore RC, Bonass WA, Smith DA, Wallwork ML, Robinson C. Connect. Tissue. Res. 1998;38:91. doi: 10.3109/03008209809017025. [DOI] [PubMed] [Google Scholar]
- (53).Chen H, Clarkson BH, Sun K, Mansfield JF. J. Colloid Interface. Sci. 2005;288:97. doi: 10.1016/j.jcis.2005.02.064. [DOI] [PubMed] [Google Scholar]
- (54).Habelitz S, Denbesten PK, Marshall SJ, Marshall GW, Li W. Orthod. Craniofac. Res. 2005;8:232. doi: 10.1111/j.1601-6343.2005.00343.x. [DOI] [PubMed] [Google Scholar]
- (55).Habelitz S, Kullar A, Marshall SJ, DenBesten PK, Balooch M, Marshall GW, Li W. J. Dent. Res. 2004;83:698. doi: 10.1177/154405910408300908. [DOI] [PubMed] [Google Scholar]
- (56).Mankani MH, Kuznetsov SA, Wolfe RM, Marshall GW, Robey PG. Stem Cells. 2006;24:2140. doi: 10.1634/stemcells.2005-0567. [DOI] [PubMed] [Google Scholar]
- (57).Barragan-Adjemian C, Nicolella D, Dusevich V, Dallas MR, Eick JD, Bonewald LF. Calcif. Tissue Int. 2006;79:340. doi: 10.1007/s00223-006-0107-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (58).Sivakumar P, Czirok A, Rongish BJ, Divakara VP, Wang YP, Dallas SL. J. Cell Sci. 2006;119:1350. doi: 10.1242/jcs.02830. [DOI] [PubMed] [Google Scholar]
- (59).Wang YH, Liu Y, Maye P, Rowe DW. Biotechnol. Prog. 2006;22:1697. doi: 10.1021/bp060274b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (60).Wang YH, Liu Y, Buhl K, Rowe DW. J. Bone Miner. Res. 2005;20:5. doi: 10.1359/JBMR.041016. [DOI] [PubMed] [Google Scholar]
- (61).Carden A, Morris MD. J. Biomed. Opt. 2000;5:259. doi: 10.1117/1.429994. [DOI] [PubMed] [Google Scholar]
- (62).Nauman EA, Ebenstein DM, Hughes KF, Pruitt L, Halloran BP, Bikle DD, Keaveny TM. Tissue Eng. 2002;8:931. doi: 10.1089/107632702320934038. [DOI] [PubMed] [Google Scholar]
- (63).Orly I, Gregoire M, Menanteau J, Heughebaert M, Kerebel B. Calcif. Tissue Int. 1989;45:20. doi: 10.1007/BF02556656. [DOI] [PubMed] [Google Scholar]
- (64).Phillips JE, Hutmacher DW, Guldberg RE, Garcia AJ. Biomaterials. 2006;27:5535. doi: 10.1016/j.biomaterials.2006.06.019. [DOI] [PubMed] [Google Scholar]
- (65).Rey C, Kim HM, Gerstenfeld L, Glimcher MJ. J. Bone Miner. Res. 1995;10:1577. doi: 10.1002/jbmr.5650101020. [DOI] [PubMed] [Google Scholar]
- (66).Rey C, Kim HM, Gerstenfeld L, Glimcher MJ. Connect. Tissue. Res. 1996;35:343. doi: 10.3109/03008209609029210. [DOI] [PubMed] [Google Scholar]
- (67).Satomura K, Hiraiwa K, Nagayama M. Bone Miner. 1991;14:41. doi: 10.1016/0169-6009(91)90101-5. [DOI] [PubMed] [Google Scholar]
- (68).Liu H, Li W, Shi S, Habelitz S, Gao C, Denbesten P. Arch. Histol. Cytol. 2005;50:923. doi: 10.1016/j.archoralbio.2005.03.003. [DOI] [PubMed] [Google Scholar]
- (69).Boskey A, Pleshko Camacho N. Biomaterials. 2007;28:2465. doi: 10.1016/j.biomaterials.2006.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (70).Boskey AL, Doty SB, Binderman I. Microsc. Res. Tech. 1994;28:492. doi: 10.1002/jemt.1070280605. [DOI] [PubMed] [Google Scholar]
- (71).Boskey AL, Doty SB, Stiner D, Binderman I. Calcif. Tissue Int. 1996;58:177. doi: 10.1007/BF02526884. [DOI] [PubMed] [Google Scholar]
- (72).Boskey AL, Paschalis EP, Binderman I, Doty SB. J. Cell Biochem. 2002;84:509. [PubMed] [Google Scholar]
- (73).Pourmand EP, Binderman I, Doty SB, Kudryashov V, Boskey AL. J. Cell Biochem. 2007;100:43. doi: 10.1002/jcb.20977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (74).Chesnick IE, Avallone FA, Leapman RD, Landis WJ, Eidelman N, Potter K. Bone. 2007;40:904. doi: 10.1016/j.bone.2006.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (75).Boyan BD, Bonewald LF, Paschalis EP, Lohmann CH, Rosser J, Cochran DL, Dean DD, Schwartz Z, Boskey AL. Calcif. Tissue Int. 2002;71:519. doi: 10.1007/s00223-001-1114-y. [DOI] [PubMed] [Google Scholar]
- (76).Magne D, Bluteau G, Lopez-Cazaux S, Weiss P, Pilet P, Ritchie HH, Daculsi G, Guicheux J. Connect. Tissue. Res. 2004;45:101. doi: 10.1080/03008200490464839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (77).Tarnowski CP, Ignelzi MA, Jr., Morris MD. J. Bone Miner. Res. 2002;17:1118. doi: 10.1359/jbmr.2002.17.6.1118. [DOI] [PubMed] [Google Scholar]
- (78).Washburn NR, Weir M, Anderson P, Potter K. J. Biomed. Mater. Res. A. 2004;69:738. doi: 10.1002/jbm.a.30054. [DOI] [PubMed] [Google Scholar]
- (79).Xu H, Othman SF, Hong L, Peptan IA, Magin RL. Phys. Med. Biol. 2006;51:719. doi: 10.1088/0031-9155/51/3/016. [DOI] [PubMed] [Google Scholar]
- (80).Potter K, Landis WJ. In: Regenerative Medicine: Translational Approaches of Tissue Engineering. 1 ed. Mao J, Vunjak-Novakovic G, Mikos A, Atala A, editors. Artech House; Norwood, MA: 2007. [Google Scholar]
- (81).Cowan CM, Aghaloo T, Chou YF, Walder B, Zhang X, Soo C, Ting K, Wu B. Tissue Eng. 2007;13:501. doi: 10.1089/ten.2006.0141. [DOI] [PubMed] [Google Scholar]
- (82).Cartmell S, Huynh K, Lin A, Nagaraja S, Guldberg R. J. Biomed. Mater. Res. A. 2004;69:97. doi: 10.1002/jbm.a.20118. [DOI] [PubMed] [Google Scholar]
- (83).Zhou H, Wu T, Dong X, Wang Q, Shen J. Biochem. Biophys. Res. Commun. 2007;361:91. doi: 10.1016/j.bbrc.2007.06.169. [DOI] [PubMed] [Google Scholar]
- (84).Declercq H, Van den Vreken N, De Maeyer E, Verbeeck R, Schacht E, De Ridder L, Cornelissen M. Biomaterials. 2004;25:757. doi: 10.1016/s0142-9612(03)00580-5. [DOI] [PubMed] [Google Scholar]
- (85).Fell HB. Proc. Nutr. Soc. 1965;24:166. doi: 10.1079/pns19650030. [DOI] [PubMed] [Google Scholar]
