Abstract
Sgk1 is an aldosterone-induced kinase that regulates epithelial sodium channel (ENaC)-mediated Na+ transport in the collecting duct and connecting tubule of the kidney. The NH2 terminus of Sgk1 contains instability motifs that direct the ubiquitination of Sgk1 resulting in a rapidly degraded protein. By bioinformatic analysis, we identified a 5′ variant alternate transcript of human Sgk1 (Sgk1_v2) that is widely expressed, is conserved from rodent to humans, and is predicted to encode an Sgk1 isoform, Sgk1_i2, with a different NH2 terminus. When expressed in HEK293 cells, Sgk1_i2 was more abundant than Sgk1 because of an increased protein half-life and this correlated with reduced ubiquitination of Sgk1_i2 and enhanced surface expression of ENaC. Immunocytochemical studies demonstrated that in contrast to Sgk1, Sgk1_i2 is preferentially targeted to the plasma membrane. When coexpressed with ENaC subunits in FRT epithelia, Sgk1_i2 had a significantly greater effect on amiloride-sensitive Na+ transport compared with Sgk1. Together, the data demonstrate that a conserved NH2-terminal variant of Sgk1 shows improved stability, enhanced membrane association, and greater stimulation of epithelial Na+ transport in a heterologous expression system.
Keywords: phosphorylation
serum and glucocorticoid-induced protein kinase 1 (Sgk1) is closely related to the PKB/Akt family of serine/threonine kinases and is highly conserved in all eukaryotes. Sgk1 performs key roles in such diverse functions as regulation of cell volume and cell survival and in epithelial ion transport (15, 17). Sgk1 expression is rapidly induced by serum, corticosteroid hormones, various growth factors, heat shock, hypertonic and oxidative stress and is activated by phosphorylation. In turn, active Sgk1 interacts with a number of downstream targets including ion channels, carriers, pumps, enzymes, and transcription factors. Among its targets is the apical heteromultimeric epithelial sodium channel (ENaC), composed of α, β, and γ subunits, which is expressed in the collecting duct and connecting tubule of the kidney and in airway and alveolar epithelia of the lung.
The central portion of Sgk1 contains the catalytic domain which includes the ATP binding site at K127 and the two principal sites of phosphorylation, T256 and S422. Phosphorylation is induced by a signaling cascade that includes PI 3-kinase, the PIP-3-dependent kinase, PDK1, and a third unknown kinase (15, 17). Sgk1 is fully activated when phosphorylated on serine 422 in a PI3-K-dependent manner and at Thr256 in a PDK-1-dependent manner. Also, within the catalytic domain is a PY motif that appears to be required for the interaction of Sgk1 with certain substrates like the E3 ubiquitin ligase, Nedd4-2 (29).
Sgk1 is an unstable protein rapidly degraded by ubiquitination leading to its very short protein half-life (9). Nedd4-2 and CHIP are E3 ubiquitin ligases that appear to mediate ubiquitination of Sgk1 at least in HEK293 cells and in mouse fibroblasts, respectively (6, 29). The NH2-terminal 60-amino acid sequence of Sgk1 confers this instability to the protein as deletion of this region significantly enhances the abundance of expressed Sgk1 (4, 9, 29). More recent work demonstrated that deletion of the first 24 amino acids of Sgk1 is sufficient to stabilize the protein and that a hydrophobic motif GMVAIL at amino acids 19 to 24 is required for rapid degradation of Sgk1 (8). Ubiquitin modification of multiple lysines within the NH2 terminus appears to mark the protein for destruction by the proteasome since mutation of six lysines within the NH2 terminus appears to reduce its ubiquitination and increase the protein half-life (9).
Given the dominant role of proteolysis in regulating the abundance of Sgk1, we hypothesized that Sgk1 isoforms that are less susceptible to degradation may exist naturally. We identified an alternate transcript of Sgk1, called Sgk1_v2, which encodes an Sgk1 isoform with a different NH2 terminus (Sgk1_i2). Sgk1_v2 is also widely expressed, is present in epithelial cells of the kidney and lung, and when expressed in HEK293 cells, is a more stable protein that is less susceptible to ubiquitination. Furthermore, Sgk1_i2 appears to be preferentially targeted to the plasma membrane and robustly stimulates amiloride-sensitive Na+ transport when reconstituted in epithelial cells.
MATERIALS AND METHODS
Materials.
Dexamethasone, selenium, sorbitol, transferrin, triiodothyronine, N-acetyl-Leu-Leu-norleucinal (ALLN), and poly-l-lysine were purchased from Sigma (St. Louis, MO) and cycloheximide was purchased from EMD Chemicals (San Diego, CA). All cell culture media were obtained from Invitrogen Life Technologies (Gaithersburg, MD). Anti-FLAG M2 antibody was obtained from Sigma, anti-HA antibody from US Biological (Swampscott, MA), anti-α-tubulin antibody and horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG were from Santa Cruz Biotechnology (Santa Cruz, CA), and HRP-conjugated goat anti-rabbit IgG from Cell Signaling Technology (Danvers, MA).
Cell culture.
Human embryonic kidney cell line HEK293 was cultured in high-glucose DMEM containing 10% FBS and 1% penicillin-streptomycin. MpkCCDc14 cells (gift from A. Vandewalle) were cultured in defined media containing 2% FBS, antibiotics, and other hormones, as previously described (7, 14). Fisher rat thyroid (FRT) cells were cultured in F-12 Coon's media (Harlan, Indianapolis, IN) with 5% fetal calf serum (Sigma), 100 U/ml penicillin, and 100 μg/ml streptomycin at 37°C, as previously described (22). H441 and A549, human lung epithelial cell lines, were cultured as previously described (13). In some cases, A549 cells were cultured with 100 nM dexamethasone or 300 mM sorbitol for various times.
Real-time PCR.
