Abstract
Studies performed using cultured cells indicate that IgE functions not only to trigger degranulation of mast cells following allergen exposure but also to enhance their survival. Such an influence of IgE on mast cell homeostasis during allergic responses in vivo has not been established. Here we show that inhalation of Aspergillus fumigatus (Af) extract in mice induced a dramatic rise in IgE accompanied by an increase in airway mast cells. These had an activated phenotype with high levels of FcεRI. Plasma mast cell protease-1 was also increased, indicating an elevated systemic mast cell load. In addition, enhanced levels of IL-5 and eosinophils were observed in the airway. Both mast cell expansion and activation were markedly attenuated in IgE−/− animals which are incapable of producing IgE in response to Af. The recruitment of eosinophils to the airways was also reduced in IgE−/− mice. Analyses of potential cellular targets of IgE revealed that IgE antibodies are not required for the induction of mast cell progenitors in response to allergen but rather act by sustaining the survival of mature mast cells. Our results identify an important role for IgE antibodies in promoting mast cell expansion during allergic responses in vivo.
Keywords: Mast cells/Basophils, Allergy, Antibodies
Introduction
Asthma commonly affects individuals with atopy, a genetic predisposition to generate allergen-specific IgE antibodies. The disease is characterized both by T cell-driven airway inflammation with eosinophil and mononuclear cell infiltration of the airway mucosa and by superimposed acute exacerbations triggered by the interaction of IgE antibodies with inhaled aeroallergens (1–4). Pedigree analyses reveal an association between IgE levels and disease activity in asthma (5–9). In allergic subjects, acute allergen-induced decreases in FEV1 are markedly blunted by IgE blockade with the monoclonal anti-IgE antibody, omalizumab (10). Mast cells are present in the airways of patients with asthma (11–13), where they are optimally situated to trigger hypersensitivity responses upon exposure to inhaled environmental antigens. A correlation has been observed between numbers of mast cells in bronchial smooth muscle and asthma severity (14).
Although IgE antibodies provide a critical antigen-specific activation function for mast cells in initiating both immediate hypersensitivity and late phase responses, there is evidence that they also regulate mast cell survival and phenotype. Several recent studies using cultured bone marrow-derived mast cells (BMMC) have established that IgE can function as a survival factor for mast cells in an FcεRI-dependent fashion (15–18). In addition to enhancing BMMC survival, IgE antibodies, even in the absence of antigen, have been shown to modulate their phenotype, increasing levels of transcripts for IL-6, IL-4, TNF-α and IL-13 as well as secretion of IL-6 protein. We have observed that the splenic mastocytosis induced by infestation with the parasite, Trichinella spiralis, is IgE dependent (19) and Kitaura and colleagues have shown that IgE can enhance mast cell responses in mice injected with an IgE-secreting hybridoma (20).
These observations led us to hypothesize that IgE antibodies produced in the course of an allergic response might serve not only to arm mast cells for allergen-triggered mediator release but also to sustain mast cell expansion, promote FcεRI expression and induce an activated phenotype. Such an IgE-driven amplification of the mast cell population and activation state might provide an increased tissue reservoir both of mediators of immediate hypersensitivity and of the cytokines and chemokines capable of initiating the recruitment of inflammatory cells in chronic allergic disease. In order to test this hypothesis and assess the role of IgE antibodies in airway mast cell responses during allergen exposure, we chose to use a model of airway inflammation in which mice are repeatedly exposed to an aqueous extract of Aspergillus fumigatus, Af, resulting in the production of levels of IgE higher than elicited in OVA models. Our analyses of the Af responses of IgE−/− mice previously established that intense airway inflammation and bronchial hyperresponsiveness (BHR) can arise in the absence of IgE (21). However neither the effect of Af inhalation on airway mast cell numbers nor the role of IgE antibodies in regulating mast cell homeostasis in this allergic disease model has been studied before.
