Abstract
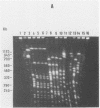
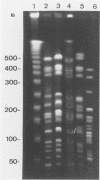
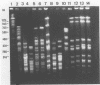
Fingerprints for 72 reference serovar strains of pathogenic Leptospira spp. were obtained by pulsed-field gel electrophoresis (PFGE) following NotI restriction digests of the chromosome. These strains included the serovar reference strains of serogroups Australis, Ballum, Bataviae, Grippotyphosa, Panama, Pomona, and Pyrogenes. Sixty-four serovars could be identified by a unique NotI restriction profile. The remaining serovars were differentiated by chromosomal digestion with SgrAI. These included four serovars from serogroup Australis, two serovars from serogroup Ballum, and two serovars from serogroup Bataviae. Thirteen of 18 recent clinical isolates identified by microagglutination test and cross-adsorption procedure were correctly typed by PFGE. The results indicate that PFGE, which is considerably more rapid than serology, should be useful for identification and epidemiological studies.
Full text
PDF


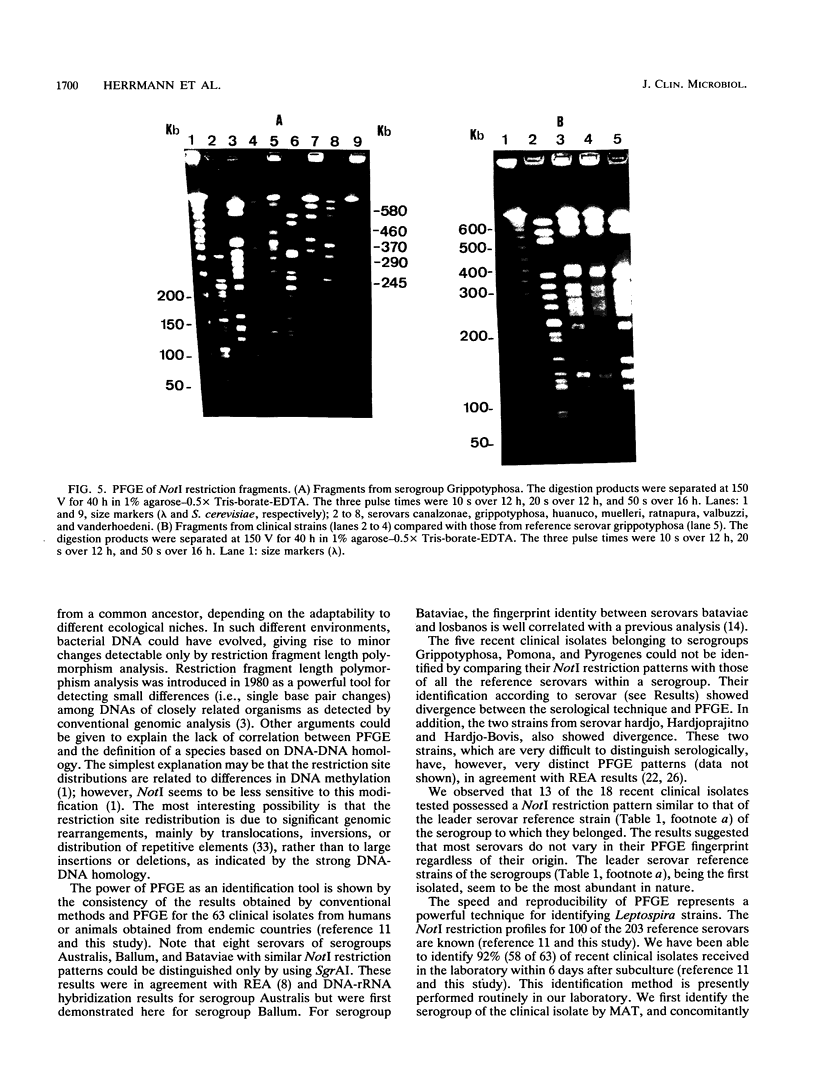
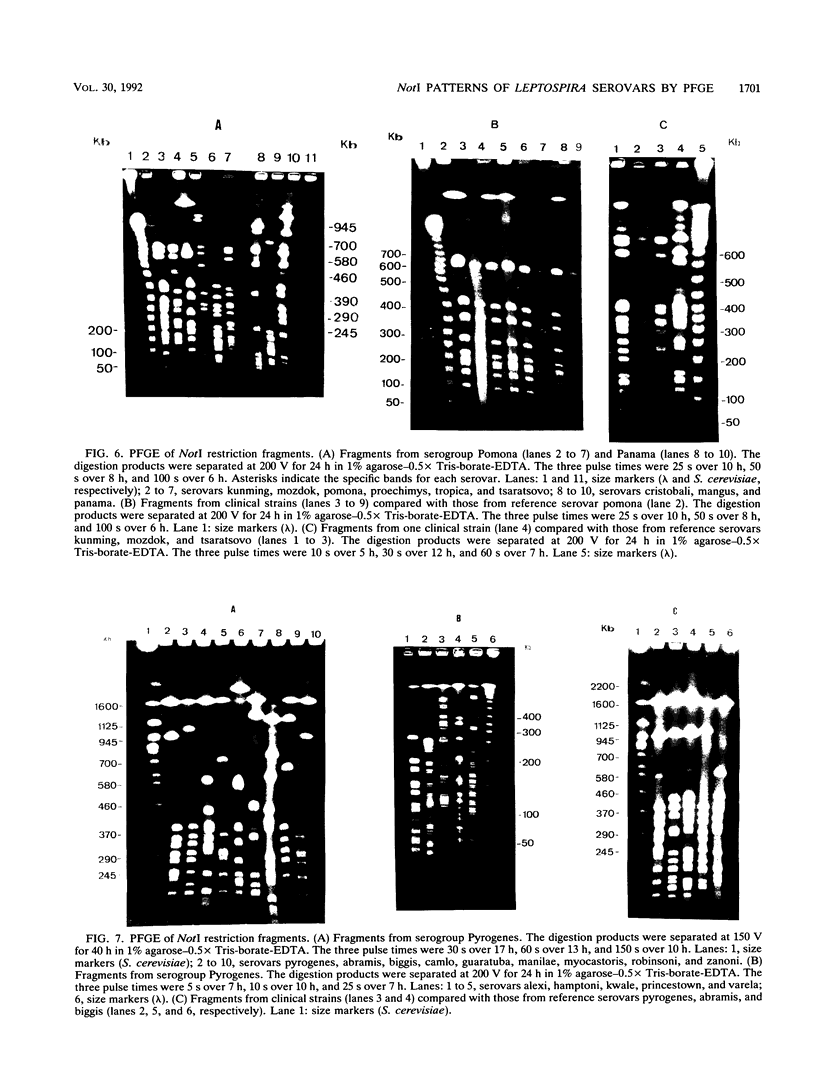

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allardet-Servent A., Bourg G., Ramuz M., Pages M., Bellis M., Roizes G. DNA polymorphism in strains of the genus Brucella. J Bacteriol. 1988 Oct;170(10):4603–4607. doi: 10.1128/jb.170.10.4603-4607.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botstein D., White R. L., Skolnick M., Davis R. W. Construction of a genetic linkage map in man using restriction fragment length polymorphisms. Am J Hum Genet. 1980 May;32(3):314–331. [PMC free article] [PubMed] [Google Scholar]
- Ellis W. A., Montgomery J. M., Thiermann A. B. Restriction endonuclease analysis as a taxonomic tool in the study of pig isolates belonging to the Australis serogroup of Leptospira interrogans. J Clin Microbiol. 1991 May;29(5):957–961. doi: 10.1128/jcm.29.5.957-961.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haapala D. K., Rogul M., Evans L. B., Alexander A. D. Deoxyribonucleic acid base composition and homology studies of Leptospira. J Bacteriol. 1969 May;98(2):421–428. doi: 10.1128/jb.98.2.421-428.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann J. L., Baril C., Bellenger E., Perolat P., Baranton G., Saint Girons I. Genome conservation in isolates of Leptospira interrogans. J Bacteriol. 1991 Dec;173(23):7582–7588. doi: 10.1128/jb.173.23.7582-7588.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hookey J. V. The detection of genetic variation in Leptospira interrogans serogroup ICTEROHAEMORRHAGIAE by ribosomal RNA gene restriction fragment patterns. FEMS Microbiol Lett. 1990 Nov;60(3):329–335. doi: 10.1016/0378-1097(90)90326-l. [DOI] [PubMed] [Google Scholar]
