Abstract
Necrotizing enterocolitis (NEC) is a devastating inflammatory condition of the gut that occurs in premature infants. Ischemia-reperfusion gut injury with production of reactive oxygen species (ROS) is thought to contribute to NEC; the exact cellular mechanisms involved are largely unknown. The purpose of this study was to determine the intracellular signaling transduction pathways involved in oxidative stress-induced intestinal epithelial cell apoptosis. H2O2 treatment resulted in rat intestinal epithelial cell apoptosis in a dose- and time-dependent manner; the caspase inhibitor, zVAD-fmk, blocked this response. Western blotting was performed to determine phosphorylation of kinases and ELISA was used to assess DNA fragmentation, as a measure of apoptosis. A rapid increase in phosphorylation of extracellular signal-related kinase (ERK)1/2, c-Jun N-terminal kinase (JNK)1/2, and Akt was noted. Inhibition of ERK and JNK decreased H2O2-induced apoptosis. Additionally, inhibition of protein kinase C (PKC) and phosphatidylinositol 3-kinase (PI3-K) attenuated and enhanced H2O2-mediated apoptosis and mitochondrial membrane potential decrease, respectively. Furthermore, activation of PKC reduced the Akt phosphorylation, while inhibition of PKC attenuated H2O2-mediated activation of caspase-3 and enhanced the H2O2-induced Akt phosphorylation. This study shows that activation of multiple signaling transduction pathways occurs during oxidative stress-induced intestinal epithelial cell injury. In contrast to ERK, JNK and PKC, PI3-K/Akt may play an important role as a protective cellular signaling pathway during this process.
Keywords: Oxidative stress, Intestinal epithelial cell injury, NEC, Signal transduction pathway, Apoptosis, Mitochondrial membrane potential
INTRODUCTION
Necrotizing enterocolitis (NEC), characterized by inflammation, ischemia and necrosis of the intestine, is a devastating condition in premature infants (1). Recent advances in the care of premature infants born with respiratory insufficiency have resulted in increased survival for extremely small premature infants and as a result, the incidence of NEC has steadily risen. There are numerous presumed risk factors for NEC which include perinatal stress, hypoxia, mesenteric ischemia-reperfusion, and hyperosmolar enteral feedings (2). In particular, a potential link between reactive oxygen species (ROS) produced by ischemia-reperfusion injury to the gut and the development of NEC has been suggested by several studies (3-5). However, the exact cellular signaling involved in oxidative stress-mediated intestinal epithelial cell apoptosis, which may occur during NEC, has not been clearly defined.
Cells undergo oxidative stress when levels of ROS exceed the counter-regulatory antioxidant capacity of the cell (6). Cellular responses to oxidative stress can vary from growth arrest to cell death depending upon the stress stimuli, duration of exposure, cell type, and surrounding cell environment (7). ROS-mediated cell damage is implicated in the pathogenesis of a variety of diseases (7). In the GI tract, ROS-induced injury endothelial dysfunction is considered to be an important cellular mechanism in indomethacin-induced gastric mucosal injury (8) as well as in the colonic inflammation associated with ulcerative colitis (9). The exact cellular signaling involved in ROS-mediated intestinal cell injury in NEC are not well defined.
Oxidative stress is known to induce apoptosis in a variety of cell types by activating intracellular cell death signaling cascades (6). On the other hand, oxidative stress can also triggerthe activation of certain signaling pathways that protect against cell death (10, 11). For example, treatment with hydrogen peroxide (H2O2) to generate ROS activates members of the mitogen-activated protein kinase (MAPK) family [extracellular signal-regulated kinases 1 and 2 (ERK1/2), c-Jun N-terminal kinases (JNK1/2) and p38 MAPKs] and phosphatidylinositol 3-kinase (PI3-K). It is generally accepted that ERK1/2 and PI3-K activation promotes cell survival by activating anti-apoptotic signaling pathways, whereas activation of JNK and p38 MAPK is associated with cell death (12-14). However, recent studies have suggested an apoptotic function for mitogen-activated protein kinase kinase (MEK)/ERK in H2O2-mediated signals (15, 16). In addition, PI3-K activation, resulting in phosphorylation of its downstream effector Akt (phospho-Akt), appears to play an essential role in cell survival by preventing the oxidative stress-induced cell death (11).
Considering the pivotal role of ROS in intestinal inflammation, we determined the intracellular signaling transduction pathways involved in the H2O2-induced rat intestinal epithelial cell (RIE-1) injury in the present study. We show that H2O2 induces apoptosis in RIE-1 cells with increased phosphorylation of ERK1/2, JNK1/2, and Akt. Inhibition of MEK/ERK1/2, JNK, and protein kinase C (PKC) attenuated, while inhibition of PI3-K enhanced H2O2-mediatedapoptosis. Moreover, we found that inhibition of PKC results in the enhanced Akt phosphorylation and attenuates H2O2-induced RIE-1 cell death.