Human tissue cDNAs were obtained from Clontech and 5 ng of each sample were used for each PCR reaction. Total RNA from mpkCCDc14, H441, and A549 were extracted with Absolutely RNA miniprep kit (Stratagene, La Jolla, CA). Reverse transcription was performed by StrataScript QPCR cDNA synthesis kit (Stratagene) under the following conditions: 5-min incubation at 25°C for primer annealing, 42°C for 45 min for cDNA synthesis, and 5-min termination at 95°C. Sgk1_v2 was amplified from human tissue cDNAs using a unique forward primer, Sgk1_v2_F1: 5′-CCCGGG TGCCAACCTGGATCTATAA and a reverse primer common to Sgk1 and its variants, Sgk1_R1: 5′-CTTGGTGGAGGAGAAGGGTTG. Sgk1 was amplified with the same reverse primer and forward primer, Sgk1wt_F: 5′-GTGATGACGGTGAAAACTGAGGCTGCT. To quantify Sgk1 from H441 and A549 mRNA, unique Sgk1_v2 primers, Sgk1_v2_F2: 5′-ATCAGAGTCCCAGCCTGAAGTACACC, and reverse primer Sgk1_v2_R2: 5′-GTTGGCATGATTACATGGCTCTCTCAC were used. Mouse sgk1 was amplified using a forward primer msgk1_F: 5′-TGCTCGAAGCACCCTTACCTACTC and reverse primer msgk1R: 5′-TTAGCGTTCATAAGCTCCGGCTCC. Mouse Sgk1_v2 was amplified with unique forward and reverse primers, mSgk1_v2_F2: 5′-GAATGGATTCCCGGTCAAGAAATGCTC and mSgk1_v2_R2: 5′-TTTGGAAAGCATGGTCACCCAGGC. 18S rRNA in human and mouse samples was amplified with 18S_F: 5′-TAACGAACGAGACTCTGGCAT and 18S_R: 5′-CGGACATCTAAGGGCATCACAG. Real-time PCR reactions were done with Brilliant SYBR Green QPCR master mix (Stratagene) in a total reaction volume of 25 μl with the following cycling conditions: 1 cycle for 10 min at 95°C followed by 30–45 cycles of 30-s denaturation at 95°C, 1-min annealing at 55–60°C, and 1- to 1.5-min extension at 72°C. The number of cycles, annealing temperature, and extension time were varied as appropriate for the abundance of transcripts, the Tm of primers, and the size of amplicons. All reactions were performed in a Mx3000 Multiplex Quantitative PCR system (Statagene) and dissociation curves were generated for all primer pairs and samples.
Plasmids and transient transfections.
ENaC subunits with COOH-terminal tags: αENaC-FLAG, βENaC-V5, and γENaC-Myc and Sgk1Δ60 have been described previously (14, 20, 23). Full-length human Sgk1 with a COOH-terminal FLAG epitope (Sgk1-FLAG) was cloned into pcDNA3 using Sgk1wt_F primer and reverse primer, Sgk1wtFLAG_R: 5′-CTTGTCGTCATCGTCTTTGTAGTCGAGGAAAG. The 5′ end of Sgk1 was removed using a flanking EcoRV site and an internal BglII site and the 5′ end of Sgk1_v2 amplified by PCR was substituted using a flanking SmaI site and the internal BglII site to generate Sgk1_i2 with a COOH-terminal FLAG epitope (Sgk1_i2-FLAG). HEK293 cells were transiently transfected with αENaC-FLAG, βENaC-V5, and γENaC-Myc, with or without Sgk1-FLAG or Sgk1_i2-FLAG and HA-ubiquitin (gift from D. Bohmann) using Lipofectamine 2000 (Invitrogen) as described previously (20). Twenty-four to forty-eight hours following transfection, cells were lysed directly for Western blotting or for immunoprecipitation followed by Western blotting. In some experiments, cells were treated with 10 μM ALLN or 100 μM chloroquine for various times before lysis. In other experiments, 20 μg/ml of cycloheximide were added to transfected cells in serum-free media for various times before cell lysis.
Western blotting.
Transfected HEK cells were washed twice in cold PBS and solubilized in 150 mM NaCl, 50 mM Tris, pH 7.4, 1% Triton X-100, 10 μM ALLN and protease inhibitor cocktail (Roche Applied Science, Indianapolis, IN) at 4°C. Protein concentrations were determined by BCA protein assay kit (Thermo Scientific, Rockford, IL). Equal amounts of protein were then boiled with SDS-PAGE sample loading buffer (30% glycerol, 50 mM Tris·HCl, pH 6.8, 7.5% SDS, 200 mM dithiothreitol and bromophenol blue) and separated on a 7% SDS-PAGE gel. Following electrophoresis, the proteins were transferred to a polyvinylidene fluoride (PVDF) membrane (Millipore, Billerica, MA) and blocked with PBS-Tween 20 (PBST; 0.05% Tween-20 in PBS) containing 5% milk for 1 h followed by overnight incubation at 4°C in 5% milk-PBST with primary antibody. Blots were then washed with PBST to remove any unbound antibodies and then incubated with HRP-conjugated secondary antibody for 1 h followed by several washings with PBST. Chemiluminescence detection of proteins was done using Supersignal West Pico/Femto chemiluminescent substrate (Thermo Scientific) and the image was captured using VisionWorksLS Image Acquisition and Analysis Software and the EC3 imaging system (UVP LLC, Upland, CA). For calculation of Sgk1 half-life, data points were plotted on a semilogarithmic scale and exponential regression lines were derived for experimental conditions. Stripping and reprobing of membranes with different antibodies were performed with Restore Western Blot Stripping Buffer (Thermo Scientific) as per manufacturer's instructions.
Coimmunoprecipitation and cell surface biotinylation.
For coimmunoprecipitation experiments, 1 mg of protein lysates from transfected HEK293 cells was incubated overnight with the appropriate antibody in 1:250 dilutions with end-over-end rotation at 4°C. The cell lysate/antibody mixture was incubated with 80 μl (40 μl bead bed volume per sample) of protein G Sepharose (GE Healthcare, Piscataway, NJ) for 1 h at 4°C followed by several washings with cold PBS and immunoprecipitates were eluted in 50–80 μl ImmunoPure nonreducing sample buffer (Thermo Scientific). Eluted proteins were separated on SDS-PAGE, transferred to membranes, and immunoblotted as previously described (20).
For cell surface biotinylation experiments, transfected HEK293 cells were treated with 10 μM ALLN for 16 h and then surface proteins were labeled with biotin by Pinpoint Cell Surface Protein Isolation Kit (Thermo Scientific) as per manufacturer's protocol. After biotinylation, cells were solubilized in lysis buffer and 1 mg of cell lysate was subjected to Neutravidin precipitation to isolate surface-labeled proteins which were then analyzed by SDS-PAGE as previously described (20).
Immunofluorescence microscopy.
HEK293 cells were grown in poly-l-lysine-coated four-well Permanox chamber slides from Lab-Tek (Nalgene Nunc International, Naperville, IL) and transiently transfected with 1 μg of Sgk1-FLAG, Sgk1_i2-FLAG, or Sgk1Δ60-FLAG using 2 μl of Lipofectamine 2000 (Invitrogen). The following day, cells were treated with 10 μM ALLN and/or 100 nM dexamethasone or vehicle and then processed for immunofluorescence microscopy the following day. Cells were first washed multiple times with PBS, fixed in 4% paraformaldehyde, permeabilized with 0.2% Triton X-100, and nonspecific epitopes were blocked with 5% normal goat serum for 30 min. Cells were then incubated with a 1:500 dilution of anti-FLAG monoclonal antibody in blocking buffer for 1 h. After several washes with PBS, AlexaFlour-488 goat anti-mouse IgG (Molecular Probes, Eugene, OR) was added at a dilution of 1:500 for 1 h. After many washes, cells were stained with nuclear stain To-Pro3 (Molecular Probes). Slides were then mounted with VectaShield (Vector Laboratories, Burlingame, CA) and fluorescent signals were captured in Bio-Rad Multi-Photon microscope.