- Le Febvre R. B., Thiermann A. B. DNA homology studies of leptospires of serogroups Sejroe and Pomona from cattle and swine. Am J Vet Res. 1986 Apr;47(4):959–963. [PubMed] [Google Scholar]
- LeFebvre R. B. DNA probe for detection of the Leptospira interrogans serovar hardjo genotype hardjo-bovis. J Clin Microbiol. 1987 Nov;25(11):2236–2238. doi: 10.1128/jcm.25.11.2236-2238.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall R. B., Wilton B. E., Robinson A. J. Identification of Leptospira serovars by restriction-endonuclease analysis. J Med Microbiol. 1981 Feb;14(1):163–166. doi: 10.1099/00222615-14-1-163. [DOI] [PubMed] [Google Scholar]
- Marshall R. B., Winter P. J., Yanagawa R. Restriction endonuclease DNA analysis of Leptospira interrogans serovars icterohaemorrhagiae and hebdomadis. J Clin Microbiol. 1984 Oct;20(4):808–810. doi: 10.1128/jcm.20.4.808-810.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen J. N., Armstrong C. H., Nielsen N. C. Relationship among selected Leptospira interrogans serogroups as determined by nucleic acid hybridization. J Clin Microbiol. 1989 Dec;27(12):2724–2729. doi: 10.1128/jcm.27.12.2724-2729.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillot J. La classification des Spirochaetales à la lumière de nouvelles données anatomiques et antigéniques. C R Acad Sci Hebd Seances Acad Sci D. 1965 Jul 12;261(2):587–590. [PubMed] [Google Scholar]
- Pérolat P., Grimont F., Regnault B., Grimont P. A., Fournié E., Thevenet H., Baranton G. rRNA gene restriction patterns of Leptospira: a molecular typing system. Res Microbiol. 1990 Feb;141(2):159–171. doi: 10.1016/0923-2508(90)90025-l. [DOI] [PubMed] [Google Scholar]
- Robinson A. J., Ramadass P., Lee A., Marshall R. B. Differentiation of subtypes within Leptospira interrogans serovars Hardjo, Balcanica and Tarassovi, by bacterial restriction-endonuclease DNA analysis (BRENDA). J Med Microbiol. 1982 Aug;15(3):331–338. doi: 10.1099/00222615-15-3-331. [DOI] [PubMed] [Google Scholar]
- Tamai T., Sada E., Kobayashi Y. Restriction endonuclease DNA analysis of Leptospira interrogans serovars Icterohaemorrhagiae and Copenhageni. Microbiol Immunol. 1988;32(9):887–894. doi: 10.1111/j.1348-0421.1988.tb01450.x. [DOI] [PubMed] [Google Scholar]
