Abstract
There is compelling evidence that peroxisome proliferator-activated receptor-β/δ (PPARβ/δ) mediates terminal differentiation and is associated with inhibition of cell growth. However, it was recently suggested that growth of two human lung cancer cell lines is enhanced by PPARβ/δ. The goal of the present study was to provide insight in resolving this controversy. Therefore, the effect of ligand activation of PPARβ/δ in A549 and H1838 human lung cancer cell lines was examined using two high affinity PPARβ/δ ligands. Ligand activation of PPARβ/δ caused up-regulation of a known PPARβ/δ target gene, angiopoietin-like 4 (Angptl4) but did not influence expression of phosphatase and tensin homolog (PTEN) or phosphorylation of protein kinase B (Akt), and did not affect cell growth. Results from this study demonstrate that two human lung cancer cell lines respond to ligand activation of PPARβ/δ by modulation of target gene expression (Angptl4), but fail to exhibit significant modulation of cell proliferation.
Keywords: peroxisome proliferator-activated receptor, lung cancer, nuclear receptor, cell proliferation, PTEN
1. Introduction
While there are reports suggesting that peroxisome proliferator-activated receptor-β/δ (PPARβ/δ) can either potentiate or attenuate cancer (reviewed in (Burdick et al. 2006; Peters et al. 2008)), there is a large body of evidence demonstrating that PPARβ/δ can mediate terminal differentiation in many cell types (reviewed in (Burdick et al. 2006; Peters et al. 2008)). The induction of terminal differentiation is associated with withdrawal from cell cycling, which is also consistent with numerous reports showing inhibition of cell proliferation through PPARβ/δ-dependent mechanisms in cell types ranging from keratinocytes, colonocytes, vascular smooth muscle cells, endothelial cells and human cancer cell lines (reviewed in (Burdick et al. 2006; Peters et al. 2008)). For example, inhibition of cell proliferation was found in a human lung adenocarcinoma cell line (A549) in response to L165041 (Fukumoto et al. 2005), a synthetic high affinity PPARβ/δ ligand. Inhibition of A549 cell proliferation was associated with reduced expression of cyclin D and proliferating cellular nuclear antigen (PCNA), proteins essential for cell cycle progression (Fukumoto et al. 2005). Given the observed inhibition of cell proliferation by ligand activation of PPARβ/δ in a human lung cancer cell line (Fukumoto et al. 2005), it is of interest to note that Raf-dependent lung cancer in a mouse transgenic model is also exacerbated in the absence of PPARβ/δ expression (Muller-Brusselbach et al. 2007). Similarly, inhibition of lung cancer is also observed in mice overexpressing prostacyclin synthase (Keith et al. 2002; Keith et al. 2004), which is consistent with the idea that activating PPARβ/δ will inhibit tumorigenesis since prostacyclin may be an endogenous ligand for PPARβ/δ (Gupta et al. 2000). In contrast to the above-mentioned findings, two recent publications suggest that ligand activation of PPARβ/δ potentiates cell proliferation of human lung cancer cell lines (Han et al. 2008; Pedchenko et al. 2008). In the first report, it was suggested that activation of PPARβ/δ causes a decrease in the expression of PTEN leading to increased cell proliferation via an interaction with the p65 subunit of NF-κB in the H1838 human lung cancer cell line (Han et al. 2008). However, these investigators quantified cell proliferation at a single timepoint and detection of PTEN expression was performed using enhanced chemilluminscence rather than quantitative radioactive detection. The mechanistic basis why H1838 cells are reported to exhibit enhanced cell proliferation by ligand activation of PPARβ/δ (Han et al. 2008), in contrast to earlier reports (Fukumoto et al. 2005; Keith et al. 2002; Keith et al. 2004; Muller-Brusselbach et al. 2007), was not thoroughly examined (Han et al. 2008). In the second report, it was suggested that ligand activation of PPARβ/δ in A549 human lung cancer cells causes down-regulation of PTEN expression, increased expression of 3-phosphoinositide-dependent kinase-1 (PDPK1), increased phosphorylation of protein kinase B (Akt) and inhibition of apoptosis (Pedchenko et al. 2008). These findings are surprising since this is in direct contrast to previous in vitro and in vivo analysis (Fukumoto et al. 2005; Keith et al. 2002; Keith et al. 2004; Muller-Brusselbach et al. 2007). Given these disparities, the present study used two high affinity ligands for PPARβ/δ (GW0742 and GW501516) and highly quantitative approaches to analyze expression of cell cycle regulatory proteins (PTEN, PDPK1 and phosphorylated Akt) and cell proliferation in two human lung cancer cell lines, H1838 and A549.