- (86).Proffit WR, Ackerman JL. Science. 1964;145:932. doi: 10.1126/science.145.3635.932. [DOI] [PubMed] [Google Scholar]
- (87).Slavkin HC. J. Craniofac. Genet. Dev. Biol. 1991;11:338. [PubMed] [Google Scholar]
- (88).Nagata T, Goldberg HA, Zhang Q, Domenicucci C, Sodek J. Matrix. 1991;11:86. doi: 10.1016/s0934-8832(11)80212-x. [DOI] [PubMed] [Google Scholar]
- (89).Hamlin NJ, Price PA. Calcif. Tissue Int. 2004;75:231. doi: 10.1007/s00223-004-0190-1. [DOI] [PubMed] [Google Scholar]
- (90).Hamlin NJ, Ong KG, Price PA. Calcif. Tissue Int. 2006;78:326. doi: 10.1007/s00223-005-0205-6. [DOI] [PubMed] [Google Scholar]
- (91).Price PA, June HH, Hamlin NJ, Williamson MK. J. Biol. Chem. 2004;279:19169. doi: 10.1074/jbc.M307880200. [DOI] [PubMed] [Google Scholar]
- (92).Bagi CM, Miller SC. J. Bone Miner. Res. 1992;7:29. doi: 10.1002/jbmr.5650070106. [DOI] [PubMed] [Google Scholar]
- (93).Bagi C, Burger EH. Calcif. Tissue Int. 1989;45:342. doi: 10.1007/BF02556004. [DOI] [PubMed] [Google Scholar]
- (94).Ben-Ami Y, von der Mark K, Franzen A, de Bernard B, Lunazzi GC, Silbermann M. Cell Tissue Res. 1993;271:317. doi: 10.1007/BF00318618. [DOI] [PubMed] [Google Scholar]
- (95).Reginato AM, Tuan RS, Ono T, Jimenez SA, Jacenko O. Dev. Dyn. 1993;198:284. doi: 10.1002/aja.1001980406. [DOI] [PubMed] [Google Scholar]
- (96).Gronowicz G, Woodiel FN, McCarthy MB, Raisz LG. J. Bone Miner. Res. 1989;4:313. doi: 10.1002/jbmr.5650040305. [DOI] [PubMed] [Google Scholar]
- (97).Bronckers AL, Bervoets TJ, Woltgens JH, Lyaruu DM. Eur. J. Oral Sci. 2006;114(Suppl 1):116. doi: 10.1111/j.1600-0722.2006.00307.x. [DOI] [PubMed] [Google Scholar]
- (98).Tjaderhane L, Palosaari H, Wahlgren J, Larmas M, Sorsa T, Salo T. Adv. Dent. Res. 2001;15:55. doi: 10.1177/08959374010150011401. [DOI] [PubMed] [Google Scholar]
- (99).Ashton BA, Allen TD, Howlett CR, Eaglesom CC, Hattori A, Owen M. Clin. Orthop. Relat. Res. 1980;151:294. [PubMed] [Google Scholar]
- (100).Howlett CR, Cave J, Williamson M, Farmer J, Ali SY, Bab I, Owen ME. Clin. Orthop. Relat. Res. 1986:251. [PubMed] [Google Scholar]
- (101).Gan OI, Soueidan A, Castagne A, Daculsi GC. R. Acad. Sci. III. 1994;317:324. [PubMed] [Google Scholar]
- (102).zur Nieden NI, Kempka G, Ahr HJ. Differentiation. 2003;71:18. doi: 10.1046/j.1432-0436.2003.700602.x. [DOI] [PubMed] [Google Scholar]
- (103).Majors AK, Boehm CA, Nitto H, Midura RJ, Muschler GF. J. Orthop. Res. 1997;15:546. doi: 10.1002/jor.1100150410. [DOI] [PubMed] [Google Scholar]
- (104).Cheng SL, Zhang SF, Avioli LV. J. Cell Biochem. 1996;61:182. doi: 10.1002/(sici)1097-4644(19960501)61:2<182::aid-jcb3>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- (105).Kim MK, Niyibizi C. Yonsei Med. J. 2001;42:338. doi: 10.3349/ymj.2001.42.3.338. [DOI] [PubMed] [Google Scholar]
- (106).Caplan AI, Dennis JE. J. Cell Biochem. 2006;98:1076. doi: 10.1002/jcb.20886. [DOI] [PubMed] [Google Scholar]
- (107).Gay CV, Lloyd QP. Comp. Biochem. Physiol. A. Physiol. 1995;111:257. doi: 10.1016/0300-9629(95)00004-q. [DOI] [PubMed] [Google Scholar]
- (108).Declercq HA, Verbeeck RM, De Ridder LI, Schacht EH, Cornelissen MJ. Biomaterials. 2005;26:4964. doi: 10.1016/j.biomaterials.2005.01.025. [DOI] [PubMed] [Google Scholar]
- (109).Bellows CG, Heersche JN, Aubin JE. Bone Miner. 1992;17:15. doi: 10.1016/0169-6009(92)90707-k. [DOI] [PubMed] [Google Scholar]
- (110).Bellows CG, Aubin JE, Heersche JN. Bone Miner. 1991;14:27. doi: 10.1016/0169-6009(91)90100-e. [DOI] [PubMed] [Google Scholar]
- (111).Whitson SW, Jenkins DB, Bowers DE, Jr., Hatton JF. Proc. Finn. Dent. Soc. 1992;88(Suppl 1):305. [PubMed] [Google Scholar]
- (112).Kasugai S, Shibata S, Suzuki S, Susami T, Ogura H. Arch. Histol. Cytol. 1993;38:769. doi: 10.1016/0003-9969(93)90073-u. [DOI] [PubMed] [Google Scholar]
- (113).Balic A, Mina M. Orthod. Craniofac. Res. 2005;8:252. doi: 10.1111/j.1601-6343.2005.00347.x. [DOI] [PubMed] [Google Scholar]
- (114).Wu LN, Guo Y, Genge BR, Ishikawa Y, Wuthier RE. J. Cell Biochem. 2002;86:475. doi: 10.1002/jcb.10240. [DOI] [PubMed] [Google Scholar]
- (115).Teixeira CC, Hatori M, Leboy PS, Pacifici M, Shapiro IM. Calcif. Tissue Int. 1995;56:252. doi: 10.1007/BF00298620. [DOI] [PubMed] [Google Scholar]
- (116).Garimella R, Bi X, Camacho N, Sipe JB, Anderson HC. Bone. 2004;34:961. doi: 10.1016/j.bone.2004.02.010. [DOI] [PubMed] [Google Scholar]
- (117).Iwamoto M, Shapiro IM, Yagami K, Boskey AL, Leboy PS, Adams SL, Pacifici M. Exp. Cell Res. 1993;207:413. doi: 10.1006/excr.1993.1209. [DOI] [PubMed] [Google Scholar]
- (118).Chen LS, Couwenhoven RI, Hsu D, Luo W, Snead ML. Arch. Histol. Cytol. 1992;37:771. doi: 10.1016/0003-9969(92)90110-t. [DOI] [PubMed] [Google Scholar]
- (119).Den Besten PK, Mathews CH, Gao C, Li W. Connect. Tissue. Res. 1998;38:3. doi: 10.3109/03008209809017011. [DOI] [PubMed] [Google Scholar]
- (120).Kukita A, Harada H, Kukita T, Inai T, Matsuhashi S, Kurisu K. Calcif. Tissue Int. 1992;51:393. doi: 10.1007/BF00316886. [DOI] [PubMed] [Google Scholar]
- (121).Bodine PV, Komm BS. Vitam. Horm. 2002;64:101. doi: 10.1016/s0083-6729(02)64004-x. [DOI] [PubMed] [Google Scholar]
- (122).Allen DD, Caviedes R, Cardenas AM, Shimahara T, Segura-Aguilar J, Caviedes PA. Drug Dev. Ind. Pharm. 2005;31:757. doi: 10.1080/03639040500216246. [DOI] [PubMed] [Google Scholar]