- Terpstra W. J., Korver H., Schoone G. J., von Leeuwen J., Schönemann C. E., de Jonge-Aglibut S., Kolk A. H. Comparative classification of Leptospira serovars of the Pomona group by monoclonal antibodies and restriction-endonuclease analysis. Zentralbl Bakteriol Mikrobiol Hyg A. 1987 Oct;266(3-4):412–421. doi: 10.1016/s0176-6724(87)80221-3. [DOI] [PubMed] [Google Scholar]
- Terpstra W. J., Schoone G. J., ter Schegget J. Detection of leptospiral DNA by nucleic acid hybridisation with 32P- and biotin-labelled probes. J Med Microbiol. 1986 Aug;22(1):23–28. doi: 10.1099/00222615-22-1-23. [DOI] [PubMed] [Google Scholar]
- Thiermann A. B., Handsaker A. L., Foley J. W., White F. H., Kingscote B. F. Reclassification of North American leptospiral isolates belonging to serogroups Mini and Sejroe by restriction endonuclease analysis. Am J Vet Res. 1986 Jan;47(1):61–66. [PubMed] [Google Scholar]
- Thiermann A. B., Handsaker A. L., Moseley S. L., Kingscote B. New method for classification of leptospiral isolates belonging to serogroup pomona by restriction endonuclease analysis: serovar kennewicki. J Clin Microbiol. 1985 Apr;21(4):585–587. doi: 10.1128/jcm.21.4.585-587.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Eys G. J., Gerritsen M. J., Korver H., Schoone G. J., Kroon C. C., Terpstra W. J. Characterization of serovars of the genus Leptospira by DNA hybridization with hardjobovis and icterohaemorrhagiae recombinant probes with special attention to serogroup sejroe. J Clin Microbiol. 1991 May;29(5):1042–1048. doi: 10.1128/jcm.29.5.1042-1048.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Eys G. J., Gravekamp C., Gerritsen M. J., Quint W., Cornelissen M. T., Schegget J. T., Terpstra W. J. Detection of leptospires in urine by polymerase chain reaction. J Clin Microbiol. 1989 Oct;27(10):2258–2262. doi: 10.1128/jcm.27.10.2258-2262.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Eys G. J., Zaal J., Schoone G. J., Terpstra W. J. DNA hybridization with hardjobovis-specific recombinant probes as a method for type discrimination of Leptospira interrogans serovar hardjo. J Gen Microbiol. 1988 Mar;134(3):567–574. doi: 10.1099/00221287-134-3-567. [DOI] [PubMed] [Google Scholar]
- Zuerner R. L., Bolin C. A. Nucleic acid probe characterizes Leptospira interrogans serovars by restriction fragment length polymorphisms. Vet Microbiol. 1990 Sep;24(3-4):355–366. doi: 10.1016/0378-1135(90)90183-v. [DOI] [PubMed] [Google Scholar]
- Zuerner R. L., Bolin C. A. Repetitive sequence element cloned from Leptospira interrogans serovar hardjo type hardjo-bovis provides a sensitive diagnostic probe for bovine leptospirosis. J Clin Microbiol. 1988 Dec;26(12):2495–2500. doi: 10.1128/jcm.26.12.2495-2500.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]