2. Experimental procedures
2.1 Cell culture
The human lung adenocarcinoma cell lines A549 (CCL-185) and H1838 (CRL-5899) were obtained from American Type Culture Collection (Manassas, VA). Cells were cultured according to the recommended procedures; A549 cells were cultured in Ham's F-12K medium supplemented with 10% fetal bovine serum (FBS) and H1838 cells were cultured in RPMI-1640 medium supplemented with 10% FBS. To examine mRNA or protein expression, cells were plated in 6-well tissue culture plates, and cultured until 80% confluency at which time they were treated with either GW0742 or GW501516 (Vehicle control (DMSO), 0.1, 1.0 or 10.0 µM) for 24 hours. After this treatment, mRNA or protein was isolated and used for quantitative realtime PCR (qPCR) analysis or western blotting, respectively, as described below. To examine cell proliferation by Coulter counting, A549 or H1838 cells were plated in 12-well tissue culture plates (25,000/well or 40,000/well, respectively). To examine cell cycle distribution by flow cytometry, A549 or H1838 cells were plated in 6-well tissue culture plates (300,000/well). Twenty-four hours after plating, cells were treated with either GW0742 or GW501516 (Vehicle control (DMSO), 0.1, 1.0 or 10.0 µM) and cell proliferation and cell cycle kinetics was quantified as described below using either a Coulter counter or flow cytometry.
2.2 qPCR analysis of PPARβ/δ target gene expression
RNA was isolated from control and ligand treated cells as previously described (Hollingshead et al. 2007). Expression of angiopoietin-like protein-4 (Angptl4), a well characterized PPARβ/δ target gene, was measured using qPCR as previously described (Hollingshead et al. 2007). Triplicate samples were examined for each treatment group.
2.3 Quantitative western blotting
Protein samples were prepared from control- and ligand-treated cells using lysis buffer containing protease and phosphatase inhibitors. Thirty µg of protein per sample was resolved using sodium dodecyl sulfate-polyacrylamide gels. After transferring the gels onto polyvinylidene fluoride using electroblotting, membranes were blocked with 5% milk or 5% bovine serum albumin in Tris Buffered Saline Tween 20 (TBST) and incubated overnight with primary antibodies at 4°C. Following incubation with the primary antibody, membranes were washed and then incubated with biotinylated secondary antibody (Jackson ImmunoResearch Laboratories, West Grove, PA). Immunoreactive proteins were detected by 125I-labeled streptavidin. Hybridization signals for specific proteins were normalized to the hybridization signal of the housekeeping gene lactate dehydrogenase (LDH). Four independent samples were analyzed for each treatment group. The following antibodies were used: anti-Akt, anti-phospho-Akt, PTEN (Cell Signaling Technology, Beverly, MA), PDPK1 (BD Transduction Laboratories, Franklin Lakes, NJ), and anti-LDH (Rockland, Gilbertsville, PA).
2.4 Quantification of cell proliferation and cell cycle distribution
Cell growth was assessed with either a Coulter counter or by flow cytometry. For quantifying cell growth with a Coulter counter, cells were seeded on a 12-well plate and cultured overnight in control medium. After this 24-hour culture period, cells were then cultured in medium containing either GW0742 or GW501516 for up to 96 hours. Cells were counted every 24 hours with a Z1 Coulter particle counter®. Triplicate samples for each treatment were used for each time point, and each replicate was counted three times. Growth was depicted as the average number of cells over a 96-hour treatment period (beginning 24 hours post-plating). For flow cytometry analysis, cells were plated on a 6-well plate and cultured overnight in control medium. After this 24-hour culture period, cells were then cultured in medium containing either GW0742 or GW501516 for 24 hours. During the last hour of cell culture in the presence of the ligands, cells were pulsed with 10 µg/mL of bromodeoxyuridine (BrdU) for 1 hour. Triplicate samples for each treatment were used. The cells were then trypsinized, washed in cold-phosphate buffered saline (PBS), and pelleted (300 × g/5 minutes). Cells were then fixed in ice-cold 70% ethanol, incubated with wash buffer (0.5% Tween-20 PBS), and pelleted. The cells were then incubated in denaturing solution (2M HCl/0.5% Triton X-100) for 20 minutes at room temperature. After incubating in wash buffer and pelleting, the cell pellet was resuspended in 0.1 M sodium-borate. The cells were washed again and pelleted. The cells then resuspended with a 1:100 dilution of FITC-labeled anti-BrdU antibody (Phoenix Flow Systems, San Diego, CA) in dilution buffer (0.5% Tween-20/0.5% BSA PBS) for 20 minutes in dark. The cells were washed again and counterstained with 10 µg/mL propidium iodide (PI) solution (0.1% Triton X-100 PBS) and analyzed by flow cytometry to detect both fluorescein and PI using a FC500 flow cytometer (Beckman Coulter, Miami Lakes, FL).