MATERIALS AND METHODS
Reagents
Wortmannin, phorbol-12-myristate-13-acetate (PMA), and H2O2 were purchased from Sigma (St. Louis, MO). Bis-indolylmaleimide (GF109203×), U0126, and SP600125 were from Calbiochem (San Diego, CA). Rabbit polyclonal anti-ERK1, rabbit polyclonal anti-phospho-ERK1/2, rabbit polyclonal anti-caspase-3, and rabbit anti-poly (ADP-ribose) polymerase (PARP) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). The anti-actin antibody was purchased from Sigma. Rabbit anti-JNK antibody, rabbit anti-phospho-JNK antibody, rabbit anti-phospho-Akt (Ser473) antibody, and rabbit anti-Akt antibody were purchased from Cell Signaling (Beverly, MA). Tissue culture media and reagents were obtained from Life Technologies, Inc. (Grand Island, NY). Polyvinylidene difluoride PVDF membranes were from Millipore Corp. (Bedford, MA). The enhanced chemiluminescence system was purchased from Amersham Biosciences (Piscataway, NJ).
Cell culture
Rat intestinal epithelial (RIE)-1 cells were maintained in Dulbecco's modified Eagle medium supplemented with 10% fetal bovine serum and cultured at 37°C under an atmosphere containing 5% CO2. All experiments were performed on cells within 6 weeks of culture from liquid nitrogen stocks and free of mycoplasma contamination. In order to eliminate the potential ROS scavenger action of dimethyl sulfoxide (DMSO) (17) in vehicle, we used the same concentrations of DMSO (0.1%) for both inhibitors and vehicle.
DNA fragmentation assay
Cells were plated in 96-well plates 24 h before treatment. DNA fragmentation was evaluated by examination of cytoplasmic histone-associated DNA fragments (mononucleosomes and oligonucleosomes) using a Cell Death Detection ELISAPlus kit (Roche Molecular Biochemicals, Indianapolis, IN) according to the manufacturer's instructions.
Protein extraction and Western blot analysis
Cells were lysed with TNN buffer [50 mM Tris-HCI (pH 7.5), 150 mM NaCl, 0.5 mM NP40, 50 mM NaF, 1 mM sodium orthovanadate, 1 mM DTT, 1 mM phenylmethylsulfonyl fluoride, and 25 μg/ml each of aprotinin, leupeptin, and pepstatin A] at 4°C for 30 min. Lysates were clarified by centrifugation (10,000 × g for 30 min at 4°C), and protein concentrations were determined using the method described by Bradford (18). Total protein (100 μg) was resolved on a 10% polyacrylamide gel and transferred to polyvinylidene difluoride membranes. Filters were incubated overnight at 4°C in a blocking solution (Tris-buffered saline containing 5% nonfat dried milk and 0.1% Tween 20), followed by a 1h incubation with primary antibodies at 4°C overnight. Filters were washed three times in a blocking solution and incubated with horseradish peroxidase-conjugated second antibodies for 1h at room temperature. After three additional washes, the immune complexes were visualized by enhanced chemiluminescence system.
JC-1 mitochondrial membrane potential detection
The mitochondrial membrane potential was analyzed using MitoProbe™ JC-1 Assay kit (Molecular Probes, Eugene, OR). The collapse in the electrochemical gradient across the mitochondrial membrane was measured using a fluorescent cationic dye 5,5’,6,6,-tetrachloro-1,1’,3,3’-tetraethyl-benzamidazolo-carbocyanin iodide, known as JC-1. This dye exhibits potential dependent accumulation in mitochondrial matrix (19). Cells (1×106) were incubated with 2 μM JC-1 for 15 min at room temperature in darkness. Cells were washed twice with PBS at 4°C, resuspended in 0.5 ml PBS, and analyzed on a FACSCalibur flowcytometer.
Statistical analysis
Results are expressed as the mean ± SEM. The data in the figures were analyzed using the Kruskal-Wallis test and assessed at the 0.05 level of significance.
RESULTS
H2O2 induces apoptosis in RIE-1 cells
H2O2 treatment resulted in RIE-1 cell death in a dose-dependent manner with a significant induction of cell death at concentrations of 100 to 500 μM (Fig 1A). The mechanism of apoptosis is mediated by sequential activation of caspases (20). To confirm whether H2O2 is inducing apoptosis in RIE-1 cells through caspase activation, we next pretreated cells with the pan-caspase inhibitor, zVAD-fmk (20), prior to H2O2 treatment. Pretreatment with zVAD-fmk effectively inhibited apoptosis induced by H2O2 in a dose-dependent manner, suggesting a role for caspase activation in H2O2-mediated induction of apoptosis (Fig. 1B).
Fig. 1. H2O2 induces RIE-1 cell apoptosis.

(A) RIE-1 cells were plated for 24 h prior to treatment with H2O2 for 3 h or (B) pretreated with zVAD-fmk for 30 min prior to treatment with H2O2. Apoptosis was estimated by a DNA fragmentation ELISA (Data represent triplicate determinations ± SEM; * = P < 0.05 vs. control; † = P < 0.05 vs. H2O2 alone). Experiments were repeated twice. (C) RIE-1 cells were treated with H2O2 for various timepoints. A representative Western blot from three separate experiments is shown here.