- (123).Atsumi T, Miwa Y, Kimata K, Ikawa Y. Cell Differ. Dev. 1990;30:109. doi: 10.1016/0922-3371(90)90079-c. [DOI] [PubMed] [Google Scholar]
- (124).Reznikoff CA, Bertram JS, Brankow DW, Heidelberger C. Cancer Res. 1973;33:3239. [PubMed] [Google Scholar]
- (125).Yoneda T, Pratt RM. Science. 1981;213:563. doi: 10.1126/science.7017936. [DOI] [PubMed] [Google Scholar]
- (126).Harris SA, Enger RJ, Riggs BL, Spelsberg TC. J. Bone Miner. Res. 1995;10:178. doi: 10.1002/jbmr.5650100203. [DOI] [PubMed] [Google Scholar]
- (127).Kodama H, Amagai Y, Sudo H, Kasai S, Yamomoto S. Jpn. J. Oral. Biol. 1981;23:899. [Google Scholar]
- (128).Ghosh-Choudhury N, Windle JJ, Koop BA, Harris MA, Guerrero DL, Wozney JM, Mundy GR, Harris SE. Endocrinology. 1996;137:331. doi: 10.1210/endo.137.1.8536632. [DOI] [PubMed] [Google Scholar]
- (129).Chen D, Chen H, Feng J, Windle J, Koop B, Harris H, Harris S. Mol. Cell Differentiation. 1995;3:193. [Google Scholar]
- (130).Majeska RJ, Rodan SB, Rodan GA. Exp. Cell Res. 1978;111:465. doi: 10.1016/0014-4827(78)90193-3. [DOI] [PubMed] [Google Scholar]
- (131).Boland CJ, Fried RM, Tashjian AH., Jr. Endocrinology. 1986;118:980. doi: 10.1210/endo-118-3-980. [DOI] [PubMed] [Google Scholar]
- (132).Murray E, Provvedini D, Curran D, Catherwood B, Sussman H, Manolagas S. J. Bone Miner. Res. 1987;2:231. doi: 10.1002/jbmr.5650020310. [DOI] [PubMed] [Google Scholar]
- (133).Rodan SB, Imai Y, Thiede MA, Wesolowski G, Thompson D, Bar-Shavit Z, Shull S, Mann K, Rodan GA. Cancer Res. 1987;47:4961. [PubMed] [Google Scholar]
- (134).McAllister RM, Gardner MB, Greene AE, Bradt C, Nichols WW, Landing BH. Cancer. 1971;27:397. doi: 10.1002/1097-0142(197102)27:2<397::aid-cncr2820270224>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- (135).Forrest SM, Ng KW, Findlay DM, Michelangeli VP, Livesey SA, Partridge NC, Zajac JD, Martin TJ. Calcif. Tissue Int. 1985;37:51. doi: 10.1007/BF02557679. [DOI] [PubMed] [Google Scholar]
- (136).Sudo H, Kodama HA, Amagai Y, Yamamoto S, Kasai S. J. Cell Biol. 1983;96:191. doi: 10.1083/jcb.96.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (137).Luppen CA, Smith E, Spevak L, Boskey AL, Frenkel B. J. Bone Miner. Res. 2003;18:1186. doi: 10.1359/jbmr.2003.18.7.1186. [DOI] [PubMed] [Google Scholar]
- (138).Franceschi RT, Iyer BS. J. Bone Miner. Res. 1992;7:235. doi: 10.1002/jbmr.5650070216. [DOI] [PubMed] [Google Scholar]
- (139).Marsh ME, Munne AM, Vogel JJ, Cui Y, Franceschi RT. J. Bone Miner. Res. 1995;10:1635. doi: 10.1002/jbmr.5650101105. [DOI] [PubMed] [Google Scholar]
- (140).Qu X, Cui W, Yang F, Min C, Shen H, Bei J, Wang S. Biomaterials. 2007;28:9. doi: 10.1016/j.biomaterials.2006.08.024. [DOI] [PubMed] [Google Scholar]
- (141).Rosa AL, Beloti MM, Van Noort R, Hatton PV, Devlin AJ. Pesqui. Odontol. Bras. 2002;16:209. doi: 10.1590/s1517-74912002000300005. [DOI] [PubMed] [Google Scholar]
- (142).Aronow MA, Gerstenfeld LC, Owen TA, Tassinari MS, Stein GS, Lian JB. J. Cell Physiol. 1990;143:213. doi: 10.1002/jcp.1041430203. [DOI] [PubMed] [Google Scholar]
- (143).Noda M. Endocrinology. 1989;124:612. doi: 10.1210/endo-124-2-612. [DOI] [PubMed] [Google Scholar]
- (144).Nishimoto SK, Stryker WF, Nimni ME. Calcif. Tissue Int. 1987;41:274. doi: 10.1007/BF02555229. [DOI] [PubMed] [Google Scholar]
- (145).Shteyer A, Gazit D, Passi-Even L, Bab I, Majeska R, Gronowicz G, Lurie A, Rodan G. Calcif. Tissue Int. 1986;39:49. doi: 10.1007/BF02555740. [DOI] [PubMed] [Google Scholar]
- (146).Hale LV, Ma YF, Santerre RF. Calcif. Tissue Int. 2000;67:80. doi: 10.1007/s00223001101. [DOI] [PubMed] [Google Scholar]
- (147).Rao LG, Liu LJ, Murray TM, McDermott E, Zhang X. Biol. Pharm. Bull. 2003;26:936. doi: 10.1248/bpb.26.936. [DOI] [PubMed] [Google Scholar]
- (148).Stanford CM, Jacobson PA, Eanes ED, Lembke LA, Midura RJ. J. Biol. Chem. 1995;270:9420. doi: 10.1074/jbc.270.16.9420. [DOI] [PubMed] [Google Scholar]
- (149).Cornelissen M, Declercq H, Ridder LD. Eur. Cell. Mat. 2003;5:60. [Google Scholar]
- (150).Kato Y, Windle JJ, Koop BA, Mundy GR, Bonewald LF. J. Bone Miner. Res. 1997;12:2014. doi: 10.1359/jbmr.1997.12.12.2014. [DOI] [PubMed] [Google Scholar]
- (151).Bonewald LF. J. Bone Miner. Metab. 1999;17:61. doi: 10.1007/s007740050066. [DOI] [PubMed] [Google Scholar]
- (152).Kato Y, Boskey A, Spevak L, Dallas M, Hori M, Bonewald LF. J. Bone Miner. Res. 2001;16:1622. doi: 10.1359/jbmr.2001.16.9.1622. [DOI] [PubMed] [Google Scholar]
- (153).Panagakos FS. J. Endod. 1998;24:171. doi: 10.1016/S0099-2399(98)80177-5. [DOI] [PubMed] [Google Scholar]
- (154).MacDougall M, Unterbrink A, Carnes D, Rani S, Luan X, Chen S. Adv. Dent. Res. 2001;15:25. doi: 10.1177/08959374010150010601. [DOI] [PubMed] [Google Scholar]
- (155).MacDougall M, Thiemann F, Ta H, Hsu P, Chen LS, Snead ML. Connect. Tissue. Res. 1995;33:97. doi: 10.3109/03008209509016988. [DOI] [PubMed] [Google Scholar]
- (156).Ritchie HH, Liu J, Kasugai S, Moller P. In Vitro Cell Dev. Biol. Anim. 2002;38:25. doi: 10.1290/1071-2690(2002)038<0025:AMRDPC>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- (157).Hao J, Narayanan K, Ramachandran A, He G, Almushayt A, Evans C, George A. J. Biol. Chem. 2002;277:19976. doi: 10.1074/jbc.M112223200. [DOI] [PubMed] [Google Scholar]
- (158).Iwata T, Yamakoshi Y, Simmer JP, Ishikawa I, Hu JC. Eur. J. Oral Sci. 2007;115:48. doi: 10.1111/j.1600-0722.2007.00426.x. [DOI] [PubMed] [Google Scholar]