3. Results
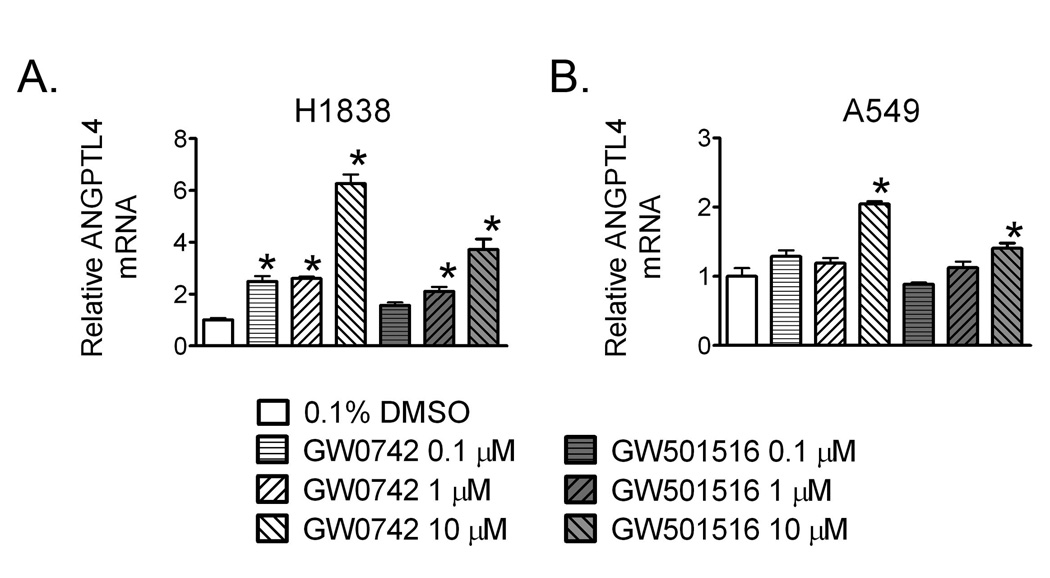
Ligand activation of PPARβ/δ by either GW0742 or GW501516 in H1838 human lung cancer cells caused an increased in the expression of the known PPARβ/δ target gene Angptl4 (Fig. 1A). Increased expression of Angptl4 was observed at concentrations ranging from 0.1 to 10.0 µM in H1838 cells by GW0742. Increased expression of Angptl4 was observed with either 1.0 or 10.0 µM GW501516 in H1838 cells. Ligand activation of PPARβ/δ in A549 human lung cancer cells caused an increased in the expression of mRNA encoding Angptl4 by both GW0742 and GW501516, but this increase was only observed in response to 10.0 µM ligand (Fig. 1B). These results demonstrate that both A549 and H1838 human lung adenocarcinoma cell lines express a functional receptor as both cell lines respond to PPARβ/δ agonists by increasing expression of a known PPARβ/δ target gene, Angptl4. While both cell lines express functional protein (Fig. 2), it is worth noting that expression of protein is considerably lower in the human lung cancer cell lines as compared to mouse tissues with relatively high expression such as intestine, liver and keratinocytes (Girroir et al. 2008b).
Fig. 1.
Ligand activation of PPARβ/δ upregulates expression of the known PPARβ/δ target gene Angptl4. H1838 or A549 cells were treated for 24 hours with the indicated concentration of either GW0742 or GW501516. qPCR was used to quantify expression of Angptl4 mRNA. *Significantly different than control, P ≤ 0.05.
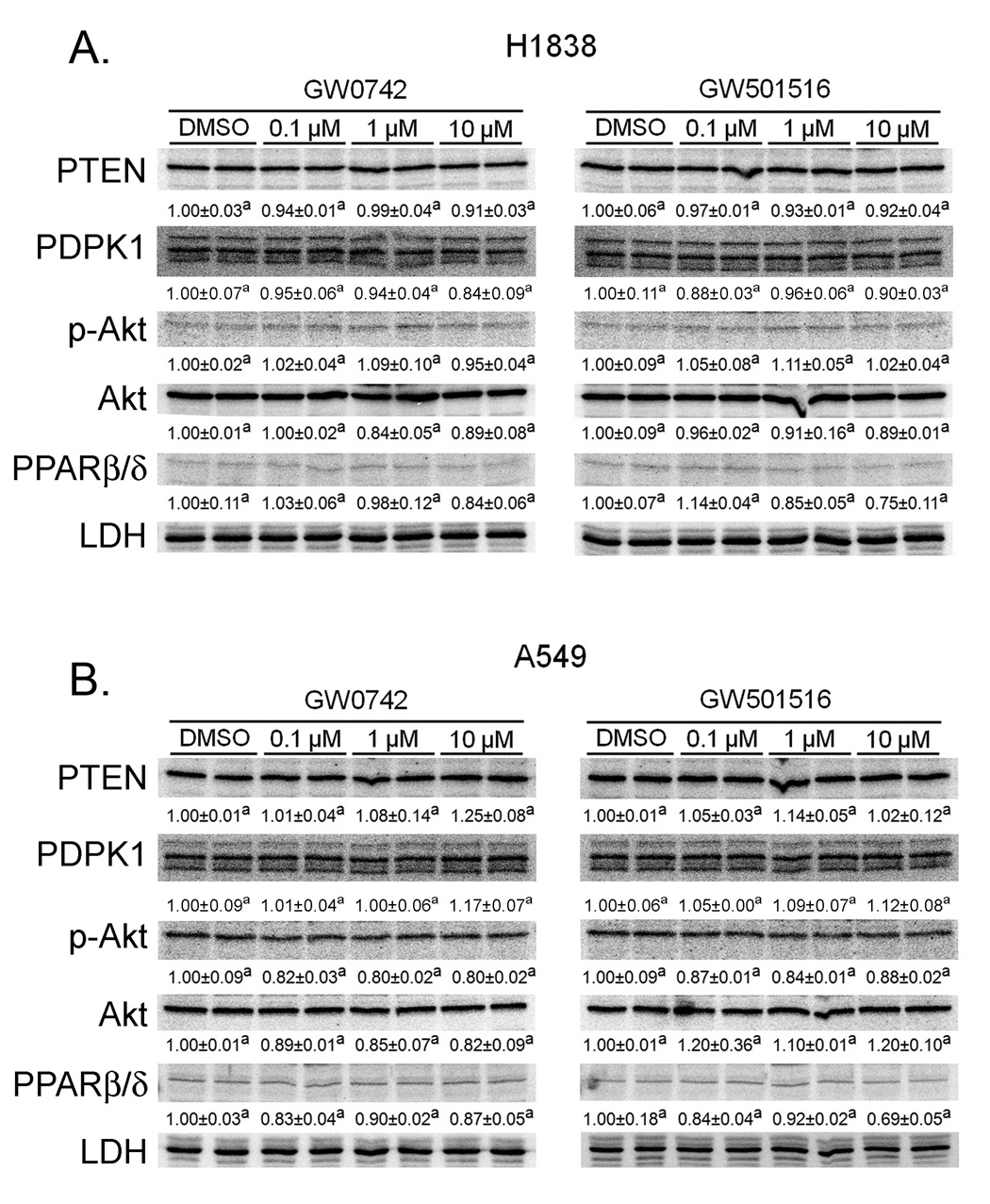
Fig. 2.