We found that mice subjected to repeated inhalation of Af displayed a vigorous IgE response accompanied by a robust expansion of mast cells in the trachea and mainstem bronchi. These mast cells expressed higher levels of FcεRI and produced elevated levels of IL-5 compared to those from saline-treated control animals. In contrast, IgE−/− mice, which were incapable of generating an IgE response to Af, exhibited neither the increased numbers of mature mast cells nor the increased FcεRI density or IL-5 expression observed in WT animals. The expansion of mast cells in Af-treated WT mice was associated with enhanced levels of IL-5 and a more vigorous recruitment of eosinophils in the airways of WT than IgE−/− mice.
We considered it likely that IgE antibodies might exert their effect on airway mast cell numbers at the level of mature mast cells (which express the IgE receptor, FcεRI) rather than on receptor-negative progenitors. This hypothesis was supported by our observation that mast cell progenitors were recruited to the inflamed airways of both WT and IgE−/− mice following Af exposure but that survival of adoptively transferred mature mast cells was preferentially sustained in mice with high IgE levels. These findings identify important novel roles for IgE antibodies in the regulation of mast cell homeostasis and phenotype during allergic responses in vivo.
Materials and methods
Reagents and mice
A sterile aqueous extract of Af (1:10 w/v at 87,000 PNU/ml) was obtained from Greer Laboratories (Lenoir, NC). IgE−/− mice (22) were bred onto a BALB/c background (ten generations). Wild-type (WT) BALB/c mice were purchased from Taconic Farms (Germantown, NY). Mice were housed in a specific pathogen-free environment and were 6 to 12 weeks old. All experiments were carried out in accordance with the IACUC policies and procedures of The Children’s and The Brigham and Women’s Hospitals.
Sensitization of mice
Mice were lightly anesthetized by isoflurane inhalation and 50 µl of Aspergillus antigen or saline was applied to the nares. Mice were immunized three times a week for 3 weeks and were studied 12–24 h after the final dose.
Histological analysis
Airway mast cells were enumerated by microscopic examination of sections of paraffin-embedded airway tissue. Briefly, tracheae and main-stem bronchi were fixed in 10% buffered formalin and embedded in paraffin. Tissue sections were stained with toluidine blue and chloroacetate esterase. Mast cells were counted in 4–5 complete cross-sections of trachea, bronchus or spleen as specified in the figure labels. The numbers of mast cells were calibrated to unit length of epithelium (airway) or tissue area (spleen).
Collection of specimens
For BAL, 1 ml of staining medium (SM) consisting of Hank’s balanced salt solution (HBSS) supplemented with 10mM HEPES buffer at pH 7.2 and 2% newborn calf serum was infused into the trachea and then retrieved. To obtain lung cell suspensions for flow cytometry, whole lungs were minced and treated with 1.3 mM EDTA in HBSS at 37°C for 30 min with shaking, followed by digestion with 100 U/ml collagenase (Life Technologies, Rockville, MD) in RPMI containing Ca++ and Mg++ and 5% FCS, at 37°C for 1 h. Released cells were mashed through a cell strainer (BD Biosciences) and washed with PBS. Erythrocytes were lysed by hypotonic shock and cells were resuspended in SM. Total cells were enumerated and viability was assessed by trypan blue exclusion. The viable cells (propidium iodide-negative) in these preparations contained 80–85% CD45+ cells and the majority of these (75–85%) resided in the mononuclear cell gate based on forward and side scatter analysis.
BAL cytology
BAL fluid was cytocentrifuged onto slides and differential cellular analysis was assessed by light microscopy after staining with DiffQuik (Baxter).
Flow cytometry
Total cells from lung, spleen, BAL and peritoneal lavage were analyzed by flow cytometry. Mast cells were stained as follows: cells were incubated with Fc-block (anti-CD16/CD32) for 10 min on ice to prevent any non-specific binding via the Fc receptor, followed by incubation with 10 µg/ml of anti-DNP IgE for 45 min at 4°C. Cells were washed and then incubated with various combinations of anti-c-Kit, anti-IgE and mAbs specific for lineage markers, including CD3, CD4, CD8, CD45R, CD11b and Gr-1 for 30 min on ice. Mast cell progenitors were similarly identified by using a cocktail of antibodies to CD45, CD34 and β7-integrin while excluding lineage markers mentioned above. All mAbs were conjugated with either FITC, PE, APC, PE-Cy5 or APC-Cy7 and were purchased from eBioscience (CA) and BD Biosciences (CA). Cells were washed and resuspended in SM containing propidium iodide to differentiate between live and dead cells. Cells were analyzed using FACS Calibur and FACS Canto flow cytometers and data was processed using either CellQuest (BD Biosciences, San Jose, CA) or FlowJo (Treestar, Inc., OR) software.