To further confirm the activation of caspase pathway in the apoptotic effect of H2O2 on RIE-1 cells, we next determined protein expression of cleavage products of caspase-3 and PARP after treatment with H2O2. Treatment with H2O2 resulted in activation of caspase-3, as demonstrated by cleavage of pro-caspase-3 noted by increased expression of the Mr 17,000 cleavage product (i.e., active caspase-3). In addition, cleavage of PARP (Mr 85,000 cleavage product) was also noted (Fig. 1C).
H2O2 activates ERK1/2, JNK, and Akt
We found that RIE-1 cells undergo apoptosis when exposed to H2O2 in a dose- and time-dependent manner. Oxidative stress is known to activate multiple signal transduction pathways in many experimental systems (6, 7). To identify the signaling mechanisms activated by H2O2 in RIE-1 cells, we assessed the phosphorylated protein expression of ERK, JNK, and Akt. Treatment with H2O2 resulted in marked increases in phosphorylation of ERK1/2 and JNK1/2, without affecting the total protein expression levels (Fig. 2). The activation of ERK1/2 and JNK1/2 occurred at 25 min after the addition of H2O2 (500 μM), peaked at 30 to 60 min, and returned to basal level by 2 h. Interestingly, H2O2 treatment resulted in sustained activation of Akt throughout the time course.
Fig. 2. Activation of signal transduction pathways by H2O2.

RIE-1 cells were treated with H2O2 over a time course for Western blot analysis. A representative blot from three separate experiments is shown here.
Effects of protein kinase inhibition on H2O2-mediated apoptosis
To further delineate the signaling pathways involved in H2O2-mediated apoptosis, RIE-1 cells were pretreated for 30 min with inhibitors to JNK (SP600125), ERK1/2 upstream kinase MEK1 (U0126), or combination of both for 30 min prior to treatment with H2O2 (500 μM). Cell death was assayed at 3 h after addition of H2O2. As expected, H2O2 induced RIE-1 cell death; this induction was partially attenuated by treatment with either SP600125 or U0126 (Fig. 3A). However, combination treatment did not show synergistic inhibition of cell death. We also confirmed the inhibitory effect of U0126 on the MEK/ERK1/2 pathway by determining phosphorylated protein level of ERK1/2 by Western blot. As noted previously, phospho-ERK1/2 was increased in H2O2-treated RIE-1 cells, and this response was completely blocked by U0126 compound (Fig. 3B).
Fig. 3. Effects of kinase inhibitors on H2O2-induced RIE-1 cell death.

(A, C) RIE-1 cells were pretreated with kinase inhibitors [JNK inhibitor SP600125, the MEK1 inhibitor U0126 or combination (A) and PI3-K inhibitor wortmannin or PKC inhibitor GF109203× (C)] for 30 min prior to treatment with H2O2 for 3 hours. Apoptosis was estimated by a DNA fragmentation ELISA (Data represent triplicate determinations ± SEM; * = P < 0.05 vs. control; † = P < 0.05 vs. H2O2 alone). Experiments were repeated twice. (B) RIE-1 cells were pretreated with the U0126 for 30 min prior to treatment with H2O2 for 30 min and Western blotting performed. (D) RIE-1 cells were pretreated with the wortmannin for 30 min prior to treatment with H2O2 for 3 hours and Western blotting performed. Representative blots from three separate experiments are shown as (B) and (D).
To delineate the role of PI3-K/Akt and PKC signaling pathways involved in H2O2-mediated apoptosis, RIE-1 cells were pretreated for 30 min with Akt upstream kinase PI3-K inhibitor, wortmannin or PKC inhibitor GF109203×, prior to addition of H2O2 (500 μM). Wortmannin significantly enhanced H2O2-mediated RIE-1 cell death at 3 h (Fig. 3C). In contrast, GF109203× markedly attenuated H2O2-induced RIE-1 cell death. The inhibitory effect of wortmannin on the PI3-K/Akt pathway was also confirmed by demonstrating the inhibition of H2O2-induced Akt phosphorylation by wortmannin in Fig. 3D. Collectively, these results suggest a proapoptotic role for the MEK/ERK, JNK, and PKC pathways and an antiapoptotic role for PI3-K/Akt pathway in H2O2-induced RIE-1 cell death.
PKC negatively regulates Akt activity in RIE-1 cells
To further determine the molecularmechanisms involved in the effects of PKC or PI3-K inhibition on H2O2-induced apoptosis, we next determined activation of caspase-3. As expected, treatment with H2O2 resulted in activation of caspase-3; this activation was attenuated by treatment with GF109203× (an inhibitor of PKC) as demonstrated by decreased cleavage of pro-caspase-3 (Fig. 4A). In contrast, pretreatment with wortmannin significantly enhanced cleavage of pro-caspase-3.
Fig. 4. Inhibition of PKC results in the activation of Akt and blocks H2O2-induced caspase-3 cleavage.

(A) RIE-1 cells were pretreated with the PI3-K inhibitor wortmannin or the PKC inhibitor GF109203× for 30 min prior to treatment with H2O2 for 3 h for Western blot analysis. (B, C) RIE-1 cells were pretreated with the GF109203× for 30 min prior to treatment with H2O2 (B) or PMA (C) for Western blot analysis. Representative blots from three separate experiments are shown here.