Ligand activation of PPARβ/δ does not influence expression of PTEN, PDPK1 or phosphorylation of Akt in H1838 or A549 cells. H1838 or A549 cells were treated for 24 hours with the indicated concentration of either GW0742 or GW501516. Quantitative western blotting was performed to quantify protein expression of PTEN, PDPK1 or phosphorylation of Akt. Hybridization signals were normalized to a loading control (LDH). Values with different superscripts are significantly different from control, P ≤ 0.05.
Previous work by others suggests that ligand activation of PPARβ/δ potentiates cell growth by decreasing PTEN expression, increasing PDPK1 expression and enhancing phosphorylation of Akt, which collectively lead to inhibition of apoptotic signalling and increased cell proliferation (Han et al. 2008; Pedchenko et al. 2008). In contrast, other studies are inconsistent with these findings (Fukumoto et al. 2005; Keith et al. 2002; Keith et al. 2004; Muller-Brusselbach et al. 2007). To begin to determine if the differences in the reported mechanisms proposed for the effect of ligand activation in human lung cancer cell lines could be due to differences in cell lines examined, the present study examined the effect of GW0742 and GW501516 on PTEN, PDPK1 and phosphorylation of Akt in both H1838 and A549 cells. Additionally, the present studies used a broad concentration range of ligand and the same timepoint, which caused maximal down-regulation of PTEN expression (Han et al. 2008). In contrast to previous studies (Han et al. 2008; Pedchenko et al. 2008), results from the present studies show that ligand activation of PPARβ/δ had no effect on the expression of PTEN, PDPK1 or phosphorylation of Akt in either H1838 or A549 cells in response to a concentration range of PPARβ/δ ligands known to specifically activate PPARβ/δ (Fig. 2). This demonstrates that quantitative expression of PTEN, PDPK1 and phosphorylation of Akt are not modulated by ligand activation of PPARβ/δ in either A549 or H1838 human lung cancer cell lines.