- (159).Katagiri T, Yamaguchi A, Ikeda T, Yoshiki S, Wozney JM, Rosen V, Wang EA, Tanaka H, Omura S, Suda T. Biochem. Biophys. Res. Commun. 1990;172:295. doi: 10.1016/s0006-291x(05)80208-6. [DOI] [PubMed] [Google Scholar]
- (160).Denker AE, Haas AR, Nicoll SB, Tuan RS. Differentiation. 1999;64:67. doi: 10.1046/j.1432-0436.1999.6420067.x. [DOI] [PubMed] [Google Scholar]
- (161).Date T, Doiguchi Y, Nobuta M, Shindo H. J. Orthop. Sci. 2004;9:503. doi: 10.1007/s00776-004-0815-2. [DOI] [PubMed] [Google Scholar]
- (162).Narayanan K, Srinivas R, Ramachandran A, Hao J, Quinn B, George A. Proc. Natl. Acad. Sci. U. S. A. 2001;98:4516. doi: 10.1073/pnas.081075198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (163).Yang S, Wei D, Wang D, Phimphilai M, Krebsbach PH, Franceschi RT. J. Bone Miner. Res. 2003;18:705. doi: 10.1359/jbmr.2003.18.4.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (164).Nochi H, Sung JH, Lou J, Adkisson HD, Maloney WJ, Hruska KA. J. Bone Miner. Res. 2004;19:111. doi: 10.1359/jbmr.2004.19.1.111. [DOI] [PubMed] [Google Scholar]
- (165).Yamashiro T, Zheng L, Shitaku Y, Saito M, Tsubakimoto T, Takada K, Takano-Yamamoto T, Thesleff I. Differentiation. 2007;75:452. doi: 10.1111/j.1432-0436.2006.00150.x. [DOI] [PubMed] [Google Scholar]
- (166).Kartsogiannis V, Ng KW. Mol. Cell Endocrinol. 2004;228:79. doi: 10.1016/j.mce.2003.06.002. [DOI] [PubMed] [Google Scholar]
- (167).Shukunami C, Ishizeki K, Atsumi T, Ohta Y, Suzuki F, Hiraki Y. J. Bone Miner. Res. 1997;12:1174. doi: 10.1359/jbmr.1997.12.8.1174. [DOI] [PubMed] [Google Scholar]
- (168).Chang W, Tu C, Pratt S, Chen TH, Shoback D. Endocrinology. 2002;143:1467. doi: 10.1210/endo.143.4.8709. [DOI] [PubMed] [Google Scholar]
- (169).Kamiya N, Jikko A, Kimata K, Damsky C, Shimizu K, Watanabe H. J. Bone Miner. Res. 2002;17:1832. doi: 10.1359/jbmr.2002.17.10.1832. [DOI] [PubMed] [Google Scholar]
- (170).Berry JE, Zhao M, Jin Q, Foster BL, Viswanathan H, Somerman MJ. Connect. Tissue. Res. 2003;44(Suppl 1):97. [PubMed] [Google Scholar]
- (171).Boabaid F, Gibson CW, Kuehl MA, Berry JE, Snead ML, Nociti FH, Jr., Katchburian E, Somerman MJ. J. Periodontol. 2004;75:1126. doi: 10.1902/jop.2004.75.8.1126. [DOI] [PubMed] [Google Scholar]
- (172).Arzate H, Alvarez-Perez MA, Alvarez-Fregoso O, Wusterhaus-Chavez A, Reyes-Gasga J, Ximenez-Fyvie LA. J. Dent. Res. 2000;79:28. doi: 10.1177/00220345000790010301. [DOI] [PubMed] [Google Scholar]
- (173).Saito M, Handa K, Kiyono T, Hattori S, Yokoi T, Tsubakimoto T, Harada H, Noguchi T, Toyoda M, Sato S, Teranaka T. J. Bone Miner. Res. 2005;20:50. doi: 10.1359/JBMR.041006. [DOI] [PubMed] [Google Scholar]
- (174).DenBesten PK, Gao C, Li W, Mathews CH, Gruenert DC. Eur. J. Oral Sci. 1999;107:276. doi: 10.1046/j.0909-8836.1999.eos107407.x. [DOI] [PubMed] [Google Scholar]
- (175).Nakata A, Kameda T, Nagai H, Ikegami K, Duan Y, Terada K, Sugiyama T. Biochem. Biophys. Res. Commun. 2003;308:834. doi: 10.1016/s0006-291x(03)01467-0. [DOI] [PubMed] [Google Scholar]
- (176).Woei Ng K, Speicher T, Dombrowski C, Helledie T, Haupt LM, Nurcombe V, Cool SM. Stem Cells Dev. 2007;16:305. doi: 10.1089/scd.2006.0044. [DOI] [PubMed] [Google Scholar]
- (177).Woll NL, Heaney JD, Bronson SK. Stem Cells Dev. 2006;15:865. doi: 10.1089/scd.2006.15.865. [DOI] [PubMed] [Google Scholar]
- (178).Woll NL, Bronson SK. Methods Mol. Biol. 2006;330:149. doi: 10.1385/1-59745-036-7:149. [DOI] [PubMed] [Google Scholar]
- (179).Yamashita A, Takada T, Narita J, Yamamoto G, Torii R. Cloning Stem Cells. 2005;7:232. doi: 10.1089/clo.2005.7.232. [DOI] [PubMed] [Google Scholar]
- (180).Descalzi Cancedda F, Gentili C, Manduca P, Cancedda R. J. Cell Biol. 1992;117:427. doi: 10.1083/jcb.117.2.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (181).Gentili C, Bianco P, Neri M, Malpeli M, Campanile G, Castagnola P, Cancedda R, Cancedda FD. J. Cell Biol. 1993;122:703. doi: 10.1083/jcb.122.3.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (182).Ahrens PB, Solursh M, Reiter RS. Dev Biol. 1977;60:69. doi: 10.1016/0012-1606(77)90110-5. [DOI] [PubMed] [Google Scholar]
- (183).Malladi P, Xu Y, Chiou M, Giaccia AJ, Longaker MT. Am. J. Physiol. Cell. Physiol. 2006;290:C1139. doi: 10.1152/ajpcell.00415.2005. [DOI] [PubMed] [Google Scholar]
- (184).Soung do Y, Dong Y, Wang Y, Zuscik MJ, Schwarz EM, O’Keefe RJ, Drissi H. J. Bone Miner. Res. 2007;22:1260. doi: 10.1359/jbmr.070502. [DOI] [PubMed] [Google Scholar]
- (185).Boskey AL, Guidon P, Doty SB, Stiner D, Leboy P, Binderman I. J. Bone Miner. Res. 1996;11:1694. doi: 10.1002/jbmr.5650111113. [DOI] [PubMed] [Google Scholar]
- (186).Boskey AL, Stiner D, Binderman I, Doty SB. J. Cell Biochem. 1997;64:632. doi: 10.1002/(sici)1097-4644(19970315)64:4<632::aid-jcb11>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- (187).Benya PD, Shaffer JD. Cell. 1982;30:215. doi: 10.1016/0092-8674(82)90027-7. [DOI] [PubMed] [Google Scholar]
- (188).Sanchez C, Deberg MA, Piccardi N, Msika P, Reginster JY, Henrotin YE. Osteoarthritis Cartilage. 2005;13:988. doi: 10.1016/j.joca.2005.07.012. [DOI] [PubMed] [Google Scholar]
- (189).Batorsky A, Liao J, Lund AW, Plopper GE, Stegemann JP. Biotechnol. Bioeng. 2005;92:492. doi: 10.1002/bit.20614. [DOI] [PubMed] [Google Scholar]