Electrophysiology.
FRT cells were grown on permeable filter supports (Millicell PCF, 0.4-μm-pore size, 12-mm diameter), as described previously (22). One day after seeding, cells were cotransfected with α-, β-, and γ-ENaC (0.07 μg each), and Sgk1 (wild-type Sgk1 or Sgk1_i2, 0–0.8 μg each). The total plasmid cDNA was held constant using GFP cDNA (expression of GFP did not alter ENaC Na+ currents). The plasmids were mixed with TFX 50 (Promega, 7.9 μg/millicell) in 360 μl/millicell serum-free F12/Coon's media for 15 min and transferred to the apical surface of the monolayer. One hour later, the apical media was replaced with F-12 Coon's media containing 5% fetal calf serum and amiloride (10 μM).
Na+ transport was measured 2 days after transfection in modified Ussing chambers (Warner Instrument). The apical and basolateral surfaces were bathed in 135 mM NaCl, 1.2 mM CaCl2, 1.2 mM MgCl2, 2.4 mM K2HPO4, 0.6 mM KH2PO4, 10 mM dextrose, and 10 mM HEPES (pH 7.4) at 37°C. Amiloride-sensitive short-circuit current was determined as the difference in current with and without amiloride in the apical bathing solution, as previously described (22).
RESULTS
Identification of an NH2-terminal variant SGK1 isoform.
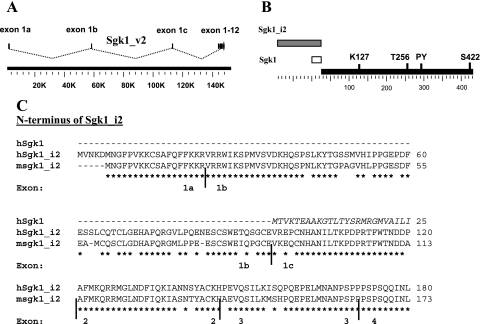
A bioinformatic analysis of gene databases using the Genome Browser (www.genome.ucsc.edu) led to the identification of a 5′-variant alternate transcript for human Sgk1 (AK055077). This variant, Sgk1_v2, arises by transcription initiation in an exon (exon 1a) far upstream of the known transcription start site for Sgk1, splices to two additional upstream exons (exons 1b and c), and then splices to exon 2 of the previously known reference sequence, Sgk1 (Fig. 1A). These three upstream exons are characterized by large introns and together span 140 kb while the entire known SGK1 gene covers just 6 kb from exon 1 through 12. The variant Sgk1_v2, when translated, is predicted to encode a protein, Sgk1_i2, where a unique 120-amino acids NH2 sequence is substituted for the first 25 amino acids of Sgk1 (Fig. 1B). The full-length Sgk1_v2 transcript is predicted to be ∼3.4 kb and the protein predicted to be ∼60 kDa compared with the reference Sgk1 sequence which is 49 kDa. The Kozak consensus sequence for translation initiation is poor for Sgk1_i2, aaATGGT as it is for Sgk1, tgATGAC. Phylogenetic analysis revealed that Sgk1_v2 is conserved in squalus acanthias and in mus musculus and the variant human NH2 terminus shares 84% homology with mouse sgk1_i2 (Fig. 1C).
Fig. 1.
Identification of an Sgk1 variant. A: intron-exon organization of human Sgk1. Upstream exons 1a, 1b, and 1c splice with exon 2 to create Sgk1_v2. The predicted translation start codon is in exon 1a. B: Sgk1 protein with the variant NH2 terminus shown as an open box (reference Sgk1) and a hatched box (Sgk1_i2) and common sequence is shown as a closed box. Known key residues and motifs are shown. Numbering of amino acids corresponds to that for Sgk1. C: comparison of the NH2 termini of human (translation of AK055077) and mouse sgk1_i2 (AAH70401) demonstrates significant homology. Identical residues are indicated by an * and the position of individual exons is shown below. Also shown are the 25 amino acids NH2 terminus of reference hSgk1 which differs completely from the NH2 terminus of Sgk1_i2.
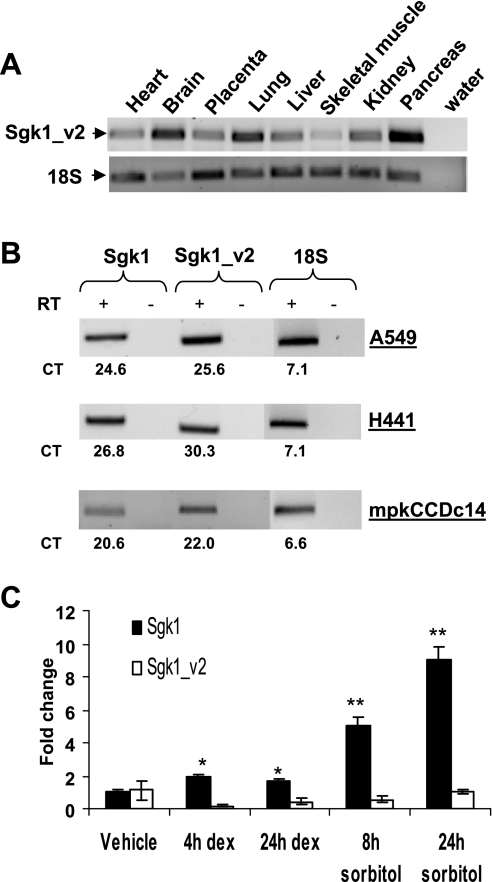
To begin to explore the significance of Sgk1_v2, we performed real-time PCR in multiple human tissues using a forward transcript-specific PCR primer and a reverse primer positioned within common Sgk1 sequence. We amplified Sgk1_v2 in each of the tissues examined, with brain and pancreas having the highest levels of this transcript (Fig. 2A). We then tested two human lung epithelial cell lines H441 and A549 and mouse collecting duct epithelial cell line mpkCCDc14 that have regulated amiloride-sensitive Na+ channels (7, 13, 14). Using transcript-specific primers and qRT-PCR, Sgk1_v2 was noted to be abundant and readily identified in each of these epithelial cell lines (Fig. 2B).
Fig. 2.
RT-PCR for Sgk1_v2 in various tissue and cell lines. A: 5 ng of cDNA from each tissue were used for PCR amplification of Sgk1_v2 and 18S rRNA and amplified products were analyzed by agarose gel electrophoresis. The amplified 562-bp Sgk1_v2 and 138-bp 18S rRNA are shown. B: Sgk1, Sgk1_v2, and 18S rRNA amplified by qRT-PCR from H441, A549 mRNA, and mpkCCDc14 and products were analyzed by agarose gel electrophoresis. Reverse transcriptase (RT) was omitted in control reactions. The threshold cycle (CT) value for each reaction is shown below. CT values are inversely proportional to abundance. C: Sgk1 and Sgk1_v2 amplified by qRT-PCR in A549 cells treated with 100 nM dexamethasone (dex) or 300 mM sorbitol for the indicated times and then corrected for 18S rRNA and expressed as fold-change compared with vehicle. *P < 0.05 compared with vehicle. **P < 0.001 compared with vehicle. Student's t-test (n = 4 ± SE).