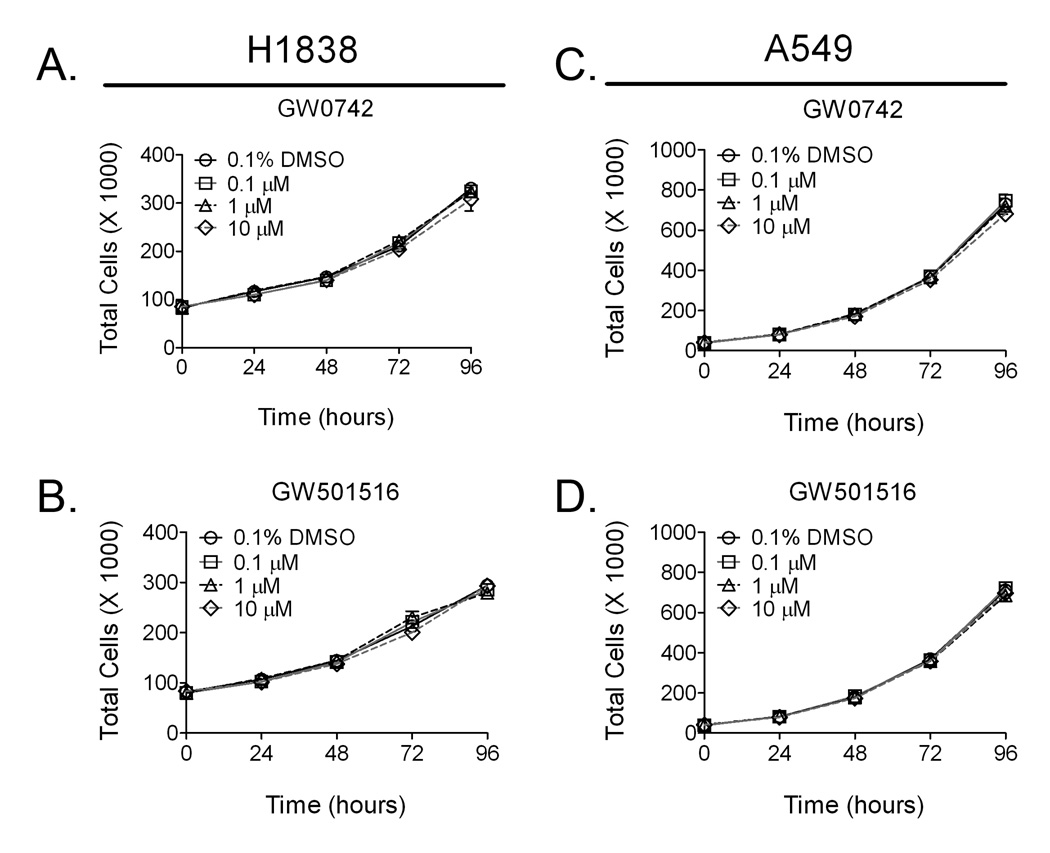
Quantitative analysis of cell proliferation was examined using two different methods. No effect of cell proliferation was observed using Coulter counting over a 96 hour period following exposure to either GW0742 or GW501516 at concentrations known to specifically activate PPARβ/δ (Fig. 3). Similarly, examination by flow cytometry revealed no differences in the percentage of cells in each phase of the cell cycle (Table 1).
Fig. 3.
Ligand activation of PPARβ/δ does not influence cell growth of H1838 or A549 cells. H1838 or A549 cells were plated and 24 hours later treated with the indicated concentration of either GW0742 or GW501516 for 96 hours.
Table 1. Cell cycle distribution of H1838 or A549 human lung adenocarcinoma cell lines following ligand activation of PPARβ/δ.
Cells were treated with either GW0742 or GW501516 for 24 hours and examined for cell cycle analysis by flow cytometry. Data are presented as the percentage of cells in each phase of the cell cycle. Values with different superscripts are significantly different than controls, P ≤ 0.05.
| H1838 cells | ||||
|---|---|---|---|---|
| Treatment | Concentration [µM] | G1 (%) | S (%) | G2/M (%) |
| 0.1% DMSO | – | 48.2 ± 0.3a | 36.0 ± 1.2a | 13.9 ± 0.8a |
| GW0742 | 0.1 | 51.0 ± 2.3a | 34.5 ± 2.5a | 12.4 ± 0.1a |
| GW0742 | 1.0 | 49.8 ± 1.2a | 35.2 ± 0.7a | 13.4 ± 0.1a |
| GW0742 | 10.0 | 50.3 ± 1.5a | 34.2 ± 0.4a | 13.5 ± 0.9a |
| GW501516 | 0.1 | 49.9 ± 1.8a | 34.3 ± 0.4a | 13.4 ± 1.2a |
| GW501516 | 1.0 | 49.3 ± 0.1a | 34.6 ± 0.4a | 13.8 ± 0.8a |
| GW501516 | 10.0 | 51.8 ± 1.8a | 33.7 ± 1.6a | 12.3 ± 0.3a |
| A549 cells | ||||
| Treatment | Concentration [µM] | G1 (%) | S (%) | G2/M (%) |
| 0.1% DMSO | – | 62.9 ± 1.1a | 26.0 ± 0.6a | 10.2 ± 0.4a |
| GW0742 | 0.1 | 62.9 ± 0.1a | 26.3 ± 0.3a | 9.7 ± 0.2a |
| GW0742 | 1.0 | 64.4 ± 0.6a | 24.8 ± 0.4a | 9.7 ± 0.1a |
| GW0742 | 10.0 | 62.7 ± 1.3a | 26.3 ± 1.2a | 10.2 ± 0.3a |
| GW501516 | 0.1 | 64.3 ± 0.3a | 24.8 ± 0.3a | 9.8 ± 0.7a |
| GW501516 | 1.0 | 63.7 ± 0.4a | 24.7 ± 1.6a | 10.4 ± 1.6a |
| GW501516 | 10.0 | 65.2 ± 0.1a | 24.2 ± 0.2a | 9.8 ± 0.1a |
4. Discussion
Results from the present study reveal significant differences with other studies reported in the literature and raise serious concern regarding the mechanisms described for PPARβ/δ in lung cancer cell growth. In the present study, no significant effect on the expression of proteins that modulate cell growth (PTEN, PDPK1, phosphorylated Akt) and no changes in cell cycle progression were observed in either H1838 or A549 human lung cancer cells. Collectively, this suggests that previous work indicating that PPARβ/δ attenuates or potentiates cell growth during lung cancer should be carefully re-examined.