Since PI3-K/Akt appears to be an important anti-apoptotic pathway during H2O2-induced RIE-1 cell death and inhibition of PKC significantly prevents H2O2-induced cell death, we next determined the regulation of Akt phosphorylation by PKC. As shown previously, treatment with H2O2 induced Akt phosphorylation (Fig. 4B). Interestingly, the treatment with GF109203× resulted in synergistic increase in H2O2-induced activation of Akt, suggesting that significant attenuation of H2O2-mediated apoptosis with PKC inhibition may act through the enhanced PI3-K/Akt activation. Pretreatment with GF109203× has no effect on H2O2-induced ERK1/2 or JNK phosphorylation, suggesting that H2O2 induces ERK1/2 or JNK phosphorylation in a PKC-independent fashion. To further delineate the regulation of Akt phosphorylation by PKC, RIE-1 cells were pretreated with the PKC inhibitor GF109203× (5 μM) for 30 min prior to treatment with PKC activator, PMA (100 nM) for additional 30 min. As shown in Fig. 4C, PMA reduced Akt phosphorylation. GF109203× alone increased Akt phosphorylation and attenuated PMA-reduced Akt phosphorylation. Treatment with PMA induced ERK1/2 but not JNK phosphorylation and this induction was blocked by the combination treatment with GF109203×, suggesting that PKC isoform(s) involved in PMA-induced ERK phosphorylation is different from that involved in H2O2-induced cell death.
Inhibition of PKC attenuates, while inhibition of PI3-K enhances, H2O2-induced mitochondrial depolarization
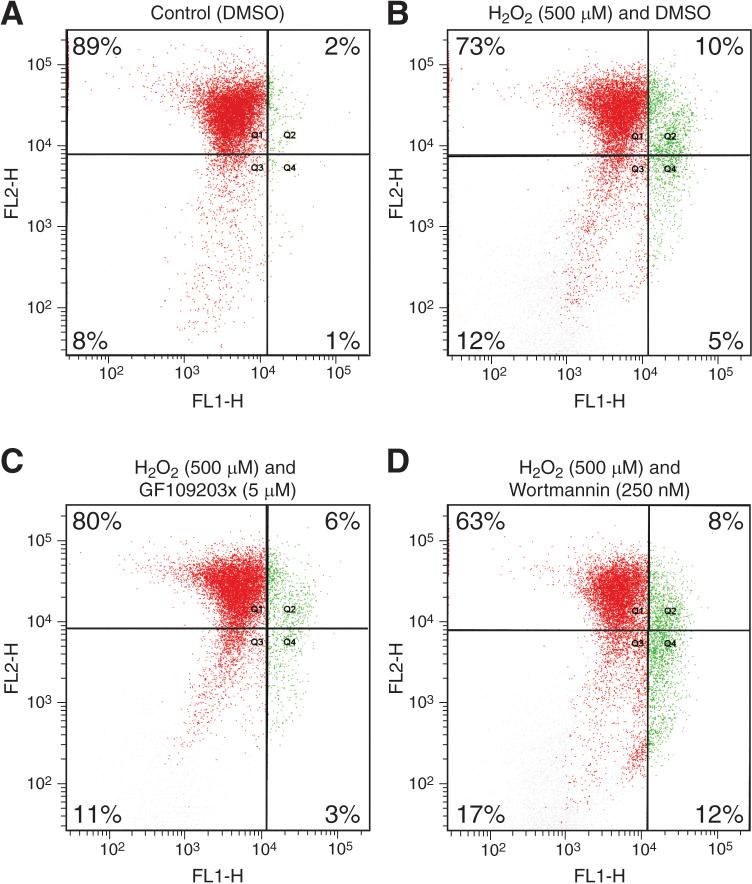
Mitochondrial membrane depolarization, which increases permeability and releases pro-apoptotic factors into the cytosol, is an early event of apoptosis. Recently, decreased mitochondrial membrane potential was found to be the early signs of apoptosis in human intestinal epithelial cell line SW-480 (21). To determine if the mitochondria-initiated pathway is involved in H2O2-induced apoptosis, we measured H2O2-induced alterations in mitochondrial membrane potential and the effect of PKC or PI3-K inhibition. In healthy cells, lipophilic cation JC-1 exists as a monomer in the cytosol (FL-1 positive; green) and also accumulates as aggregates in the mitochondria (FL-2 positive; red). In apoptotic and necrotic cells, JC-1 exists exclusively in monomer form and produces a green cytosolic signal (22). As shown in Figure 5, treatment with H2O2 resulted in a decline in the mitochondrial membrane potential (Fig. A, B), implicating altered mitochondrial membrane permeability as an important event for H2O2-induced apoptosis in RIE-1 cells. Pretreatment with PKC inhibitor GF109203× attenuated H2O2-induced mitochondrial depolarization (Fig. 5C). In contrast, pretreatment with wortmannin significantly enhanced H2O2-induced mitochondrial depolarization (Fig. 5D). These results suggest that regulation of H2O2-induced apoptosis by PKC or PI3-K may lie upstream of mitochondria.
Fig. 5. Inhibition of PKC attenuates, while inhibition of PI3-K enhances, H2O2-induced mitochondrial depolarization.