Sgk1 is known to be transcriptionally regulated by glucocorticoids and by hypertonicity (5, 13, 25, 27). We tested the effect of the dexamethasone, to stimulate the glucocorticoid receptor (GR), and sorbitol, to increase extracellular tonicity, on Sgk1 and Sgk1_v2 expression in A549 cells. We note that both stimuli significantly increase Sgk1, as we and others previously reported but neither had an effect on Sgk1_v2 expression (Fig. 2C). Since Sgk1 and Sgk1_v2 arise from separate transcription start sites that are more than 140 kb apart, each transcript is likely to be regulated by its cognate promoter and distinct transcriptional regulatory elements. In fact, the glucocorticoid response element (GRE) that mediates GR activation of Sgk1 expression and the p38 MAPK-responsive Sp1 binding site that mediates the hypertonic response are immediately upstream of the Sgk1 transcription start site and thus far downstream of the start site for Sgk1_v2 (5, 13).
Enhanced stability of Sgk1_i2 compared with sgk1.
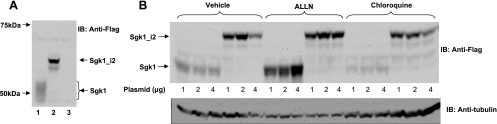
To better understand the functional relevance of Sgk1_i2, full-length human Sgk1 and Sgk1_v2 cDNAs were cloned with a COOH-terminal FLAG epitope and transiently expressed in HEK293 cells. We found that Sgk1 protein levels were quite low and detected as faint multiple bands ranging from 45 to 54 kDa (Fig. 3A), in keeping with prior reports that Sgk1 protein is unstable and may be translated from multiple internal start codons (2). In contrast, levels of Sgk1_i2 protein appeared higher and were clearly seen as a single discrete band of ∼60 kDa.
Fig. 3.
Relative abundance of expressed Sgk1 and Sgk1_i2 in HEK293 cells. A: HEK293 cells transfected with Sgk1-FLAG (lane 1), Sgk1_i2-FLAG (lane 2), or an empty vector (lane 3) followed by Western blotting using a FLAG antibody that shows Sgk1 detected as relatively faint multiple sized bands ranging from 45 to 54 kDa. Sgk1_i2 is clearly detectable as a ∼60-kDa protein. B: HEK293 cells transiently transfected with the indicated amounts of Sgk1 or Sgk1_i2 and then treated with 10 μM ALLN or 100 μM chloroquine or vehicle. Cell lysates were immunoblotted with FLAG antibody and then stripped and reprobed with tubulin. Sgk1 levels increased in the presence of ALLN but not chloroquine. The data with Sgk1 are representative of 3 separate experiments.
Since we observed significant dissimilarity in immunoblottable levels of Sgk1 and Sgk1_i2, we wondered whether this related to differences in degradation of Sgk1 proteins. To determine whether this was due to enhanced stability of expressed Sgk1_i2, we examined Sgk1 and Sgk1_i2 protein levels in HEK293 cells with or without treatment with a proteasomal inhibitor, ALLN, or a lysosomal inhibitor, chloroquine. We saw a dramatic increase in Sgk1 proteins with the addition of ALLN that increased with increasing transfected plasmid DNA and saw no effect of chloroquine (Fig. 3B), as has been described by others (9). In contrast to Sgk1, Sgk1_i2 levels were not significantly altered by either ALLN or with chloroquine. Taken together, these data suggest that Sgk1_i2 is more resistant to proteasomal degradation which may be mediated by the sequences within the variant 120-amino acid NH2 terminus.
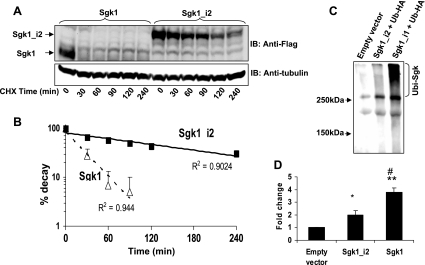
To confirm that differences seen were due to increased protein stability and not enhanced transcription or translation, we measured protein turnover in transfected HEK293 cells after inhibiting protein translation with cycloheximide (Fig. 4A). Our data clearly demonstrate that the turnover of Sgk1 is significantly faster than Sgk1_i2 as Sgk1 levels had substantially diminished within 30 min of cycloheximide treatment, whereas Sgk1_i2 was still detectable even after 4 h of cycloheximide. The calculated half-life of Sgk1 is 16 min while that of Sgk1_i2 is 119 min (Fig. 4B).
Fig. 4.
Measurement of Sgk1 and Sgk1_i2 turnover and ubiquitination. A: HEK293 cells transfected with Sgk1-FLAG or Sgk1_i2-FLAG and then treated with 20 μg/ml cycloheximide (CHX) for the indicated time periods. Cell lysates were immunoblotted with FLAG antibody and then stripped and reprobed with tubulin. Following CHX, there is a rapid decline in Sgk1 with a relatively slower decline in Sgk1_i2 levels. B: intensity of signals was quantitated by densitometry and used to derive an exponential regression line for both conditions. The t1/2 for sgk1 is 16 min and for Sgk1_i2 is 119 min. The plotted data show the means ± SE of 3 experiments. C: HEK293 cells transfected with emtpy vector, Sgk1-FLAG, or Sgk1_i2-FLAG and then immunoprecipitated with an anti-FLAG antibody and immunoblotted with anti-HA to detect ubiquitinated Sgk1. Ubiquitinated proteins appear as high molecular mass-like smear. Sgk1 is relatively more ubiquitinated than Sgk1_i2. D: ubiquitinated broad band was quantitated by densitometry and confirms enhanced ubiquitination of Sgk1 compared with Sgk1_i2. *P = 0.051 compared with empty vector. **P < 0.001 compared with empty vector. #P < 0.005 compared with Sgk1_i2. Tukey's test (means ± SE, n = 4).
Sgk1 is a better target for ubiquitination.
The NH2 terminus of Sgk1 contains multiple lysine residues that are ubiquitinated and are thought to underlie the intrinsic instability of Sgk1 since removal of the first 60 amino acids leads to a significant increase in the abundance of Sgk1 (4, 9, 29). We then asked whether NH2 terminus of Sgk1_i2 was a poor target for ubiquitination compared with Sgk1. To test this hypothesis, we expressed FLAG-tagged Sgk1 or Sgk1_i2 along with HA-tagged ubiquitin in HEK293 cells and ubiquitinated Sgk1 proteins were identified by immunoprecipitation with an anti-FLAG antibody followed by immunoblotting with anti-HA antibody. We detected a high molecular mass smear indicative of ubiquitination and this ubiquitination was increased with Sgk1 compared with Sgk1_i2 (Fig. 4, C and D). Together, these results indicate that Sgk1 is a better target of ubiquitination and this modification by ubiquitination is altered by having a different NH2-terminal sequence in Sgk1_i2. Since ubiquitination can be a signal for proteasomal degradation, a reduction in ubiquitination of Sgk1_i2 is consistent with the results showing increased abundance and increased half-life of Sgk1_i2 (Figs. 3 and 4).