One of the earlier reports suggesting a link between PPARβ/δ and lung cancer cell growth showed that L165041 inhibited cell proliferation of A549 cells using concentrations greater than 20 µM (Fukumoto et al. 2005). At this concentration, a significant down-regulation of cyclin D and PCNA was observed and this correlated well with the observed inhibition of cell growth, in particular a decrease in the percentage of cells entering S phase (Fukumoto et al. 2005). In contrast, no changes in the percentage of cells in S phase were observed in the present study and no significant differences in cell proliferation were found in either H1838 or A549 cells using two high affinity ligands and highly quantitative approaches. It is important to note that the present study used concentrations of PPARβ/δ agonists that will activate PPARβ/δ, and that the concentrations between 0.1 and 1.0 µM are very specific for PPARβ/δ as demonstrated using mouse primary keratinocytes (Kim et al. 2006). Thus, it remains possible that higher concentrations of GW0742 or GW501516 could lead to inhibition of cell proliferation in either H1838 or A549 cells as observed in the previous study (Fukumoto et al. 2005). It is also possible that inhibition of cell growth might be observed in the absence of serum in the culture medium, as this has previously been shown to cause more growth inhibition by ligand activation of PPARβ/δ in other human cancer cell lines (Girroir et al. 2008a; Hollingshead et al. 2007). However, this model may not be suitable as it is unlikely that cells encounter an environment that lacks the presence of serum growth factors. The results from cell culture suggest that in vivo models are more appropriate to examine the role of PPARβ/δ in lung cancer. In fact there is evidence from in vivo models suggesting that ligand activation of PPARβ/δ inhibits lung cancer. For example, in the absence of PPARβ/δ expression, over-expression of oncogenic Raf leads to exacerbated lung carcinogenesis (Muller-Brusselbach et al. 2007). However, it is also possible that this effect is due to ligand-independent modulation of cell signaling pathways. Indeed, PPARβ/δ is known to interfere with NF-κB signaling and can also repress gene expression in the absence of exogenous ligand (reviewed in (Burdick et al. 2006; Kilgore and Billin 2008; Peters et al. 2008)). The observation that lung cancer is inhibited in mice over-expressing prostacyclin synthase (Keith et al. 2002; Keith et al. 2004) supports the hypothesis that ligand activation attenuates lung cancer, as it is thought that prostacyclin may act as an endogenous ligand for PPARβ/δ (Gupta et al. 2000). Importantly, there is evidence from many other models showing that PPARβ/δ mediates the induction of terminal differentiation, which is associated with inhibition of cell proliferation (reviewed in (Burdick et al. 2006; Peters et al. 2008)) and that PPARβ/δ has potent anti-inflammatory activities (reviewed in (Kilgore and Billin 2008)). Collectively, results from the present studies and these reports in the literature (Burdick et al. 2006; Fukumoto et al. 2005; Keith et al. 2002; Keith et al. 2004; Kilgore and Billin 2008; Muller-Brusselbach et al. 2007; Peters et al. 2008) strongly support the hypothesis that PPARβ/δ attenuates carcinogenesis. Nevertheless, further studies are necessary to more definitively examine the hypothesis that ligand activation can attenuate lung cancer.