RIE-1 cells were pretreated with the PI3-K inhibitor wortmannin or the PKC inhibitor GF109203× for 30 min prior to treatment with H2O2. for 3 h. Cells were labeled with 2 μM JC-1 for 15 min at room temperature, and intensities of FL-1 and FL-2 fluorescence were measured. JC-1 fluorescence in the FL-1 channel increases as the mitochondrial membrane potential drops while its fluorescence in FL-2 channel decreases. Percentage numbers in Q1 and Q2, Q4 indicate proportion of cells with normal and depolarized mitochondria, respectively. Representative data from three separate experiments are shown here.
DISCUSSION
In this study, we found that H2O2 treatment induces the phosphorylation of ERK, JNK, and Akt, resulting in RIE-1 cell apoptosis via the caspase pathway. The activation of ERK and JNK may be involved in the H2O2-induced intestinal epithelial cell apoptosis since the inhibition of either pathway resulted in attenuation of apoptotic response. In contrast, the activation of Akt may be important as the cell survival signal given the fact that inhibition of PI3-K significantly enhanced H2O2-induced cell death. Moreover, inhibition of PKC, which resulted in activation of Akt, was found to attenuate the apoptosis induced by H2O2.
NEC is the most common gastrointestinal tract surgical emergencies in neonates. Despite recent advances, the overall mortality rate remains high, especially in extremely small premature infants. The numerous risk factors for the pathogenesis of NEC have been well described; however, the exact cellular mechanisms involved in intestinal epithelial cell injury during NEC are still not clearly elucidated. Transient mesenteric ischemia-reperfusion injury resulting in the production of ROS has been implicated to contribute to NEC (3-5). Additionally, extensive apoptosis occurs in enterocytes in the apical villi of infants with NEC (23); however, the exact role of ROS on the intestinal epithelial cell fate during NEC as well as the cellular mechanisms that are involved in this process are largely unknown. Therefore, in the present study, we attempted to investigate the role of intracellular signal transduction pathways involved in oxidative stress-induced intestinal epithelial cell apoptosis.
Oxidative stress can trigger the activation of multiple signaling pathways that influence the cytotoxicity observed in affected cells, including the activation of the MAPK cascade. The MAPK signaling, which is stimulated by growth factor receptors, is also linked to the protection of various cell types against apoptosis. Having established that H2O2 triggers ERK1/2, JNK1/2 and PI3-K activation in RIE-1 cells, we then investigated role of these protein kinase pathways in H2O2-induced cell death using a range of pharmacological inhibitors. Our results show that pre-treatment with the selective MEK1 inhibitor U0126 (10 μM) or JNK inhibitor SP600125 (20 μM) partially reduced H2O2-induced apoptosis in RIE-1 cells. Similar results were found with the use of PD98059 compound, another MEK inhibitor (data not shown). These data clearly suggest that an inhibition of ERK1/2 and JNK pathways protect RIE-1 cells from H2O2-induced cell death.
Our results are consistent with the findings from other cell-type studies. For example, ERK1/2 is involved in neuronal cell death triggered by oxidative stress (24), and inhibition of the JNK pathway improves cell viability in response to oxidant injury (25). Furthermore, inhibition of MEK PD98059 attenuates H2O2-induced cell death of the human colon cancer cell line HT-29 through inhibition of JNK (16). However, several studies have also shown the opposite role of ERK1/2 pathway during oxidative stress-induced cell injury. Treatment of primary cortical neurons with the MEK1/2 inhibitors, U0126 and PD98059, have been shown to significantly increase neuronal cell loss induced by H2O2 and hypoxia, respectively (14). Therefore, the role of ERK1/2 in determining the fate of cells (survival or death) following oxidative stress injury is cell-type specific. Moreover, several reports have also demonstrated that H2O2-induced activation of ERK is mediated by both PKC-dependent and -independent mechanisms (26, 27). In our study, treatment with PKC inhibitor, GF109203×, which attenuated H2O2-induced RIE-1 cell death, blocked PMA- but not H2O2-mediated ERK1/2 phosphorylation, suggesting that H2O2 may activate ERK1/2 via PKC-independent pathway in RIE-1 cells.
Apoptotic signals by H2O2 also up-regulated antiapoptotic signaling, such as the PI3-K/Akt pathway. PI3-K/Akt pathway plays a critical role in human intestinal epithelial cell survival and differentiation (28, 29). Furthermore, the role of the PI3-K/Akt pathway in protecting against ROS-induced cell death has been extensively documented (6, 11, 30). In this study, we show that H2O2 stimulates Akt phosphorylation in RIE-1 cells via a PI3-K-dependent pathway suggesting that H2O2-induced Akt activation may be involved in protecting RIE-1 cells against oxidative stress-induced cell death. In order to investigate this possibility, we used the PI3-K inhibitor, wortmannin and found that it significantly increases cell death triggered by H2O2. Our findings suggest that the ultimate fate of intestinal epithelial cells in response to oxidative stress-induced injury is determined by the balance between activation of apoptotic and antiapoptotic signals.