Distinct subcellular localization of Sgk1 and Sgk1_i2.
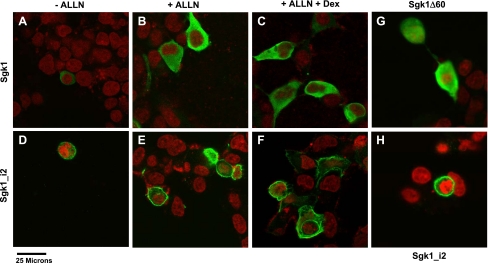
To determine the subcellular localization of Sgk1_i2, we compared the expression of Sgk1 with Sgk1_i2 by confocal immunofluorescence microscopy in transfected HEK293 cells. Sgk1 appears as a faint homogeneously distributed protein throughout the cytosol without the proteasomal inhibitor, ALLN, and the addition of ALLN enhanced Sgk1 fluorescence, consistent with our immunoblotting data (Fig. 5, A and B). In contrast, Sgk1_i2 protein was predominantly localized at or near the plasma membrane and was clearly visible without ALLN (Fig. 5D). Occasional cells revealed nuclear membrane localization of Sgk1_i2 as well (data not shown). The addition of ALLN did not increase the fluorescent signal or change the distribution in Sgk1_i2 (Fig. 5E). Furthermore, treatment of transfected cells with dexamethasone did not change the distribution or fluorescence intensity of either Sgk1 or Sgk1_i2 (Fig. 5, C and F). In contrast to prior reports, we did not detect any Sgk1 expression within the nucleus. To determine whether the cytosolic localization of Sgk1 was mediated via its NH2 terminus, we tested Sgk1 after deletion of the NH2-terminal 60 amino acids and noted some redistribution of Sgk1Δ60 to the nucleus with most of the signal remaining with the cytosol (Fig. 5G). Our data suggest that the membrane association of Sgk1_i2 is mediated specifically via its NH2 terminus.
Fig. 5.
Subcellular localization of Sgk1, Sgk1_i2, and Sgk1Δ60. A–F: HEK293 cells grown on chamber slides were transfected with Sgk1-FLAG or Sgk1_i2-FLAG and Sgk1 isoforms were detected by fluorescence confocal microscopy using an anti-FLAG monoclonal antibody (green). Nuclei are stained with To-Pro3 (red). In the absence of ALLN, Sgk1 expression is relatively weak (A) and in the presence of ALLN, there is increased abundance of Sgk1 (B) and the additional treatment with dex does not alter Sgk1 intensity or localization of Sgk1 (C). Neither ALLN nor dex has any apparent effect on Sgk1_i2 (D, E, and F). Overall, Sgk1 is distributed homogeneously throughout the cytoplasm, whereas Sgk1_i2 is predominantly located on the plasma membrane with some distribution seen in cytoplasm. G and H: comparison of Sgk1 where the 1st 60 amino acids are deleted (Sgk1Δ60) with Sgk1_i2 shows that Sgk1Δ60 (G) has a predominantly cytosolic distribution with some nuclear staining also seen, whereas Sgk_i2 (H) is localized to the cell membrane.
Sgk1 and Sgk1_i2 induce the surface expression of ENaC.
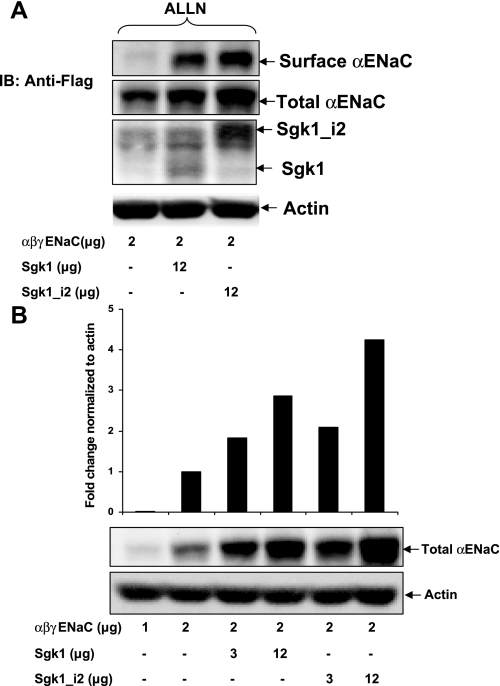
Previous published work showed that Sgk1 phosphorylates Nedd4-2 and reduces its affinity for ENaC which, in turn, leads to increasing plasma membrane retention of ENaC and, in part, explains the stimulatory effect of Sgk1 on amiloride-sensitive Na+ transport (11, 24). To examine the effect of Sgk1_i2 on surface expression of ENaC, we expressed α-, β-, and γ-ENaC in HEK293 cells along with either Sgk1 or Sgk1_i2. Biotin-labeled surface proteins were affinity-purified and immunoblotted using an anti-FLAG antibody to detect surface α-ENaC. Under the conditions tested, Sgk1 and Sgk1_i2 increased α-ENaC at the cell surface with Sgk_i2 having the greater effect (Fig. 6A). These results correlated with increased expression of Sgk1_i2 compared with Sgk1 in these cells and are consistent with an effect of Sgk1 and to a greater extent Sgk1_i2 to inhibit Nedd4-2-mediated ENaC retrieval from the cell surface and reduce proteasomal degradation of ENaC subunits. We also observed a smaller increase in total α-ENaC expression with Sgk1 isoforms. To examine the effect on total ENaC expression further, we expressed ENaC with two different concentrations of either Sgk1 or Sgk_i2 and demonstrate a dose-dependent effect on the abundance of α-ENaC with Sgk1_i2 having the greater effect (Fig. 6B).
Fig. 6.
Expression of ENaC is increased by Sgk1 isoforms. A: HEK293 cells transfected with α-, β-, and γ-ENaC subunits and Sgk1 or Sgk1_i2 and then treated with 10 μM ALLN. Biotin-labeled surface proteins or total cell lysates were then immunoblotted with an anti-FLAG antibody to detect α-ENaC. Surface α-ENaC is dramatically increased in the presence of Sgk1 with a greater effect of Sgk1_i2 (top) and total cellular α-ENaC is marginally increased by Sgk1 and Sgk1_i2. As shown previously, levels of expressed Sgk1_i2 are greater than Sgk1. B: both Sgk1 and Sgk1_i2 dose dependently increase α-ENaC expression in HEK293 cells. Representative blot below with quantitation normalized for actin above. Similar results were obtained from 2 separate experiments.