Murine mast-cell protease 1 (mMCP-1), IL-5 and total IgE ELISA
mMCP-1 ELISA was performed on serum samples obtained after three weeks of treatment with Af or normal saline using a kit (Moredun Scientific Ltd., Scotland) according to the manufacturer’s instructions. IL-5 ELISA was performed on BAL supernatants using the BDBiosciences murine IL-5 ELISA set. Total IgE levels in the sera were quantified by sandwich ELISA as previously described (23).
Intracellular cytokine staining
Peritoneal lavage cells from WT or IgE−/− mice were incubated with Brefeldin A for 5 hrs at 37°C. Cells were then washed, incubated with anti-DNP IgE (20 µg/ml) at 4°C and stained for the surface markers c-Kit and IgE. They were then washed, permeabilized and fixed using the Cytofix/Cytoperm buffer from BD Biosciences, and incubated with an antibody to IL-5. Cells were washed again, resuspended in fixative and read using a FACS Calibur.
Culture of bone marrow mast cells (BMMC)
BMMC were generated as previously described (24). Briefly, marrow was obtained from the femurs and tibiae of naïve WT mice, and cultured with10 ng/ml IL-3 and 10 ng/ml SCF (Peprotech) for 4–6 weeks. Harvested BMMC were > 95% positive for c-Kit and FcεRI by flow cytometry and had characteristic appearance of mast cells on microscopic examination of toluidine blue-stained cytospun specimens.
Quantitative PCR analysis of IL-5
Total RNA was extracted from BMMC using TRIzol® Reagent (Invitrogen, Carlsbad, CA) following the manufacturer’s instructions. cDNA was generated with iScript cDNA synthesis kit (Bio-Rad Laboratory, Hercules, CA). Quantitative real-time PCR was done using Taqman® Gene Expression Assay probe, Taqman® PCR master mix and the ABI Prism 7300 sequence detection system, all from Applied Biosystems, Fostercity, CA. Relative expression of IL-5 was calculated in comparison to a reference gene transcript, β2-microglobulin, using the method described by Pfaffl (25).
Mast cell progenitor assay
The mast cell progenitor (MCp) assay was performed as previously described (24, 26). Briefly, a single cell suspension of lung mononuclear cells (MNCs) was obtained from saline and Af-treated WT and IgE−/− mice. The cells were serially diluted 2-fold and 100 µl of each dilution was plated in 96-well-flat-bottomed microtiter plates in 24 replicates. Cells were cultured with 100 µl of 105 naïve irradiated splenic feeder cells and recombinant cytokines, IL-3 (20 ng/ml) and SCF (100 ng/ml) at 37°C for 12–14 days. Wells containing mast cell colonies were enumerated using an inverted microscope. The MCp concentration was expressed as the number of MCps obtained per million MNCs. The total number of MCp/lung was derived by multiplying the concentration of MCp by the MNC yield per lung for individual mice or divided by the total number of lungs when MNCs were pooled from more than one mouse.
CFSE labeling and transfer of mast cells
BMMC were treated with 5µM CFSE/DMSO in PBS and incubated at 37°C for 5 minutes without shaking. CFSE-labeling was quenched by addition of ice-cold media. CFSE labeling was confirmed to be greater than 99% before adoptive transfer. Approximately 2.0 × 10 6 cells were injected i.p. into WT and IgE−/− mice, which had been subjected to the 3-week Af regimen followed by a week of rest. Similarly, a single mouse in each group received equivalent numbers of unlabeled cells to compare background signal in the FL-1 channel. Following transfer, 5 experimental mice from each group were sacrificed on days 1, 2, 3 and 6, and peritoneal cells were examined for the presence of CFSE+c-Kit+FcεRI+ cells by flow cytometry. Total numbers of positive cells were enumerated as a fraction of the total peritoneal cellular inflammation.