The ERK and JNK inhibition did not completely block H2O2-induced cell death, suggesting the involvement of multiple pathways in H2O2-induced apoptosis in RIE-1 cells. Indeed, our results show that inhibition of PKC using GF109203× maximally attenuated H2O2-induced apoptosis, indicating that H2O2-mediated RIE-1 cell death also requires PKC activation. The involvement of various isoforms of PKC in the regulation of H2O2-mediated processes has been documented in a number of cell types (31, 32); however, its role in the regulation of H2O2-induced intestinal epithelial cell death is undefined. The activation of selective PKC isoforms, δ and ε, have been implicated in tumor necrosis factor (TNF)-α-induced intestinal epithelial cell death as proapoptotic signals (33). Further studies are required to determine the role of PKC isoforms that are involved in H2O2-induced intestinal epithelial cell death.
Another important finding identified in our study is that PKC may play a role in H2O2-mediated apoptosis through the regulation of PI3-K/Akt, a critical pathway in cell survival. Our results indicated that the H2O2-induced apoptosis required PKC activation. Moreover, we show that inhibition of PKC contributed to the activation of Akt and in contrast, activation of PKC by PMA reduced Akt activity (Fig 4B &C). Consistent with our findings, recent studies have shown that non-selective PKC inhibitors stimulate and PMA attenuates Akt phosphorylation in A549 and HEK293 cells (34) and PKC negatively modulates the hepatocyte growth factor-induced migration, integrin expression and PI3-K activation in human hepatoma cells (35).
Lastly, intracellular signaling pathways directly and indirectly regulate mitochondrial membrane stability and function to ultimately dictate apoptotic fate of a cell. Activation of PKC in some cell types initiates an apoptotic pathway that involves alteration of mitochondrial membrane potential (36). Moreover, the major mechanism involved in the antiapoptotic effect of PI3-K/Akt is the phosphorylation of BAD, which is then sequestered in the cytosol and preventedfrom interacting with Bcl-2/Bcl-xL and from disrupting the mitochondrial membrane (37). Inhibition of PI3-K/Akt signaling potentiates cytochrome c release from mitochondria and induces mitochondrial transmembrane potential decrease in bovine carotid artery endothelial cells (38). In agreement with these mechanisms, we observed that activation of PKC decreased Akt phosphorylation while inhibition of PKC increases Akt phosphorylation and attenuates H2O2-induced apoptosis and mitochondrial depolarization. In contrast, inhibition of PI3-K/Akt signaling significantly potentiated H2O2-induced apoptosis and induced significant mitochondrial membrane potential decrease. Our results suggest that the regulation of H2O2-induced apoptosis by PKC may act through down-regulation of PI3-K/Akt and this regulation may occur upstream of mitochondria.
In conclusion, our study demonstrates that H2O2-induced apoptosis involves the activation of ERK, JNK, and PKC signaling pathways in intestinal epithelial cells, and that inhibition of PI3-K enhances apoptosis. Moreover, PKC may play an important role in H2O2-induced intestinal epithelial cell death through negative regulation of PI3-K/Akt and this may occur upstream of mitochondria. Further studies will be directed towards identifying the isoform(s) of PKC and the downstream mediators responsible for intestinal cell death. A better understanding of the signal transduction pathways involved in H2O2-induced intestinal cell death will potentially allow us to develop novel therapy in the treatment of oxidative stress-mediated gut injury.
ACKNOWLEDGMENTS
The authors thank Karen Martin for manuscript preparation and Tatsuo Uchida for assistance with statistical analysis.
This work was supported by grants RO1 DK61470, RO1 DK48498 and PO1 DK35608 from the National Institutes of Health and grants 8560, 8800 from Shriners Burns Hospital.
ABBREVIATIONS
- GF109203×
Bis-indolylmaleimide
- JNK1/2
c-Jun N-terminal kinases 1 and 2
- MAPK
Mitogen-activated protein kinase
- NEC
Necrotizing enterocolitis
- PI3-K
Phosphatidylinositol 3-kinase
- PARP
Poly (ADP-ribose) polymerase
- RIE-1
Rat intestinal epithelial
- ROS
Reactive oxygen species
- ERK1/2
Extracellular signal-regulated kinases 1 and 2
- PMA
phorbol-12-myristate-13-acetate
REFERENCES
- 1.Henry MC, Moss RL. Current issues in the management of necrotizing enterocolitis. Semin Perinatol. 2004;28:221–233. doi: 10.1053/j.semperi.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 2.Noerr B. Current controversies in the understanding of necrotizing enterocolitis. Part 1. Adv Neonatal Care. 2003;3:107–120. doi: 10.1016/s1536-0903(03)00072-9. [DOI] [PubMed] [Google Scholar]