Sgk1_i2 stimulates Na+ transport in FRT cells.
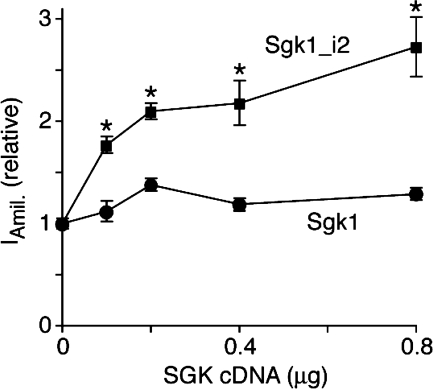
Our data thus far indicate that Sgk1_i2 has a longer half-life, is preferentially targeted to the cell surface, and enhances plasma membrane expression of ENaC. We therefore hypothesized that Sgk1_i2 would have a more robust effect on ENaC-mediated Na+ transport. We tested the functional relevance of Sgk1_i2 in FRT cells where we cotransfected α-, β-, and γ-ENaC, along with increasing amounts of Sgk1 or Sgk1_i2. Wild-type Sgk1 produced a small increase in amiloride-sensitive current (Fig. 7). In contrast, Sgk1_i2 produced a much larger increase in amiloride-sensitive current in a concentration-dependent manner (2.73- vs. 1.29-fold at 1:4 ratio of ENaC:Sgk1). Together, these data indicate that a natural Sgk1 variant better stimulates Na+ transport, an effect that may be mediated via its enhanced stability leading to increased expression and its preferential plasma membrane localization.
Fig. 7.
Sgk1_i2 stimulates ENaC activity. Fisher rat thyroid epithelia were transfected with α-, β-, and γ-ENaC (0.07 μg each) and Sgk1 (Sgk1 or Sgk1_i2, as indicated, 0–0.8 μg). Total cDNA was kept constant using GFP cDNA. Amiloride-sensitive short-circuit current is plotted vs. quantity of Sgk1 cDNA (means ± SE, n = 11–18, *P < 0.002 by Student's t-test).
DISCUSSION
Since the NH2 terminus of Sgk1 is critical in regulating its abundance and thereby its function, we searched the genome databases for NH2-terminal Sgk1 variants that may be less susceptible to proteolysis. We identified an alternate transcript, Sgk1_v2, which is conserved from mouse to humans. This mRNA uses a different transcription start site, splicing to additional upstream exons and, as a consequence has a distinct 5′ end that is predicted to encode an Sgk1 isoform with a different NH2 terminus. We identified Sgk1_v2 in several human tissues and human and mouse epithelial cell lines with regulated Na+ transport. Higher levels of Sgk1_v2 were detected in pancreas and brain and lower levels in skeletal muscle and heart and this expression profile differed somewhat from the expression of the reference Sgk1, where expression in the brain was relatively low compared with other tissues (data not shown). In earlier studies, human Sgk1 was detected as a 2.6-kb transcript by Northern blotting, studies that also confirmed high levels of expression of Sgk1 in pancreas and relatively low levels of expression in brain (26). Sgk1_v2 with a predicted size of ∼3.4 kb did not appear to be detected in that study. Interestingly, a 7-kb transcript was detected in that study using an Sgk1-specific cDNA probe and this may represent yet another alternate transcript of Sgk1 or a paralog.
We also noted differences in stimuli that induce expression: glucocorticoids and hypertonicity increased Sgk1 expression but had no effect on Sgk1_v2 expression. Differences in expression and transcriptional regulation of these 5′ variant transcripts are not surprising since they have distinct transcription start sites and have differing 5′ flanking regulatory sequences directing transcription.
We compared the levels of expressed epitope-tagged Sgk1_i2 with Sgk1 protein and confirmed that Sgk1_i2 was more stable than Sgk1. We noted multiple faint bands for Sgk1 consistent with translation from multiple start codons as have been recently reported (2). The 49- and 47-kDa isoforms are much more abundant and have short half-life, whereas the 45- and 42-kDa isoforms exhibit relatively longer half-life (2). Although both Sgk1 and Sgk1_i2 appear to have a poor Kozak sequence surrounding the initial start codon, we observed a single band for Sgk1_i2, suggesting that translation of Sgk1_i2 always begins at the first start codon. We noted that inhibitors of the proteasome, but not the lysosome, markedly increased Sgk1 expression with a minor increase in Sgk1_i2. We also demonstrated that Sgk1_i2, compared with Sgk1, had an increased half-life correlating with decreased ubiquitination. Overall, the data confirm that transiently expressed Sgk1_i2 is more stable that Sgk1 in a heterologous expression system. However, in cells or tissues where both isoforms are naturally expressed, the steady-state levels of these isoforms will reflect differences in rates of transcription and translation as well as differences in the kinetics of mRNA and protein turnover.
Previous studies reported that Sgk1 may localize to many subcellular compartments including the nucleus, plasma membrane, endoplasmic reticulum, and diffusely in the cytoplasm. Progression through the cell cycle appears to require shuttling of sgk1 between nucleus and the cytoplasm, at least in mammary epithelial cells (10, 19). Redistribution of Sgk1 also appears to occur in mammary epithelial cells with a number of other stimuli: nuclear localization was seen in response to dexamethasone and sorbitol and cytosolic localization seen in response to heat shock and UV radiation (16). Sgk1 appears to be preferentially localized to the basolateral plasma membrane in rat renal tubule sections, at least in one study, under control conditions. In another study, sgk1 was not detectable in the connecting tubule and collecting duct in adrenalectomized rats and upon aldosterone stimulation, sgk1 appeared diffusely throughout the cytoplasm (1, 18). In transfected Chinese hamster ovary and COS-7 cells, sgk1 is expressed diffusely through the cytoplasm and associates with the ER membrane, although after deletion of the first 60 amino acids, sgk1 is present homogenously in the cytoplasm and nucleus, results that we now confirm in HEK cells (4, 6). We were also unable to detect any differences in subcellular localization of Sgk1 with dexamethasone or serum and speculate that these differences reflect the use of different expression systems. Interestingly, localization of Sgk1_i2 was distinctly different with preferential plasma membrane expression in most cells with rare cells exhibiting nuclear membrane staining as well (Fig. 5F). We were unable to detect any intranuclear localization for Sgk1_i2 even though this form is lacking the first 25 amino acids of sgk1. It is thus likely that the absence of 25 amino acids from NH2 terminus is insufficient for nuclear distribution or that the presence of the NH2-terminal 120 amino acids in Sgk1_i2 masked or inhibited residues involved in nuclear localization.