- (190).Randle WL, Cha JM, Hwang YS, Chan KL, Kazarian SG, Polak JM, Mantalaris A. Tissue Eng. 2007;13:2957. doi: 10.1089/ten.2007.0072. [DOI] [PubMed] [Google Scholar]
- (191).Boskey AL, Stiner D, Binderman I, Doty SB. J. Cell Biochem. 2000;79:89. doi: 10.1002/1097-4644(2000)79:1<89::aid-jcb90>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- (192).Jikko A, Aoba T, Murakami H, Takano Y, Iwamoto M, Kato Y. Dev. Biol. 1993;156:372. doi: 10.1006/dbio.1993.1084. [DOI] [PubMed] [Google Scholar]
- (193).Stephansson SN, Byers BA, Garcia AJ. Biomaterials. 2002;23:2527. doi: 10.1016/s0142-9612(01)00387-8. [DOI] [PubMed] [Google Scholar]
- (194).Keselowsky BG, Collard DM, Garcia AJ. Proc. Natl. Acad. Sci. U. S. A. 2005;102:5953. doi: 10.1073/pnas.0407356102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (195).Ogura N, Kawada M, Chang WJ, Zhang Q, Lee SY, Kondoh T, Abiko Y. J. Oral Sci. 2004;46:207. doi: 10.2334/josnusd.46.207. [DOI] [PubMed] [Google Scholar]
- (196).Lenhert S, Meier MB, Meyer U, Chi L, Wiesmann HP. Biomaterials. 2005;26:563. doi: 10.1016/j.biomaterials.2004.02.068. [DOI] [PubMed] [Google Scholar]
- (197).Schneider GB, Perinpanayagam H, Clegg M, Zaharias R, Seabold D, Keller J, Stanford C. J. Dent. Res. 2003;82:372. doi: 10.1177/154405910308200509. [DOI] [PubMed] [Google Scholar]
- (198).Liao H, Andersson AS, Sutherland D, Petronis S, Kasemo B, Thomsen P. Biomaterials. 2003;24:649. doi: 10.1016/s0142-9612(02)00379-4. [DOI] [PubMed] [Google Scholar]
- (199).Gray C. Tissue Eng. 1998;4:315. doi: 10.1089/ten.1998.4.315. [DOI] [PubMed] [Google Scholar]
- (200).Perizzolo D, Lacefield WR, Brunette DM. J. Biomed. Mater. Res. 2001;56:494. doi: 10.1002/1097-4636(20010915)56:4<494::aid-jbm1121>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- (201).Schwartz Z, Lohmann CH, Oefinger J, Bonewald LF, Dean DD, Boyan BD. Adv. Dent. Res. 1999;13:38. doi: 10.1177/08959374990130011301. [DOI] [PubMed] [Google Scholar]
- (202).Takeuchi K, Saruwatari L, Nakamura HK, Yang JM, Ogawa T. J. Biomed. Mater. Res. A. 2005;72:296. doi: 10.1002/jbm.a.30227. [DOI] [PubMed] [Google Scholar]
- (203).Sautier JM, Kokubo T, Ohtsuki T, Nefussi JR, Boulekbache H, Oboeuf M, Loty S, Loty C, Forest N. Calcif. Tissue Int. 1994;55:458. doi: 10.1007/BF00298560. [DOI] [PubMed] [Google Scholar]
- (204).Jones JR, Tsigkou O, Coates EE, Stevens MM, Polak JM, Hench LL. Biomaterials. 2007;28:1653. doi: 10.1016/j.biomaterials.2006.11.022. [DOI] [PubMed] [Google Scholar]
- (205).Brown S, Tong W, Krebsbach PH. Cells Tissues Organs. doi: 10.1159/000151746. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (206).Honda MJ, Shinohara Y, Hata K, Ueda M. Cells Tissues Organs. doi: 10.1159/000151743. in press. [DOI] [PubMed] [Google Scholar]
- (207).Honda MJ, Shimodaira T, Ogaeri T, Shinohara Y, Hata K, Ueda M. Arch. Histol. Cytol. 2006;51:282. doi: 10.1016/j.archoralbio.2005.09.005. [DOI] [PubMed] [Google Scholar]
- (208).Justesen J, Stenderup K, Eriksen EF, Kassem M. Calcif. Tissue Int. 2002;71:36. doi: 10.1007/s00223-001-2059-x. [DOI] [PubMed] [Google Scholar]
- (209).Ishikawa Y, Wuthier RE. Bone Miner. 1992;17:152. doi: 10.1016/0169-6009(92)90727-u. [DOI] [PubMed] [Google Scholar]
- (210).Igarashi M, Kamiya N, Hasegawa M, Kasuya T, Takahashi T, Takagi M. J. Mol. Histol. 2004;35:3. doi: 10.1023/b:hijo.0000020883.33256.fe. [DOI] [PubMed] [Google Scholar]
- (211).Boskey A. J. Phys. Chem. 1989;93:1628. [Google Scholar]
- (212).Price PA, Lim JE. J. Biol. Chem. 2003;278:22144. doi: 10.1074/jbc.M300744200. [DOI] [PubMed] [Google Scholar]
- (213).Khouja HI, Bevington A, Kemp GJ, Russell RG. Bone. 1990;11:385. doi: 10.1016/8756-3282(90)90131-h. [DOI] [PubMed] [Google Scholar]
- (214).DenBesten PK, Machule D, Zhang Y, Yan Q, Li W. Arch. Histol. Cytol. 2005;50:689. doi: 10.1016/j.archoralbio.2004.12.008. [DOI] [PubMed] [Google Scholar]
- (215).Park JC, Park JT, Son HH, Kim HJ, Jeong MJ, Lee CS, Dey R, Cho MI. Eur. J. Oral Sci. 2007;115:153. doi: 10.1111/j.1600-0722.2007.00435.x. [DOI] [PubMed] [Google Scholar]
- (216).Fujiwara N, Sakakura Y, Nawa T. Arch. Histol. Cytol. 1991;54:411. doi: 10.1679/aohc.54.411. [DOI] [PubMed] [Google Scholar]
- (217).Honn KV, Singley JA, Chavin W. Proc. Soc. Exp. Biol. Med. 1975;149:344. doi: 10.3181/00379727-149-38804. [DOI] [PubMed] [Google Scholar]
- (218).Bettger WJ, McKeehan WL. Physiol. Rev. 1986;66:1. doi: 10.1152/physrev.1986.66.1.1. [DOI] [PubMed] [Google Scholar]
- (219).Bohensky J, Shapiro IM, Leshinsky S, Terkhorn SP, Adams CS, Srinivas V. Autophagy. 2007;3:207. doi: 10.4161/auto.3708. [DOI] [PubMed] [Google Scholar]
- (220).Hirao M, Hashimoto J, Yamasaki N, Ando W, Tsuboi H, Myoui A, Yoshikawa H. J. Bone Miner. Metab. 2007;25:266. doi: 10.1007/s00774-007-0765-9. [DOI] [PubMed] [Google Scholar]
- (221).Hirao M, Tamai N, Tsumaki N, Yoshikawa H, Myoui A. J. Biol. Chem. 2006;281:31079. doi: 10.1074/jbc.M602296200. [DOI] [PubMed] [Google Scholar]
- (222).Howell DS, Pita JC. Clin. Orthop. Relat. Res. 1976;118:208. [PubMed] [Google Scholar]
- (223).Brandao-Burch A, Utting JC, Orriss IR, Arnett TR. Calcif. Tissue Int. 2005;77:167. doi: 10.1007/s00223-004-0285-8. [DOI] [PubMed] [Google Scholar]
- (224).van’t Veen SJ, Hagen JW, van Ginkel FC, Prahl-Andersen B, Burger EH. Bone. 1995;17:461. doi: 10.1016/8756-3282(95)00334-6. [DOI] [PubMed] [Google Scholar]