In contrast to several studies (Fukumoto et al. 2005; Keith et al. 2002; Keith et al. 2004; Muller-Brusselbach et al. 2007) including the present suggesting that PPARβ/δ attenuates cell proliferation of lung cancer cells, the outcome of two recent reports suggests that PPARβ/δ potentiates cell proliferation through similar mechanisms (Han et al. 2008; Pedchenko et al. 2008). These authors proposed that ligand activation of PPARβ/δ causes a down-regulation of PTEN expression (Han et al. 2008; Pedchenko et al. 2008) and increased expression of PDPK1 (Pedchenko et al. 2008), leading to increased phosphorylation of Akt (Pedchenko et al. 2008) and anti-apoptotic activity and increased cell proliferation (Han et al. 2008; Pedchenko et al. 2008). The present study provided mechanistic insight that can resolve this controversy. First, analysis of cell cycle distribution by flow cytometry demonstrated that neither ligand influenced cell cycle progression, which was also consistent with the lack of changes in cell growth as determined by cell counting. If ligand activation of PPARβ/δ caused anti-apoptotic activities, then significant differences in cell number and cell cycle kinetics should have been observed but this was not the case. Secondly, no changes in PTEN, PDPK1 expression or phosphorylation of Akt were observed following ligand activation of PPARβ/δ by either GW0742 or GW501516 in either H1838 or A549 cells, despite demonstrating up-regulation of the known PPARβ/δ target gene Angptl4. Di-Poi et al suggested that PPARβ/δ mediates anti-apoptotic signaling via down regulation of PTEN expression and increased expression of PDPK1 and phosphorylation of Akt (Di-Poi et al. 2002), which was based on analysis in keratinocytes that did not express normal markers of cell growth (e.g. keratin 6). This is important to note because in primary keratinocytes that do express normal markers of growth and differentiation, these changes in the PTEN/PDPK1/Akt pathway do not occur in response to ligand activation of PPARβ/δ (Burdick et al. 2007). A lack of change in this putative PPARβ/δ-dependent signaling pathway has also been reported in a number of model systems including normal intestinal epithelium and vascular smooth muscle cells (Lim et al. 2008; Peters et al. 2008). Why one research group observes changes in the PTEN/PDPK1/Akt pathway that lead to anti-apoptotic activity while other groups do not is an important topic that needs to be closely examined in the future. It remains a possibility that this could be due to cell context-specific changes. For example, while normal keratinocytes do not exhibit alterations in the PTEN/PDPK1/Akt pathway in response to ligand activation of PPARβ/δ (Burdick et al. 2007), perhaps keratinocytes that have undergone molecular changes (e.g. events associated with the lack of keratin 6) become sensitive to this regulation. Similarly, cancer cell lines can undergo genetic alterations after significant passages, and perhaps these changes account for the reported disparities. Further work is necessary to examine this idea. Lastly, the idea that ligand activation of PPARβ/δ will potentiate cell proliferation of lung cancer cell lines as suggested by others (Han et al. 2008; Pedchenko et al. 2008), is also inconsistent with a large body of literature (Burdick et al. 2006; Fukumoto et al. 2005; Keith et al. 2002; Keith et al. 2004; Kilgore and Billin 2008; Muller-Brusselbach et al. 2007; Peters et al. 2008). There is compelling evidence that PPARβ/δ mediates terminal differentiation in many cell types, and that PPARβ/δ can inhibit of cell proliferation (reviewed in (Burdick et al. 2006; Peters et al. 2008)). Additionally, since it is well accepted that inhibiting inflammatory signaling is an effective approach to inhibit tumor growth and progression, the idea that ligand activation of PPARβ/δ will potentiate cell proliferation of lung cancer is inconsistent with numerous reports demonstrating potent anti-inflammatory activities of PPARβ/δ (reviewed in (Kilgore and Billin 2008)).
Is it possible that PPARβ/δ exhibits varied effects in function similar to those observed with transforming growth factor-β (TGFβ)? Expression of TGFβ during early cancer progression is known to be preventive whereas increased expression during the latter stages of tumorigenesis is known to exacerbate the process (Glick et al. 2008). This change in function is thought to occur through unidentified mechanisms that alter the normal cellular response to TGFβ and force the cell to use this signaling to enhance the growth of the tumor cell rather than inhibit growth. The potential of PPARβ/δ to exert varied effects during various stages of carcinogenesis in a well-defined model of lung adenocarcinomas deserves a thorough examination in the future.
Acknowledgements
Funding source
Supported in part by the National Institutes of Health grants CA124533 (J.M.P.).
The authors gratefully acknowledge Drs. Andrew Billin and Timothy Willson for providing the GW0742 used for these studies and Elaine Kunze, Susan Magargee, and Nicole Bem from the Center for Quantitative Cell Analysis at the Huck Institutes of Life Sciences of The Pennsylvania State University for their technical support with flow cytometry and data analysis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest
None
References
- Burdick AD, Bility MT, Girroir EE, Billin AN, Willson TM, Gonzalez FJ, Peters JM. Ligand activation of peroxisome proliferator-activated receptor-beta/delta(PPARbeta/delta) inhibits cell growth of human N/TERT-1 keratinocytes. Cell Signal. 2007;19:1163–1171. doi: 10.1016/j.cellsig.2006.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdick AD, Kim DJ, Peraza MA, Gonzalez FJ, Peters JM. The role of peroxisome proliferator-activated receptor-beta/delta in epithelial cell growth and differentiation. Cell Signal. 2006;18:9–20. doi: 10.1016/j.cellsig.2005.07.009. [DOI] [PubMed] [Google Scholar]
- Di-Poi N, Tan NS, Michalik L, Wahli W, Desvergne B. Antiapoptotic role of PPARβ in keratinocytes via transcriptional control of the Akt1 signaling pathway. Molecular Cell. 2002;10:721–733. doi: 10.1016/s1097-2765(02)00646-9. [DOI] [PubMed] [Google Scholar]
- Fukumoto K, Yano Y, Virgona N, Hagiwara H, Sato H, Senba H, Suzuki K, Asano R, Yamada K, Yano T. Peroxisome proliferator-activated receptor delta as a molecular target to regulate lung cancer cell growth. FEBS Lett. 2005;579:3829–3836. doi: 10.1016/j.febslet.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Girroir EE, Hollingshead HE, Billin AN, Willson TM, Robertson GP, Sharma AK, Amin S, Gonzalez FJ, Peters JM. Peroxisome proliferator-activated receptor-beta/delta (PPARbeta/delta) ligands inhibit growth of UACC903 and MCF7 human cancer cell lines. Toxicology. 2008a;243:236–243. doi: 10.1016/j.tox.2007.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girroir EE, Hollingshead HE, He P, Zhu B, Perdew GH, Peters JM. Quantitative expression patterns of peroxisome proliferator-activated receptor-beta/delta (PPARbeta/delta) protein in mice. Biochem Biophys Res Commun. 2008b;371:456–461. doi: 10.1016/j.bbrc.2008.04.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glick AB, Perez-Lorenzo R, Mohammed J. Context-dependent regulation of cutaneous immunological responses by TGFbeta1 and its role in skin carcinogenesis. Carcinogenesis. 2008;29:9–14. doi: 10.1093/carcin/bgm215. [DOI] [PubMed] [Google Scholar]
- Gupta RA, Tan J, Krause WF, Geraci MW, Willson TM, Dey SK, DuBois RN. Prostacyclin-mediated activation of peroxisome proliferator-activated receptor delta in colorectal cancer. Proc Natl Acad Sci U S A. 2000;97:13275–13280. doi: 10.1073/pnas.97.24.13275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S, Ritzenthaler JD, Zheng Y, Roman J. PPARbeta/delta agonist stimulates human lung carcinoma cell growth through inhibition of PTEN expression: the involvement of PI3K and NF-kappaB signals. Am J Physiol Lung Cell Mol Physiol. 2008;294:L1238–L1249. doi: 10.1152/ajplung.00017.2008. [DOI] [PubMed] [Google Scholar]
- Hollingshead HE, Killins RL, Borland MG, Girroir EE, Billin AN, Willson TM, Sharma AK, Amin S, Gonzalez FJ, Peters JM. Peroxisome proliferator-activated receptor-beta/delta (PPARbeta/delta) ligands do not potentiate growth of human cancer cell lines. Carcinogenesis. 2007;28:2641–2649. doi: 10.1093/carcin/bgm183. [DOI] [PubMed] [Google Scholar]
- Keith RL, Miller YE, Hoshikawa Y, Moore MD, Gesell TL, Gao B, Malkinson AM, Golpon HA, Nemenoff RA, Geraci MW. Manipulation of pulmonary prostacyclin synthase expression prevents murine lung cancer. Cancer Res. 2002;62:734–740. [PubMed] [Google Scholar]
- Keith RL, Miller YE, Hudish TM, Girod CE, Sotto-Santiago S, Franklin WA, Nemenoff RA, March TH, Nana-Sinkam SP, Geraci MW. Pulmonary prostacyclin synthase overexpression chemoprevents tobacco smoke lung carcinogenesis in mice. Cancer Res. 2004;64:5897–5904. doi: 10.1158/0008-5472.CAN-04-1070. [DOI] [PubMed] [Google Scholar]
- Kilgore KS, Billin AN. PPARbeta/delta ligands as modulators of the inflammatory response. Curr Opin Investig Drugs. 2008;9:463–469. [PubMed] [Google Scholar]
- Kim DJ, Bility MT, Billin AN, Willson TM, Gonzalez FJ, Peters JM. selectively induces differentiation and inhibits cell proliferation. Cell Death Differ. 2006;13:53–60. doi: 10.1038/sj.cdd.4401713. [DOI] [PubMed] [Google Scholar]
- Lim HJ, Lee S, Park JH, Lee KS, Choi HE, Chung KS, Lee HH, Park HY. PPARdelta agonist L-165041 inhibits rat vascular smooth muscle cell proliferation and migration via inhibition of cell cycle. Atherosclerosis. 2008 doi: 10.1016/j.atherosclerosis.2008.05.023. [DOI] [PubMed] [Google Scholar]
- Muller-Brusselbach S, Ebrahimsade S, Jakel J, Eckhardt J, Rapp UR, Peters JM, Moll R, Muller R. Growth of transgenic RAF-induced lung adenomas is increased in mice with a disrupted PPARbeta/delta gene. Int J Oncol. 2007;31:607–611. [PubMed] [Google Scholar]
- Pedchenko TV, Gonzalez AL, Wang D, Dubois RN, Massion PP. Peroxisome Proliferator-Activated Receptor {beta}/{delta} Expression and Activation in Lung Cancer. Am J Respir Cell Mol Biol. 2008 doi: 10.1165/rcmb.2007-0426OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters JM, Hollingshead HE, Gonzalez FJ. Role of peroxisome-proliferator-activated receptor beta/delta (PPARbeta/delta) in gastrointestinal tract function and disease. Clin Sci (Lond) 2008;115:107–127. doi: 10.1042/CS20080022. [DOI] [PMC free article] [PubMed] [Google Scholar]