We examined the effect of Sgk1 and Sgk1_i2 on ENaC using a surface biotinylation assay. We found that the cell surface α-ENaC is dramatically upregulated by both Sgk1 and Sgk1_i2 (Fig. 6A). Sgk1 and Sgk1_i2 appeared to increase total α-ENaC expression as well, with Sgk1_i2 appearing to be relatively more efficient at stimulation of α-ENaC. Although we did not directly test this, Sgk1 phosphorylates Nedd4-2, reducing its affinity for ENaC subunits and leading to increased surface retention of ENaC (11, 24). The reduction in internalization of ENaC is expected to reduce the proteasomal degradation of ENaC.
We demonstrated that Sgk1_i2 exhibited a significantly greater effect on Na+ transport compared with Sgk1 in FRT epithelia. The increase in Na+ transport is similar to that seen with an NH2-terminal deleted version of Sgk1 and suggests that the increased abundance of Sgk1_i2 is the likely reason for the enhanced effect on Na+ transport, although the preferential membrane localization of Sgk1_i2 may also contribute to the increase seen (29). Stimulation of Na+ transport with Sgk1 is now known to occur through multiple pathways. First, as mentioned earlier, phosphorylation of Nedd4-2 by Sgk1 leads to greater surface expression of ENaC. Second, Sgk1 appears to directly interact with the COOH terminus of α-ENaC in a phosphorylation-dependent manner and converts silent channels to active channels with a Po of ∼0.5 (12). Both of these effects of Sgk1 require the kinase function of Sgk1 which resides in its COOH-terminal portion and is common to both Sgk1 and Sgk1_i2 and can explain the stimulatory effect of Sgk1_i2. Sgk1 can also phosphorylate Af9 leading to derepression of the α-ENaC promoter by the Dot1-AF9 complex and increased transcription of α-ENaC and presumably increased translation and assembly of ENaC subunits (28). Since a heterologous promoter drives α-ENaC expression in FRT and HEK293 epithelia, enhanced transcription of α-ENaC is unlikely to contribute to the increased effect of Sgk1_i2 in our experiments.
Sgk1 is an important regulator of several cellular functions, many of which are mediated via its kinase function (15, 17). Since Sgk1_i2 and Sgk1 share the same kinase domain and Sgk1_i2 is widely expressed, we would predict that Sgk1_i2 is a more potent regulator of other functions ascribed to Sgk1. Furthermore, as transcriptional regulation is likely to be different, Sgk1_v2 expression may not change in parallel with changes in Sgk1. For example, dexamethasone and sorbitol increase Sgk1 but not Sgk1_v2 mRNA expression in A549 cells (Fig. 2C). Thus, the physiological relevance of this isoform in the collecting duct or elsewhere remains to be elucidated.
While this manuscript was being prepared for submission, the existence of three 5′ variant transcripts of sgk1 was described (3, 21). These transcripts arise by alternate transcription initiation and differ exclusively in their first exons. The variant transcripts described by Simon et al. (21) have a transcription start site 850 and 2981 nt upstream of the reference human Sgk1 and they named these transcripts Sgk1 (−850) and Sgk1 (−2981), respectively. The transcripts are expressed in myoblasts and myotubes and appeared to be upregulated in various neuronal tumor cell lines. Although these 5′ variants are predicted to encode NH2-terminal sgk1 isoforms, this study did not report on the expression, function, or other attributes of these variants. The variant transcript described by Arteaga et al. (3) which they termed sgk1.1 appears to be the mouse ortholog of Sgk1_v2. Similar to our studies, they report that sgk1.1 is more stable than sgk1 and that sgk1.1 preferentially associated with the cell membrane which they demonstrate to be secondary to certain positively charged residues in the NH2 terminus of sgk1.1. There are, however, a number of differences between their study and ours which merit discussion. First, they examined sgk1.1 by qRT-PCR of various mouse tissues and reported that Sgk1_v2 was expressed almost exclusively in the brain, although qRT-PCR was done with one primer that was shared with sgk1. Second, they report that sgk1.1 has no effect on ENaC current, although their experiments were performed in Xenopus laevis oocytes and were not compared with the reference sequence sgk1. Perhaps the difference in functional effect on ENaC compared with our studies reflects differences in protein stability or membrane localization in oocytes compared with polarized epithelial cells.
In summary, we report that Sgk1_i2, a conserved Sgk1 NH2-terminal variant, is expressed in multiple tissues including epithelial cells with regulated Na+ transport, is inherently more stable that Sgk1, is preferentially localized to the plasma membrane, and can better stimulate Na+ transport in a reconstituted epithelial cell model.
GRANTS
This work was supported in part by United States Public Health Service Grants HL-058812 (to P. M. Snyder) and HL-71664 (to C. P. Thomas) and by a VA Merit Review Award (to C. P. Thomas).
The nucleotide sequence reported in this paper will appear in DDBJ, EMBL, Genbank, and GSDB Nucleotide Sequence Databases with accession number EU518415. The suggested names for the alternate Sgk1 transcript (Sgk1_v2) and protein isoform (Sgk1_i2) in this manuscript are based on guidelines from the HUGO Gene Nomenclature Committee (HGNC) at http://www.genenames.org.