- (225).Walboomers XF, Elder SE, Bumgardner JD, Jansen JA. J. Biomed. Mater. Res. A. 2006;76:16. doi: 10.1002/jbm.a.30304. [DOI] [PubMed] [Google Scholar]
- (226).Walboomers XF, Habraken WJ, Feddes B, Winter LC, Bumgardner JD, Jansen JA. J. Biomed. Mater. Res. A. 2004;69:131. doi: 10.1002/jbm.a.20127. [DOI] [PubMed] [Google Scholar]
- (227).Winter LC, Walboomers XF, Bumgardner JD, Jansen JA. J. Biomed. Mater. Res. A. 2003;67:1269. doi: 10.1002/jbm.a.20028. [DOI] [PubMed] [Google Scholar]
- (228).Uemura T, Dong J, Wang Y, Kojima H, Saito T, Iejima D, Kikuchi M, Tanaka J, Tateishi T. Biomaterials. 2003;24:2277. doi: 10.1016/s0142-9612(03)00039-5. [DOI] [PubMed] [Google Scholar]
- (229).Torigoe I, Sotome S, Tsuchiya A, Yoshii T, Takahashi M, Kawabata S, Shinomiya K. Cell Transplant. 2007;16:729. doi: 10.3727/000000007783465109. [DOI] [PubMed] [Google Scholar]
- (230).Porter BD, Lin AS, Peister A, Hutmacher D, Guldberg RE. Biomaterials. 2007;28:2525. doi: 10.1016/j.biomaterials.2007.01.013. [DOI] [PubMed] [Google Scholar]
- (231).Case ND, Duty AO, Ratcliffe A, Muller R, Guldberg RE. Tissue Eng. 2003;9:587. doi: 10.1089/107632703768247296. [DOI] [PubMed] [Google Scholar]
- (232).Duty AO, Oest ME, Guldberg RE. J. Biomech. Eng. 2007;129:531. doi: 10.1115/1.2746375. [DOI] [PubMed] [Google Scholar]
- (233).van Loon JJ, Veldhuijzen JP, Windgassen EJ, Brouwer T, Wattel K, van Vilsteren M, Maas P. Adv. Space. Res. 1994;14:289. doi: 10.1016/0273-1177(94)90413-8. [DOI] [PubMed] [Google Scholar]
- (234).Kacena MA, Todd P, Landis WJ. In Vitro Cell Dev. Biol. Anim. 2003;39:454. doi: 10.1290/1543-706X(2003)039<0454:OSTSAS>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- (235).Van Loon JJ, Bervoets DJ, Burger EH, Dieudonne SC, Hagen JW, Semeins CM, Doulabi BZ, Veldhuijzen JP. J. Bone Miner. Res. 1995;10:550. doi: 10.1002/jbmr.5650100407. [DOI] [PubMed] [Google Scholar]
- (236).Kasahara H, Aoki H. Methods Mol. Med. 2005;112:155. doi: 10.1007/978-1-59259-879-3_9. [DOI] [PubMed] [Google Scholar]
- (237).Bronckers AL, Price PA, Schrijvers A, Bervoets TJ, Karsenty G. Eur. J. Oral Sci. 1998;106:795. doi: 10.1046/j.0909-8836.1998.eos106306.x. [DOI] [PubMed] [Google Scholar]
- (238).Achenbach TV, Brunner B, Heermeier K. Chembiochem. 2003;4:928. doi: 10.1002/cbic.200300708. [DOI] [PubMed] [Google Scholar]
- (239).Diekwisch T, David S, Bringas P, Jr., Santos V, Slavkin HC. Development. 1993;117:471. doi: 10.1242/dev.117.2.471. [DOI] [PubMed] [Google Scholar]
- (240).Torii Y, Hitomi K, Yamagishi Y, Tsukagoshi N. Cell Biol. Int. 1996;20:459. doi: 10.1006/cbir.1996.0060. [DOI] [PubMed] [Google Scholar]
- (241).Ueno A, Miwa Y, Miyoshi K, Horiguchi T, Inoue H, Ruspita I, Abe K, Yamashita K, Hayashi E, Noma T. J. Cell Physiol. 2006;209:322. doi: 10.1002/jcp.20735. [DOI] [PubMed] [Google Scholar]
- (242).Peek AS, Behlke MA. Curr. Opin. Mol. Ther. 2007;9:110. [PubMed] [Google Scholar]
- (243).Wang W, Xu J, Kirsch T. Exp. Cell Res. 2005;305:156. doi: 10.1016/j.yexcr.2004.12.022. [DOI] [PubMed] [Google Scholar]
- (244).Kato C, Kojima T, Komaki M, Mimori K, Duarte WR, Takenaga K, Ishikawa I. Biochem. Biophys. Res. Commun. 2005;326:147. doi: 10.1016/j.bbrc.2004.11.010. [DOI] [PubMed] [Google Scholar]
- (245).Kahler RA, Galindo M, Lian J, Stein GS, van Wijnen AJ, Westendorf JJ. J. Cell Biochem. 2006;97:969. doi: 10.1002/jcb.20702. [DOI] [PubMed] [Google Scholar]
- (246).Yang X, Walboomers XF, van den Dolder J, Yang F, Bian Z, Fan M, Jansen JA. Tissue Eng. Part A. 2008;14:71. doi: 10.1089/ten.a.2007.0102. [DOI] [PubMed] [Google Scholar]
- (247).Orth P, Weimer A, Kaul G, Kohn D, Cucchiarini M, Madry H. Mol. Biotechnol. 2008;38:137. doi: 10.1007/s12033-007-0071-8. [DOI] [PubMed] [Google Scholar]
- (248).Yamamoto M, Tabata Y. Adv. Drug Deliv. Rev. 2006;58:535. doi: 10.1016/j.addr.2006.03.003. [DOI] [PubMed] [Google Scholar]
- (249).Noll T, Jelinek N, Schmid S, Biselli M, Wandrey C. Adv. Biochem. Eng. Biotechnol. 2002;74:111. doi: 10.1007/3-540-45736-4_6. [DOI] [PubMed] [Google Scholar]
- (250).Yoon DM, Fisher JP. Tissue Eng. Part A. 2008;14:1263. doi: 10.1089/ten.tea.2007.0172. [DOI] [PubMed] [Google Scholar]
- (251).Selim AA, Abdelmagid SM, Kanaan RA, Smock SL, Owen TA, Popoff SN, Safadi FF. Crit. Rev. Eukaryot. Gene Expr. 2003;13:265. doi: 10.1615/critreveukaryotgeneexpr.v13.i24.180. [DOI] [PubMed] [Google Scholar]
- (252).Cooper LF, Yliheikkila PK, Felton DA, Whitson SW. J. Bone Miner. Res. 1998;13:620. doi: 10.1359/jbmr.1998.13.4.620. [DOI] [PubMed] [Google Scholar]
- (253).Malaval L, Gupta AK, Liu F, Delmas PD, Aubin JE. J. Bone Miner. Res. 1998;13:175. doi: 10.1359/jbmr.1998.13.2.175. [DOI] [PubMed] [Google Scholar]
- (254).Andre-Frei V, Chevallay B, Orly I, Boudeulle M, Huc A, Herbage D. Calcif. Tissue Int. 2000;66:204. doi: 10.1007/s002230010041. [DOI] [PubMed] [Google Scholar]
- (255).Hamade E, Azzar G, Radisson J, Buchet R, Roux B. Eur. J. Biochem. 2003;270:2082. doi: 10.1046/j.1432-1033.2003.03585.x. [DOI] [PubMed] [Google Scholar]
- (256).Orimo H, Shimada T. Mol. Cell Biochem. 2006;282:101. doi: 10.1007/s11010-006-1520-6. [DOI] [PubMed] [Google Scholar]
- (257).Zheng Q, Zhou G, Morello R, Chen Y, Garcia-Rojas X, Lee B. J. Cell Biol. 2003;162:833. doi: 10.1083/jcb.200211089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (258).Ortega N, Behonick DJ, Colnot C, Cooper DN, Werb Z. Mol. Biol. Cell. 2005;16:3028. doi: 10.1091/mbc.E04-12-1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (259).Cole DE, Gundberg CM. Clin. Chim. Acta. 1985;151:1. doi: 10.1016/0009-8981(85)90228-1. [DOI] [PubMed] [Google Scholar]