BrdU incorporation and transfer of mast cells
During the final week of BMMC generation, BMMC were cultured in the presence of 10 µM BrdU (BD Biosciences). BrdU+ WT BMMC were subsequently harvested and ~ 1 × 106 cells were injected i.p. into WT and IgE−/− mice, which had been subjected to the 3-week Af regimen followed by a week of rest. Six days following transfer of BrdU+ mast cells, mice were sacrificed and peritoneal cells were examined for the presence of BrdU+ cells, expressing c-Kit and FcεRI by flow cytometry using the BrdU Detection Kit (BD Biosciences). Background fluorescence was assessed by similarly injecting Af-treated mice with WT BMMC, which had been cultured in the absence of BrdU.
Determination of apoptosis in transferred mast cells
Six days after transfer, transferred mast cells were isolated from the peritoneum and examined for the induction of apoptosis using AnnexinV staining (BDBiosciences). Cells were stained with Annexin V and the total numbers of AnnexinV-positive mast cells was enumerated.
Statistical analysis
Differences in values for various experimental groups were examined for significance using the two-tailed Student's t test. All data are shown as the mean response ± SEM.
Results
IgE antibodies support airway mast cell expansion following Aspergillus fumigatus inhalation
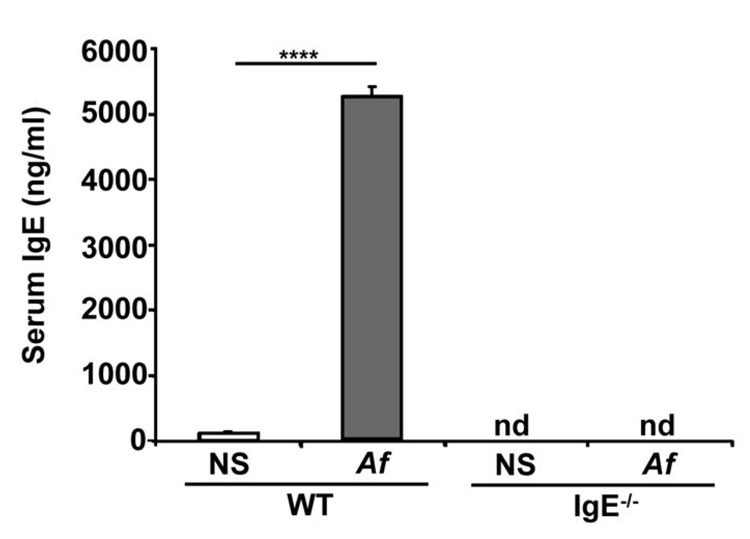
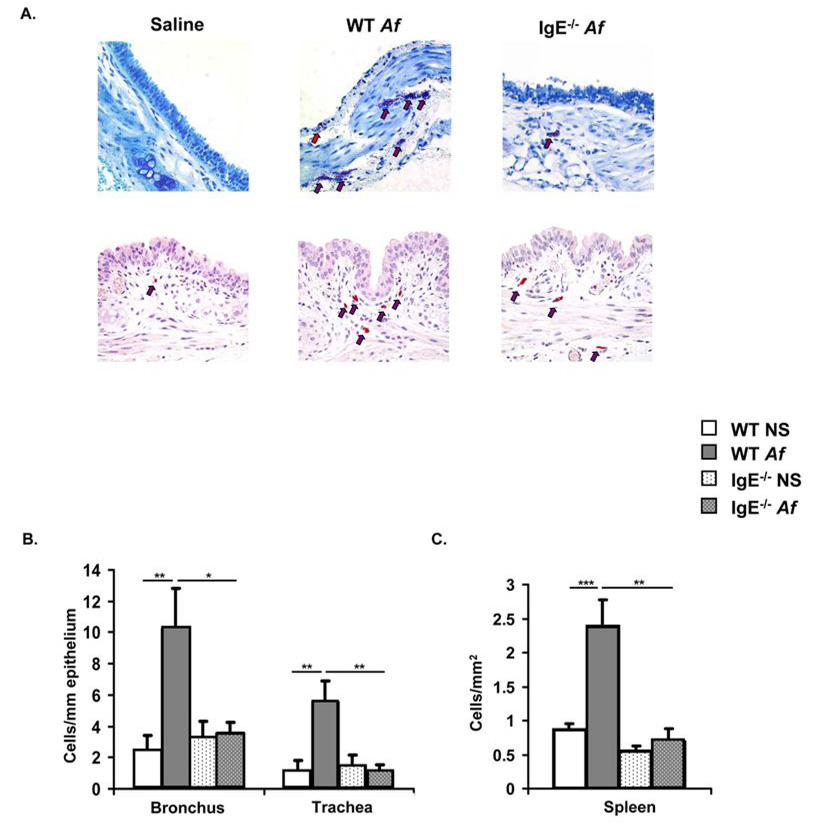
Mice were treated either with saline or Af intranasally over a period of 3 weeks resulting in a vigorous IgE response in wild-type (WT) but not IgE−/− animals (Fig. 1). A dramatic expansion of toluidine blue- and chloroacetate esterase-positive spindle-shaped mast cells containing heterochromatic granules was seen in the trachea and mainstem bronchi of WT mice after Af exposure (Fig. 2A). Enumeration of mast cells in bronchi revealed increases from <3 cells/mm to approximately 10 cells/mm, with a similar increase from 1 cell/mm to >5 cells/mm in tracheae (Fig. 2B). Numerous degranulating cells were evident. Intraepithelial mast cells were also seen in some sections and many of the mast cells were located around tracheal smooth muscle. In contrast, Af treatment did not significantly alter mast cell numbers in the airways of IgE−/− mice. Mast cell expansion was not restricted to the tissue site of initial allergen encounter. In the spleens of Af-treated mice, mast cell numbers were also about three-fold higher in WT mice than in IgE−/− animals (Fig. 2C).
Figure 1.