- 3.Kelly N, Friend K, Boyle P, Zhang XR, Wong C, Hackam DJ, Zamora R, Ford HR, Upperman JS. The role of the glutathione antioxidant system in gut barrier failure in a rodent model of experimental necrotizing enterocolitis. Surgery. 2004;136:557–566. doi: 10.1016/j.surg.2004.05.034. [DOI] [PubMed] [Google Scholar]
- 4.Okur H, Kucukaydin M, Kose K, Kontas O, Dogam P, Kazez A. Hypoxia-induced necrotizing enterocolitis in the immature rat: the role of lipid peroxidation and management by vitamin E. J Pediatr Surg. 1995;30:1416–1419. doi: 10.1016/0022-3468(95)90395-x. [DOI] [PubMed] [Google Scholar]
- 5.Clark DA, Fornabaio DM, McNeill H, Mullane KM, Caravella SJ, Miller MJ. Contribution of oxygen-derived free radicals to experimental necrotizing enterocolitis. Am J Pathol. 1988;130:537–542. [PMC free article] [PubMed] [Google Scholar]
- 6.Martindale JL, Holbrook NJ. Cellular response to oxidative stress: signaling for suicide and survival. J Cell Physiol. 2002;192:1–15. doi: 10.1002/jcp.10119. [DOI] [PubMed] [Google Scholar]
- 7.Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- 8.Yoshikawa T, Naito Y, Kishi A, Tomii T, Kaneko T, Iinuma S, Ichikawa H, Yasuda M, Takahashi S, Kondo M. Role of active oxygen, lipid peroxidation, and antioxidants in the pathogenesis of gastric mucosal injury induced by indomethacin in rats. Gut. 1993;34:732–737. doi: 10.1136/gut.34.6.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sedghi S, Fields JZ, Klamut M, Urban G, Durkin M, Winship D, Fretland D, Olyaee M, Keshavarzian A. Increased production of luminol enhanced chemiluminescence by the inflamed colonic mucosa in patients with ulcerative colitis. Gut. 1993;34:1191–1197. doi: 10.1136/gut.34.9.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang X, Martindale JL, Liu Y, Holbrook NJ. The cellular response to oxidative stress: influences of mitogen-activated protein kinase signalling pathways on cell survival. Biochem J. 1998;333(Pt 2):291–300. doi: 10.1042/bj3330291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qin S, Chock PB. Implication of phosphatidylinositol 3-kinase membrane recruitment in hydrogen peroxide-induced activation of PI3K and Akt. Biochemistry. 2003;42:2995–3003. doi: 10.1021/bi0205911. [DOI] [PubMed] [Google Scholar]
- 12.Xia Z, Dickens M, Raingeaud J, Davis RJ, Greenberg ME. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science. 1995;270:1326–1331. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]
- 13.Harper SJ, LoGrasso P. Signalling for survival and death in neurones: the role of stress-activated kinases, JNK and p38. Cell Signal. 2001;13:299–310. doi: 10.1016/s0898-6568(01)00148-6. [DOI] [PubMed] [Google Scholar]
- 14.Crossthwaite AJ, Hasan S, Williams RJ. Hydrogen peroxide-mediated phosphorylation of ERK1/2, Akt/PKB and JNK in cortical neurones: dependence on Ca(2+) and PI3-kinase. J Neurochem. 2002;80:24–35. doi: 10.1046/j.0022-3042.2001.00637.x. [DOI] [PubMed] [Google Scholar]
- 15.Bhat NR, Zhang P. Hydrogen peroxide activation of multiple mitogen-activated protein kinases in an oligodendrocyte cell line: role of extracellular signal-regulated kinase in hydrogen peroxide-induced cell death. J Neurochem. 1999;72:112–119. doi: 10.1046/j.1471-4159.1999.0720112.x. [DOI] [PubMed] [Google Scholar]
- 16.Salh BS, Martens J, Hundal RS, Yoganathan N, Charest D, Mui A, Gomez-Munoz A. PD98059 attenuates hydrogen peroxide-induced cell death through inhibition of Jun N-Terminal Kinase in HT29 cells. Mol Cell Biol Res Commun. 2000;4:158–165. doi: 10.1006/mcbr.2001.0271. [DOI] [PubMed] [Google Scholar]
- 17.Zegura B, Lah TT, Filipic M. The role of reactive oxygen species in microcystin-LR-induced DNA damage. Toxicology. 2004;200:59–68. doi: 10.1016/j.tox.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 18.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 19.Smiley ST, Reers M, Mottola-Hartshorn C, Lin M, Chen A, Smith TW, Steele GD, Jr., Chen LB. Intracellular heterogeneity in mitochondrial membrane potentials revealed by a J-aggregate-forming lipophilic cation JC-1. Proc Natl Acad Sci U S A. 1991;88:3671–3675. doi: 10.1073/pnas.88.9.3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kluck RM, Bossy-Wetzel E, Green DR, Newmeyer DD. The release of cytochrome c from mitochondria: a primary site for Bcl-2 regulation of apoptosis. Science. 1997;275:1132–1136. doi: 10.1126/science.275.5303.1132. [DOI] [PubMed] [Google Scholar]