- (260).Horiuchi K, Amizuka N, Takeshita S, Takamatsu H, Katsuura M, Ozawa H, Toyama Y, Bonewald LF, Kudo A. J. Bone Miner. Res. 1999;14:1239. doi: 10.1359/jbmr.1999.14.7.1239. [DOI] [PubMed] [Google Scholar]
- (261).Chen JK, Shapiro HS, Wrana JL, Reimers S, Heersche JN, Sodek J. Matrix. 1991;11:133. doi: 10.1016/s0934-8832(11)80217-9. [DOI] [PubMed] [Google Scholar]
- (262).Petersen DN, Tkalcevic GT, Mansolf AL, Rivera-Gonzalez R, Brown TA. J. Biol. Chem. 2000;275:36172. doi: 10.1074/jbc.M003622200. [DOI] [PubMed] [Google Scholar]
- (263).Guo R, Quarles LD. J. Bone Miner. Res. 1997;12:1009. doi: 10.1359/jbmr.1997.12.7.1009. [DOI] [PubMed] [Google Scholar]
- (264).Byers BA, Pavlath GK, Murphy TJ, Karsenty G, Garcia AJ. J. Bone Miner. Res. 2002;17:1931. doi: 10.1359/jbmr.2002.17.11.1931. [DOI] [PubMed] [Google Scholar]
- (265).Mikami Y, Omoteyama K, Kato S, Takagi M. Biochem. Biophys. Res. Commun. 2007;362:368. doi: 10.1016/j.bbrc.2007.07.192. [DOI] [PubMed] [Google Scholar]
- (266).Nakashima K, Zhou X, Kunkel G, Zhang Z, Deng JM, Behringer RR, de Crombrugghe B. Cell. 2002;108:17. doi: 10.1016/s0092-8674(01)00622-5. [DOI] [PubMed] [Google Scholar]
- (267).Sottile V, Thomson A, McWhir J. Cloning Stem Cells. 2003;5:149. doi: 10.1089/153623003322234759. [DOI] [PubMed] [Google Scholar]
- (268).Poole KE, van Bezooijen RL, Loveridge N, Hamersma H, Papapoulos SE, Lowik CW, Reeve J. FASEB J. 2005;19:1842. doi: 10.1096/fj.05-4221fje. [DOI] [PubMed] [Google Scholar]
- (269).Sevetson B, Taylor S, Pan Y. J. Biol. Chem. 2004;279:13849. doi: 10.1074/jbc.M306249200. [DOI] [PubMed] [Google Scholar]
- (270).Kalajzic I, Braut A, Guo D, Jiang X, Kronenberg MS, Mina M, Harris MA, Harris SE, Rowe DW. Bone. 2004;35:74. doi: 10.1016/j.bone.2004.03.006. [DOI] [PubMed] [Google Scholar]
- (271).Tanaka-Kamioka K, Kamioka H, Ris H, Lim SS. J. Bone Miner. Res. 1998;13:1555. doi: 10.1359/jbmr.1998.13.10.1555. [DOI] [PubMed] [Google Scholar]
- (272).Westbroek I, De Rooij KE, Nijweide PJ. J. Bone Miner. Res. 2002;17:845. doi: 10.1359/jbmr.2002.17.5.845. [DOI] [PubMed] [Google Scholar]
- (273).Sreenath TL, Cho A, Thyagarajan T, Kulkarni AB. Genesis. 2003;35:94. doi: 10.1002/gene.10170. [DOI] [PubMed] [Google Scholar]
- (274).Wu L, Zhu F, Wu Y, Lin Y, Nie X, Jing W, Qiao J, Liu L, Tang W, Zheng X, Tian W. Cells Tissues Organs. 2008;187:103. doi: 10.1159/000110079. [DOI] [PubMed] [Google Scholar]
- (275).Hao J, Narayanan K, Muni T, Ramachandran A, George A. J. Biol. Chem. 2007;282:15357. doi: 10.1074/jbc.M701547200. [DOI] [PubMed] [Google Scholar]
- (276).Wei X, Ling J, Wu L, Liu L, Xiao Y. J. Endod. 2007;33:703. doi: 10.1016/j.joen.2007.02.009. [DOI] [PubMed] [Google Scholar]
- (277).Alvarez-Perez MA, Narayanan S, Zeichner-David M, Rodriguez Carmona B, Arzate H. Bone. 2006;38:409. doi: 10.1016/j.bone.2005.09.009. [DOI] [PubMed] [Google Scholar]
- (278).Lee SK, Krebsbach PH, Matsuki Y, Nanci A, Yamada KM, Yamada Y. Int. J. Dev. Biol. 1996;40:1141. [PubMed] [Google Scholar]
- (279).MacDougall M, Simmons D, Dodds A, Knight C, Luan X, Zeichner-David M, Zhang C, Ryu OH, Qian Q, Simmer JP, Hu CC. J. Dent. Res. 1998;77:1970. doi: 10.1177/00220345980770120401. [DOI] [PubMed] [Google Scholar]
- (280).Zeichner-David M, MacDougall M, Slavkin HC. Differentiation. 1983;25:148. doi: 10.1111/j.1432-0436.1984.tb01350.x. [DOI] [PubMed] [Google Scholar]
- (281).Iwasaki K, Bajenova E, Somogyi-Ganss E, Miller M, Nguyen V, Nourkeyhani H, Gao Y, Wendel M, Ganss B. J. Dent. Res. 2005;84:1127. doi: 10.1177/154405910508401207. [DOI] [PubMed] [Google Scholar]
- (282).Honda MJ, Sumita Y, Kagami H, Ueda M. Arch. Histol. Cytol. 2005;68:89. doi: 10.1679/aohc.68.89. [DOI] [PubMed] [Google Scholar]
- (283).Ecarot-Charrier B, Shepard N, Charette G, Grynpas M, Glorieux FH. Bone. 1988;9:147. doi: 10.1016/8756-3282(88)90004-x. [DOI] [PubMed] [Google Scholar]
- (284).Kuhn LT, Wu Y, Rey C, Gerstenfeld LC, Grynpas MD, Ackerman JL, Kim HM, Glimcher MJ. J. Bone Miner. Res. 2000;15:1301. doi: 10.1359/jbmr.2000.15.7.1301. [DOI] [PubMed] [Google Scholar]
- (285).Maniatopoulos C, Sodek J, Melcher AH. Cell Tissue Res. 1988;254:317. doi: 10.1007/BF00225804. [DOI] [PubMed] [Google Scholar]
- (286).Ohgushi H, Dohi Y, Katuda T, Tamai S, Tabata S, Suwa Y. J. Biomed. Mater. Res. 1996;32:333. doi: 10.1002/(SICI)1097-4636(199611)32:3<333::AID-JBM5>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- (287).Oliveira JM, Rodrigues MT, Silva SS, Malafaya PB, Gomes ME, Viegas CA, Dias IR, Azevedo JT, Mano JF, Reis RL. Biomaterials. 2006;27:6123. doi: 10.1016/j.biomaterials.2006.07.034. [DOI] [PubMed] [Google Scholar]
- (288).Chesnutt BM, Yuan Y, Brahmandam N, Yang Y, Ong JL, Haggard WO, Bumgardner JD. J. Biomed. Mater. Res. A. 2007;82:343. doi: 10.1002/jbm.a.31070. [DOI] [PubMed] [Google Scholar]
- (289).Sakai S, Yamada Y, Yamaguchi T, Kawakami K. Biotechnol J. 2006;1:958. doi: 10.1002/biot.200600054. [DOI] [PubMed] [Google Scholar]
- (290).George J, Kuboki Y, Miyata T. Biotechnol. Bioeng. 2006;95:404. doi: 10.1002/bit.20939. [DOI] [PubMed] [Google Scholar]