Acknowledgments
The authors thank E. Kelley for excellent technical support, C. Zurzulo for the FRT cell line, A. Vandewalle for the mpkCCDc14 cell line, and D. Bohmann for the HA-tagged ubiquitin vector. The authors also acknowledge the University of Iowa DNA Core facility and Vector Core facility for services provided.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Alvarez de la Rosa D, Coric T, Todorovic N, Shao D, Wang T, Canessa CM. Distribution and regulation of expression of serum- and glucocorticoid-induced kinase-1 in the rat kidney. J Physiol 551: 455–466, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arteaga MF, Alvarez de la Rosa D, Alvarez JA, Canessa CM. Multiple translational isoforms give functional specificity to serum- and glucocorticoid-induced kinase 1. Mol Biol Cell 18: 2072–2080, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arteaga MF, Coric T, Straub C, Canessa CM. A brain-specific SGK1 splice isoform regulates expression of ASIC1 in neurons. Proc Natl Acad Sci USA 105: 4459–4464, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arteaga MF, Wang L, Ravid T, Hochstrasser M, Canessa CM. An amphipathic helix targets serum and glucocorticoid-induced kinase 1 to the endoplasmic reticulum-associated ubiquitin conjugation machinery. Proc Natl Acad Sci USA 103: 11178–11183, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bell LM, Leong ML, Kim B, Wang E, Park J, Hemmings BA, Firestone GL. Hyperosmotic stress stimulates promoter activity and regulates cellular utilization of the serum- and glucocorticoid-inducible protein kinase (Sgk) by a p38 MAPK-dependent pathway. J Biol Chem 275: 25262–25272, 2000. [DOI] [PubMed] [Google Scholar]
- 6.Belova L, Sharma S, Brickley DR, Nicolarsen JR, Patterson C, Conzen SD. Ubiquitin-proteasome degradation of serum- and glucocorticoid-regulated kinase-1 (SGK-1) is mediated by the chaperone-dependent E3 ligase CHIP. Biochem J 400: 235–244, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bens M, Vallet V, Cluzeaud F, Pascual-Letallec L, Kahn A, Rafestin-Oblin ME, Rossier BC, Vandewalle A. Corticosteroid-dependent sodium transport in a novel immortalized mouse collecting duct principal cell line. J Am Soc Nephrol 10: 923–934, 1999. [DOI] [PubMed] [Google Scholar]
- 8.Bogusz AM, Brickley DR, Pew T, Conzen SD. A novel N-terminal hydrophobic motif mediates constitutive degradation of serum- and glucocorticoid-induced kinase-1 by the ubiquitin-proteasome pathway. FEBS J 273: 2913–2928, 2006. [DOI] [PubMed] [Google Scholar]
- 9.Brickley DR, Mikosz CA, Hagan CR, Conzen SD. Ubiquitin modification of serum and glucocorticoid-induced protein kinase-1 (SGK-1). J Biol Chem 277: 43064–43070, 2002. [DOI] [PubMed] [Google Scholar]
- 10.Buse P, Tran SH, Luther E, Phu PT, Aponte GW, Firestone GL. Cell cycle and hormonal control of nuclear cytoplasmic localization of the serum- and glucocorticoid-inducible protein kinase, Sgk, in mammary tumor cells. A novel convergence point of anti-proliferative and proliferative cell signaling pathways. J Biol Chem 274: 7253–7263, 1999. [DOI] [PubMed] [Google Scholar]
- 11.Debonneville C, Flores SY, Kamynina E, Plant PJ, Tauxe C, Thomas MA, Munster C, Chraibi A, Pratt JH, Horisberger JD, Pearce D, Loffing J, Staub O. Phosphorylation of Nedd4-2 by Sgk1 regulates epithelial Na+ channel cell surface expression. EMBO J 20: 7052–7059, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Diakov A, Korbmacher C. A novel pathway of ENaC activation involves an SGK1 consensus motif in the C-terminus of the channel's α-subunit. J Biol Chem 279: 38134–38142, 2004. [DOI] [PubMed] [Google Scholar]
- 13.Itani OA, Liu KZ, Cornish KL, Campbell JR, Thomas CP. Glucocorticoids stimulate human sgk1 gene expression by activation of a hormone response element in its 5′ flanking region. Am J Physiol Endocrinol Metab 283: E971–E979, 2002. [DOI] [PubMed] [Google Scholar]
- 14.Itani OA, Stokes JB, Thomas CP. Nedd4-2 isoforms differentially associate with ENaC and regulate its activity. Am J Physiol Renal Physiol 289: F334–F346, 2005. [DOI] [PubMed] [Google Scholar]
- 15.Lang F, Bohmer C, Palmada M, Seebohm G, Strutz-Seebohm N, Vallon V. (Patho)physiological significance of the serum- and glucocorticoid-inducible kinase isoforms. Physiol Rev 86: 1151–1178, 2006. [DOI] [PubMed] [Google Scholar]
- 16.Leong MLL, Maiyar AC, Kim B, O'Keeffe BA, Firestone GL. Expression of the serum- and glucocorticoid-inducible protein kinase, Sgk, is a cell survival response to multiple types of environmental stress stimuli in mammary epithelial cells. J Biol Chem 278: 5871–5882, 2003. [DOI] [PubMed] [Google Scholar]
- 17.Loffing J, Flores SY, Staub O. Sgk kinases and their role in epithelial transport. Annu Rev Physiol 68: 16.11–16.30, 2006. [DOI] [PubMed] [Google Scholar]
- 18.Loffing J, Zecevic M, Feraille E, Kaissling B, Asher C, Rossier BC, Firestone GL, Pearce D, Verrey F. Aldosterone induces rapid apical translocation of ENaC in early portion of renal collecting system: possible role of SGK. Am J Physiol Renal Physiol 280: F675–F682, 2001. [DOI] [PubMed] [Google Scholar]
- 19.Maiyar AC, Leong MLL, Firestone GL. Importin-alpha mediates the regulated nuclear targeting of serum- and glucocorticoid-inducible protein kinase (Sgk) by recognition of a nuclear localization signal in the kinase central domain. Mol Biol Cell 14: 1221–1239, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Raikwar NS, Thomas CP. Nedd4-2 isoforms ubiquitinate individual epithelial sodium channel subunits and reduce surface expression and function of the epithelial sodium channel. Am J Physiol Renal Physiol 294: F1157–F1165, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Simon P, Schneck M, Hochstetter T, Koutsouki E, Mittelbronn M, Merseburger A, Weigert C, Niess A, Lang F. Differential regulation of serum- and glucocorticoid-inducible kinase 1 (SGK1) splice variants based on alternative initiation of transcription. Cell Physiol Biochem 20: 715–728, 2007. [DOI] [PubMed] [Google Scholar]
- 22.Snyder PM Liddle's syndrome mutations disrupt cAMP-mediated translocation of the epithelial Na+ channel to the cell surface. J Clin Invest 105: 45–53, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Snyder PM, Olson DR, Kabra R, Zhou R, Steines JC. cAMP and serum and glucocorticoid-inducible kinase (SGK) regulate the epithelial Na+ channel through convergent phosphorylation of Nedd4-2. J Biol Chem 279: 45753–45758, 2004. [DOI] [PubMed] [Google Scholar]
- 24.Snyder PM, Olson DR, Thomas BC. SGK modulates Nedd4-2-mediated inhibition of ENaC. J Biol Chem 277: 5–8, 2002. [DOI] [PubMed] [Google Scholar]
- 25.Waldegger S, Barth P, Forrest JN Jr, Greger R, Lang F. Cloning of sgk serine-threonine protein kinase from shark rectal gland–a gene induced by hypertonicity and secretagogues. Pflügers Arch 436: 575–580, 1998. [DOI] [PubMed] [Google Scholar]
- 26.Waldegger S, Barth P, Raber G, Lang F. Cloning and characterization of a putative human serine/threonine protein kinase transcriptionally modified during anisotonic and isotonic alterations of cell volume. Proc Natl Acad Sci USA 94: 4440–4445, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Webster MK, Goya L, Ge Y, Maiyar AC, Firestone GL. Characterization of sgk, a novel member of the serine/threonine protein kinase gene family which is transcriptionally induced by glucocorticoids and serum. Mol Cell Biol 13: 2031–2040, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang W, Xia X, Reisenauer MR, Rieg T, Lang F, Kuhl D, Vallon V, Kone BC. Aldosterone-induced Sgk1 relieves Dot1a-Af9-mediated transcriptional repression of epithelial Na+ channel alpha. J Clin Invest 117: 773–783, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou R, Snyder PM. Nedd4-2 phosphorylation induces serum and glucocorticoid-regulated kinase (SGK) ubiquitination and degradation. J Biol Chem 280: 4518–4523, 2005. [DOI] [PubMed] [Google Scholar]