Serum IgE levels are elevated in Af-treated WT but not IgE−/− mice. Total serum IgE was determined by sandwich ELISA in wild-type (WT) or IgE−/− mice treated with Af or normal saline (NS) for 3 weeks. 8–10 mice were analyzed in each group. nd = none detected, **** = p<0.0001
Figure 2.
Expansion of mast cells is induced in the trachea, bronchus and spleen of Af-treated WT but not IgE−/− mice. WT and IgE−/− mice were treated intranasally with either Af or saline (NS) for 3 weeks and mast cells were enumerated. (A) Toluidine blue (upper panels) and chloroacetate esterase (lower panels) positive mast cells in representative cross-sections of trachea. Mast cells indicated by arrows (red arrow for an intraepithelial mast cell) (B & C) Toluidine blue positive mast cells in the main-stem bronchi, tracheae and spleens of Af-treated animals. n=5 mice per group; * = p<0.05; ** = p<0.01; *** = p<0.001
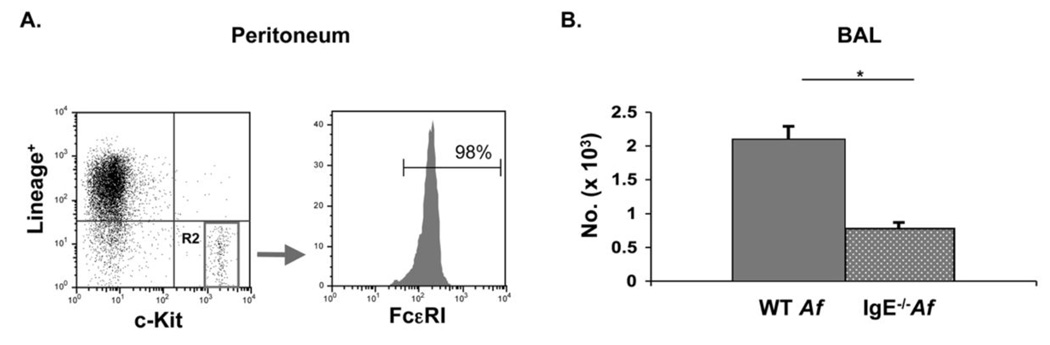
The requirement for IgE in mast cell expansion was further assessed by flow-cytometric analysis of bronchoalveolar lavage (BAL) fluid. Mast cells are typically identified on the basis of expression of c-Kit and FcεRI. However, as we and others have observed, the levels of FcεRI are so diminished on mast cells from IgE−/− mice that enumeration of mast cells cannot be reliably performed in IgE−/− mice using FcεRI expression (27, 28). In order to circumvent this technical challenge, we identified mast cells on the basis of expression of c-Kit and the absence of lineage markers for T (CD3, CD4, CD8) and B (B220) lymphocytes as well as granulocytes (Gr-1) as we have reported (29). We established that this staining specifically identified mast cells by showing that the Lineage−c-Kit+ cells obtained from WT mice indeed uniformly expressed FcεRI (Fig. 3A). CD34 was considered as an alternative mast cell marker. However, we and others have observed downregulation of CD34 on mucosal mast cells under certain circumstances, precluding reliance upon this antigen for their enumeration. Analysis of the BAL of Af –treated animals by this flow cytometric approach revealed that recruitment of mast cells to the airway of IgE−/− mice (770 ± 100 cells/ml) was significantly blunted compared with WT controls (2,090 ± 200 cells/ml) (Fig. 3B). Taken together with the results of our histologic enumeration of mast cells, these results provide strong evidence that Af-induced mast cell expansion in the airways is enhanced in the presence of IgE antibodies.