- (291).Bringas P, Jr., Nakamura M, Nakamura E, Evans J, Slavkin HC. Scanning Microsc. 1987;1:1103. [PubMed] [Google Scholar]
- (292).Evans J, Bringas P, Jr., Nakamura M, Nakamura E, Santos V, Slavkin HC. Calcif. Tissue Int. 1988;42:220. doi: 10.1007/BF02553747. [DOI] [PubMed] [Google Scholar]
- (293).About I, Mitsiadis TA. Adv. Dent. Res. 2001;15:59. doi: 10.1177/08959374010150011501. [DOI] [PubMed] [Google Scholar]
- (294).Biggers JD, Gwatkin RB, Heyner S. Exp. Cell Res. 1961;25:41. doi: 10.1016/0014-4827(61)90305-6. [DOI] [PubMed] [Google Scholar]
- (295).Hinek A, Reiner A, Poole AR. J. Cell Biol. 1987;104:1435. doi: 10.1083/jcb.104.5.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (296).Lechner J, LaVeck M. J. Tissue Culture Meth. 1985;9:43. [Google Scholar]
- (297).Bosetti M, Boccafoschi F, Leigheb M, Cannas MF. Biomol. Eng. 2007;24:613. doi: 10.1016/j.bioeng.2007.08.019. [DOI] [PubMed] [Google Scholar]
- (298).Tsuboi T, Mizutani S, Nakano M, Hirukawa K, Togari A. Calcif. Tissue Int. 2003;73:496. doi: 10.1007/s00223-002-4070-2. [DOI] [PubMed] [Google Scholar]
- (299).van Driel M, Koedam M, Buurman CJ, Roelse M, Weyts F, Chiba H, Uitterlinden AG, Pols HA, van Leeuwen JP. J. Cell Biochem. 2006;99:922. doi: 10.1002/jcb.20875. [DOI] [PubMed] [Google Scholar]
- (300).Balk ML, Bray J, Day C, Epperly M, Greenberger J, Evans CH, Niyibizi C. Bone. 1997;21:7. doi: 10.1016/s8756-3282(97)00075-6. [DOI] [PubMed] [Google Scholar]
- (301).Haaijman A, Karperien M, Lanske B, Hendriks J, Lowik CW, Bronckers AL, Burger EH. Bone. 1999;25:397. doi: 10.1016/s8756-3282(99)00189-1. [DOI] [PubMed] [Google Scholar]
- (302).Fu H, Doll B, McNelis T, Hollinger JO. J. Biomed. Mater. Res. A. 2007;83:770. doi: 10.1002/jbm.a.31356. [DOI] [PubMed] [Google Scholar]
- (303).Sato K, Nohtomi K, Shizume K, Demura H, Kanatani H, Kiyoki M, Ohashi Y, Ejiri S, Ozawa H. Bone. 1996;19:213. doi: 10.1016/8756-3282(96)00197-4. [DOI] [PubMed] [Google Scholar]
- (304).Chien HH, Lin WL, Cho MI. Calcif. Tissue Int. 1999;64:402. doi: 10.1007/pl00005822. [DOI] [PubMed] [Google Scholar]
- (305).Ellies LG, Heersche JN, Pruzanski W, Vadas P, Aubin JE. J. Dent. Res. 1993;72:18. doi: 10.1177/00220345930720010101. [DOI] [PubMed] [Google Scholar]
- (306).Sangadala S, Boden SD, Viggeswarapu M, Liu Y, Titus L. J. Biol. Chem. 2006;281:17212. doi: 10.1074/jbc.M511013200. [DOI] [PubMed] [Google Scholar]
- (307).Gordeladze JO, Drevon CA, Syversen U, Reseland JE. J. Cell Biochem. 2002;85:825. doi: 10.1002/jcb.10156. [DOI] [PubMed] [Google Scholar]
- (308).Ohazama A, Courtney JM, Sharpe PT. J. Dent. Res. 2004;83:241. doi: 10.1177/154405910408300311. [DOI] [PubMed] [Google Scholar]
- (309).Schiller PC, D’Ippolito G, Balkan W, Roos BA, Howard GA. Bone. 2001;28:362. doi: 10.1016/s8756-3282(00)00458-0. [DOI] [PubMed] [Google Scholar]
- (310).Ishikawa Y, Wu LN, Genge BR, Mwale F, Wuthier RE. J. Bone Miner. Res. 1997;12:356. doi: 10.1359/jbmr.1997.12.3.356. [DOI] [PubMed] [Google Scholar]
- (311).Zerega B, Cermelli S, Bianco P, Cancedda R, Cancedda FD. J. Bone Miner. Res. 1999;14:1281. doi: 10.1359/jbmr.1999.14.8.1281. [DOI] [PubMed] [Google Scholar]
- (312).Mada Y, Miyauchi M, Oka H, Kitagawa M, Sakamoto K, Iizuka S, Sato S, Noguchi K, Somerman MJ, Takata T. J. Periodontol. 2006;77:2051. doi: 10.1902/jop.2006.060148. [DOI] [PubMed] [Google Scholar]
- (313).Iba K, Chiba H, Yamashita T, Ishii S, Sawada N. Cell Struct. Funct. 2001;26:227. doi: 10.1247/csf.26.227. [DOI] [PubMed] [Google Scholar]
- (314).Shibuya N, Nemoto E, Kanaya S, Kunii R, Shimauchi H. J. Periodontal Res. 2005;40:432. doi: 10.1111/j.1600-0765.2005.00811.x. [DOI] [PubMed] [Google Scholar]
- (315).Wu LN, Ishikawa Y, Nie D, Genge BR, Wuthier RE. J. Cell Biochem. 1997;65:209. doi: 10.1002/(sici)1097-4644(199705)65:2<209::aid-jcb7>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- (316).Nuka S, Sawada N, Iba K, Chiba H, Ishii S, Mori M. Cell Struct. Funct. 1997;22:27. doi: 10.1247/csf.22.27. [DOI] [PubMed] [Google Scholar]
- (317).Wennberg C, Hessle L, Lundberg P, Mauro S, Narisawa S, Lerner UH, Millan JL. J. Bone Miner. Res. 2000;15:1879. doi: 10.1359/jbmr.2000.15.10.1879. [DOI] [PubMed] [Google Scholar]
- (318).Schinke T, Amendt C, Trindl A, Poschke O, Muller-Esterl W, Jahnen-Dechent W. J. Biol. Chem. 1996;271:20789. doi: 10.1074/jbc.271.34.20789. [DOI] [PubMed] [Google Scholar]
- (319).Huffman NT, Keightley JA, Chaoying C, Midura RJ, Lovitch D, Veno PA, Dallas SL, Gorski JP. J. Biol. Chem. 2007;282:26002. doi: 10.1074/jbc.M701332200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (320).Midura RJ, Wang A, Lovitch D, Law D, Powell K, Gorski JP. J. Biol. Chem. 2004;279:25464. doi: 10.1074/jbc.M312409200. [DOI] [PubMed] [Google Scholar]
- (321).Gordon JA, Tye CE, Sampaio AV, Underhill TM, Hunter GK, Goldberg HA. Bone. 2007;41:462. doi: 10.1016/j.bone.2007.04.191. [DOI] [PubMed] [Google Scholar]
- (322).Yagami K, Suh JY, Enomoto-Iwamoto M, Koyama E, Abrams WR, Shapiro IM, Pacifici M, Iwamoto M. J. Cell Biol. 1999;147:1097. doi: 10.1083/jcb.147.5.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (323).Addison WN, Azari F, Sorensen ES, Kaartinen MT, McKee MD. J. Biol. Chem. 2007;282:15872. doi: 10.1074/jbc.M701116200. [DOI] [PubMed] [Google Scholar]
- (324).Wada T, McKee MD, Steitz S, Giachelli CM. Circ. Res. 1999;84:166. doi: 10.1161/01.res.84.2.166. [DOI] [PubMed] [Google Scholar]