- 21.Li JM, Zhou H, Cai Q, Xiao GX. Role of mitochondrial dysfunction in hydrogen peroxide-induced apoptosis of intestinal epithelial cells. World J Gastroenterol. 2003;9:562–567. doi: 10.3748/wjg.v9.i3.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nihal M, Ahmad N, Mukhtar H, Wood GS. Anti-proliferative and proapoptotic effects of (−)-epigallocatechin-3-gallate on human melanoma: possible implications for the chemoprevention of melanoma. Int J Cancer. 2005;114:513–521. doi: 10.1002/ijc.20785. [DOI] [PubMed] [Google Scholar]
- 23.Ford H, Watkins S, Reblock K, Rowe M. The role of inflammatory cytokines and nitric oxide in the pathogenesis of necrotizing enterocolitis. J Pediatr Surg. 1997;32:275–282. doi: 10.1016/s0022-3468(97)90194-9. [DOI] [PubMed] [Google Scholar]
- 24.Stanciu M, DeFranco DB. Prolonged nuclear retention of activated extracellular signal-regulated protein kinase promotes cell death generated by oxidative toxicity or proteasome inhibition in a neuronal cell line. J Biol Chem. 2002;277:4010–4017. doi: 10.1074/jbc.M104479200. [DOI] [PubMed] [Google Scholar]
- 25.Li Y, Arita Y, Koo HC, Davis JM, Kazzaz JA. Inhibition of c-Jun N-terminal kinase pathway improves cell viability in response to oxidant injury. Am J Respir Cell Mol Biol. 2003;29:779–783. doi: 10.1165/rcmb.2003-0087RC. [DOI] [PubMed] [Google Scholar]
- 26.Song HJ, Lee TS, Jeong JH, Min YS, Shin CY, Sohn UD. Hydrogen peroxide-induced extracellular signal-regulated kinase activation in cultured feline ileal smooth muscle cells. J Pharmacol Exp Ther. 2005;312:391–398. doi: 10.1124/jpet.104.074401. [DOI] [PubMed] [Google Scholar]
- 27.Zhang J, Jin N, Liu Y, Rhoades RA. Hydrogen peroxide stimulates extracellular signal-regulated protein kinases in pulmonary arterial smooth muscle cells. Am J Respir Cell Mol Biol. 1998;19:324–332. doi: 10.1165/ajrcmb.19.2.3209. [DOI] [PubMed] [Google Scholar]
- 28.Gauthier R, Harnois C, Drolet JF, Reed JC, Vezina A, Vachon PH. Human intestinal epithelial cell survival: differentiation state-specific control mechanisms. Am J Physiol Cell Physiol. 2001;280:C1540–1554. doi: 10.1152/ajpcell.2001.280.6.C1540. [DOI] [PubMed] [Google Scholar]
- 29.Wang Q, Wang X, Hernandez A, Kim S, Evers BM. Inhibition of the phosphatidylinositol 3-kinase pathway contributes to HT29 and Caco-2 intestinal cell differentiation. Gastroenterology. 2001;120:1381–1392. doi: 10.1053/gast.2001.24044. [DOI] [PubMed] [Google Scholar]
- 30.Wang X, McCullough KD, Franke TF, Holbrook NJ. Epidermal growth factor receptor-dependent Akt activation by oxidative stress enhances cell survival. J Biol Chem. 2000;275:14624–14631. doi: 10.1074/jbc.275.19.14624. [DOI] [PubMed] [Google Scholar]
- 31.Gopalakrishna R, Jaken S. Protein kinase C signaling and oxidative stress. Free Radic Biol Med. 2000;28:1349–1361. doi: 10.1016/s0891-5849(00)00221-5. [DOI] [PubMed] [Google Scholar]
- 32.Niwa K, Inanami O, Yamamori T, Ohta T, Hamasu T, Karino T, Kuwabara M. Roles of protein kinase C delta in the accumulation of P53 and the induction of apoptosis in H2O2-treated bovine endothelial cells. Free Radic Res. 2002;36:1147–1153. doi: 10.1080/1071576021000016409. [DOI] [PubMed] [Google Scholar]
- 33.Chang Q, Tepperman BL. Effect of selective PKC isoform activation and inhibition on TNF-alpha-induced injury and apoptosis in human intestinal epithelial cells. Br J Pharmacol. 2003;140:41–52. doi: 10.1038/sj.bjp.0705398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wen HC, Huang WC, Ali A, Woodgett JR, Lin WW. Negative regulation of phosphatidylinositol 3-kinase and Akt signalling pathway by PKC. Cell Signal. 2003;15:37–45. doi: 10.1016/s0898-6568(02)00047-5. [DOI] [PubMed] [Google Scholar]
- 35.Gujdar A, Sipeki S, Bander E, Buday L, Farago A. Protein kinase C modulates negatively the hepatocyte growth factor-induced migration, integrin expression and phosphatidylinositol 3-kinase activation. Cell Signal. 2004;16:505–513. doi: 10.1016/j.cellsig.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 36.Li L, Lorenzo PS, Bogi K, Blumberg PM, Yuspa SH. Protein kinase Cdelta targets mitochondria, alters mitochondrial membrane potential, and induces apoptosis in normal and neoplastic keratinocytes when overexpressed by an adenoviral vector. Mol Cell Biol. 1999;19:8547–8558. doi: 10.1128/mcb.19.12.8547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alessi DR, Cohen P. Mechanism of activation and function of protein kinase B. Curr Opin Genet Dev. 1998;8:55–62. doi: 10.1016/s0959-437x(98)80062-2. [DOI] [PubMed] [Google Scholar]
- 38.Zhang L, Himi T, Morita I, Murota S. Inhibition of phosphatidylinositol-3 kinase/Akt or mitogen-activated protein kinase signaling sensitizes endothelial cells to TNF-alpha cytotoxicity. Cell Death Differ. 2001;8:528–536. doi: 10.1038/sj.cdd.4400838. [DOI] [PubMed] [Google Scholar]