Figure 3.
Airway mast cells, enumerated by flow-cytometry, are increased in WT vs. IgE−/− Af-treated mice. Peritoneal lavage and BAL fluid from Af-treated mice were examined by flow cytometry. (A) Confirmation of the mast cell phenotype of Lineage (Lin)− c-Kit+ peritoneal cells. Cells were stained with lineage-specific antibodies (CD3, B220, CD4, CD8, Gr-1) and anti-c-Kit. Cells residing in the Lin−c-Kit+ gate, (R2), were analyzed for FcεRI expression. (B). Lin− c-Kit+ mast cells are increased in the BAL of Af-treated WT mice. Cells from control NS mice could not be analyzed because inadequate numbers were present in the BAL. n=5 mice per group; * = p<0.05
The expression of FcεRI on mast cells during Af-induced allergic disease is regulated by IgE
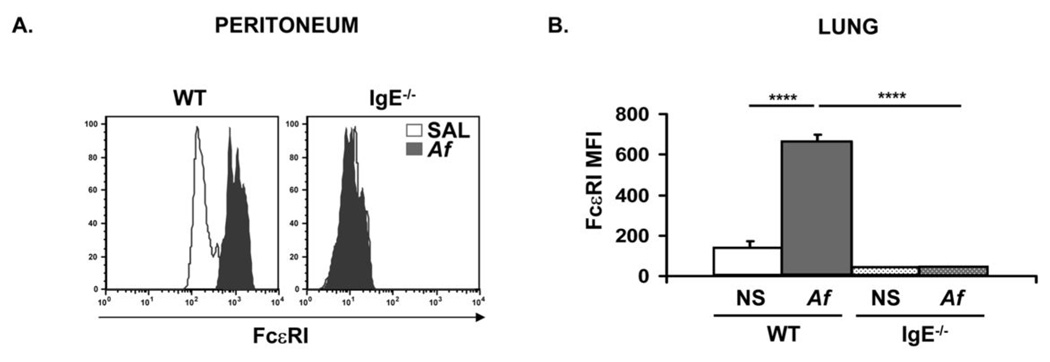
We have previously shown that IgE acts as a positive regulator of its high-affinity receptor, FcεRI, on mast cells (28). FcεRI-mediated signaling has been shown to be critical for the in vitro effects of IgE on the survival of cultured mast cells (15, 16). Therefore, we examined the expression of FcεRI on peritoneal and lung mast cells in Af-treated mice. FcεRI was significantly upregulated on both peritoneal (Fig. 4A) and lung (Fig. 4B) mast cells in WT mice following Af exposure (FcεRI MFI increased from 133 ± 40 to 663 ± 36 on lung cells). In contrast, FcεRI expression was unchanged following Af treatment in IgE−/− mice. As we have previously observed, FcεRI levels were lower in IgE−/− than WT mast cells even at physiologic baseline IgE levels. The intensity of staining was lower on both peritoneal and lung mast cells from IgE−/− saline control mice than in cells from their WT counterparts.
Figure 4.
FcεRI expression on mast cells from Af treated mice is increased in the presence of IgE. Peritoneal and lung cells were isolated from normal saline (NS) or Af-treated mice and mast cells were examined by flow cytometry. (A) FcεRI staining on peritoneal mast cells from Af-treated (solid histograms) WT and IgE−/− mice is shown overlaid on expression in NS control animals (unfilled histograms) of the same genotypes. The levels of FcεRI were assessed by staining with anti-DNP IgE followed by anti-IgE. (B). Median Fluorescence Intensity (MFI) of FcεRI expression on c-Kit+ cells from collagenase-digested lung tissue in normal saline (NS) or Af-treated WT and IgE−/− mice. n=5 mice per group; **** = p<0.0001
Murine mast cell protease-1 (mMCP-1) levels are increased during Af-induced allergic inflammation
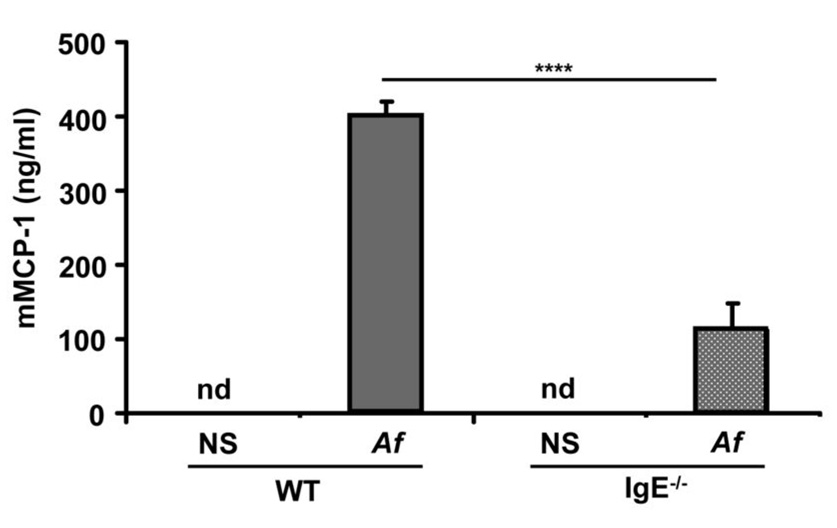
The release of mast cell proteases during allergic inflammation provides a serum marker of mast cell activation (30, 31). We assessed the levels of serum mMCP-1 in WT and IgE−/− mice after Af-treatment and found that serum mMCP-1 was undetectable in the serum of control mice (the detection limit of the mMCP-1 ELISA is 0.25 ng/ml) but rose significantly following Af treatment (399 ± 20 ng/ml). This increase was significantly blunted in mice lacking IgE which displayed only 28% (111 ± 36 ng/ml) of the WT mMCP-1 levels (Fig. 5). These data confirm our histologic and flow cytometric evidence that mast cell expansion in the setting of allergen exposure is supported by IgE antibodies.
Figure 5.
Af-induced increases in murine mast cell protease-1 levels in Af-treated mice are enhanced in the presence of IgE. Sera were isolated from normal saline (NS) or Af-treated WT and IgE−/− mice after three weeks of treatment and mMCP-1 levels were quantified by ELISA. n=5 mice per group. nd = none detected **** = p<0.0001.
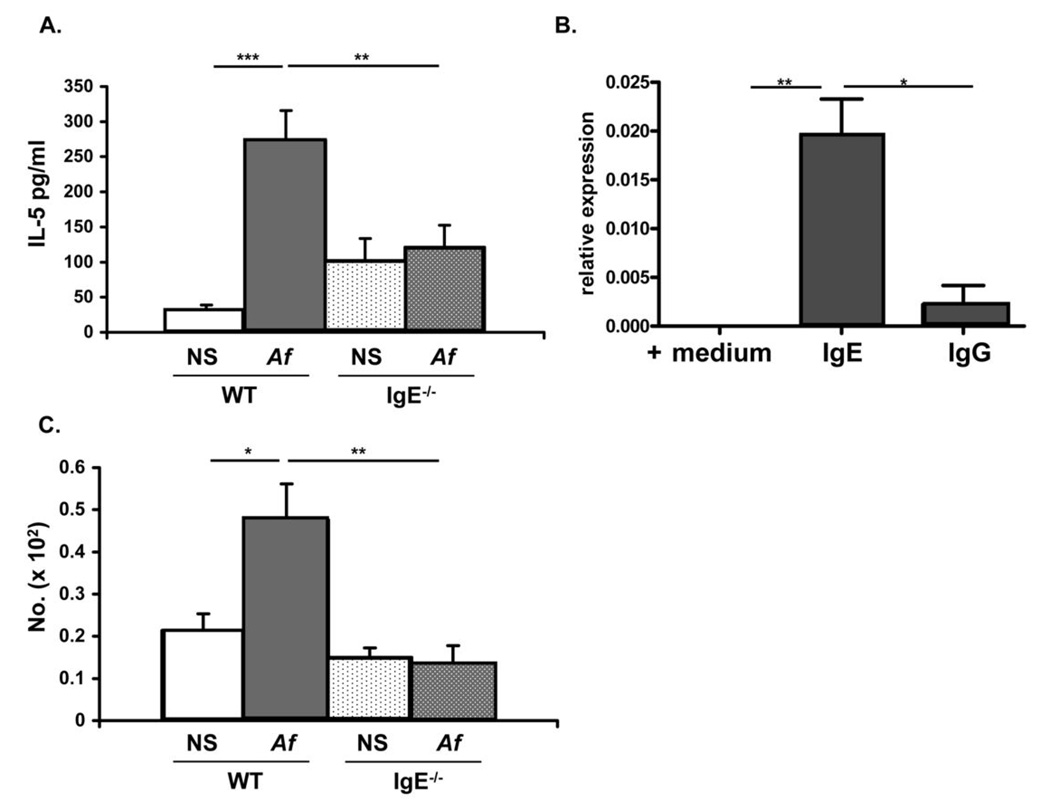
IL-5 production by mast cells is enhanced by IgE antibodies
Allergic inflammation is characterized by enhanced production of Th2 cytokines. One of these, IL-5 is important both for eosinophilopoiesis and eosinophil recruitment. We observed significantly increased levels of IL-5 in the airways of WT animals as compared to their IgE−/− counterparts (Fig. 6 A). In contrast, no difference was observed in the levels of IL-13 between the two groups of mice (data not shown). We considered airway mast cells as a potential source of IL-5 and examined the effects of IgE antibodies on their capacity to produce this cytokine. Mast cells cultured in the presence of IgE have previously been shown to produce IL-6, TNF-α, IL-4 and IL-13 (16). We observed that BMMC could similarly be induced to produce mRNA for IL-5 when cultured with IgE (Fig. 6B). In order to establish whether IgE antibody levels similarly modulate mast cell IL-5 production in vivo, we performed intracellular IL-5 staining on freshly isolated mast cells from WT and IgE−/− mice. As it was not possible to isolate sufficient numbers of pulmonary lineage−, c-Kit+ mast cells for this analysis, we examined more readily available peritoneal mast cells which, we reasoned, had been exposed to the same ambient IgE levels. Intracellular IL-5 staining of peritoneal mast cells revealed that significantly more mast cells from Af-treated WT mice stained IL-5-positive than from NS controls and this induction was observed only in WT but not IgE−/− mice (Fig. 6 C). These findings indicate that IgE antibody levels are important in regulating mast cell IL-5 production.
Figure 6.
BAL IL-5 levels and IL-5 expression by cultured mast cells and mast cells in vivo are regulated by IgE antibodies. (A) BAL fluid was obtained from normal saline (NS) or Af-treated WT and IgE−/− mice and the levels of IL-5 were determined by sandwich ELISA. (B) Bone-marrow derived mast cells were cultured in the presence of medium or 10 µg/ml IgE for 60 min. IL-5 mRNA was measured using real time quantitative PCR. IgG was used as a control. (C) Peritoneal cells were isolated from NS or Af-treated WT and IgE−/− mice and number of IL-5-positive cells assessed by intracellular staining.. * = p<0.05; ** = p<0.01; *** = p<0.001.
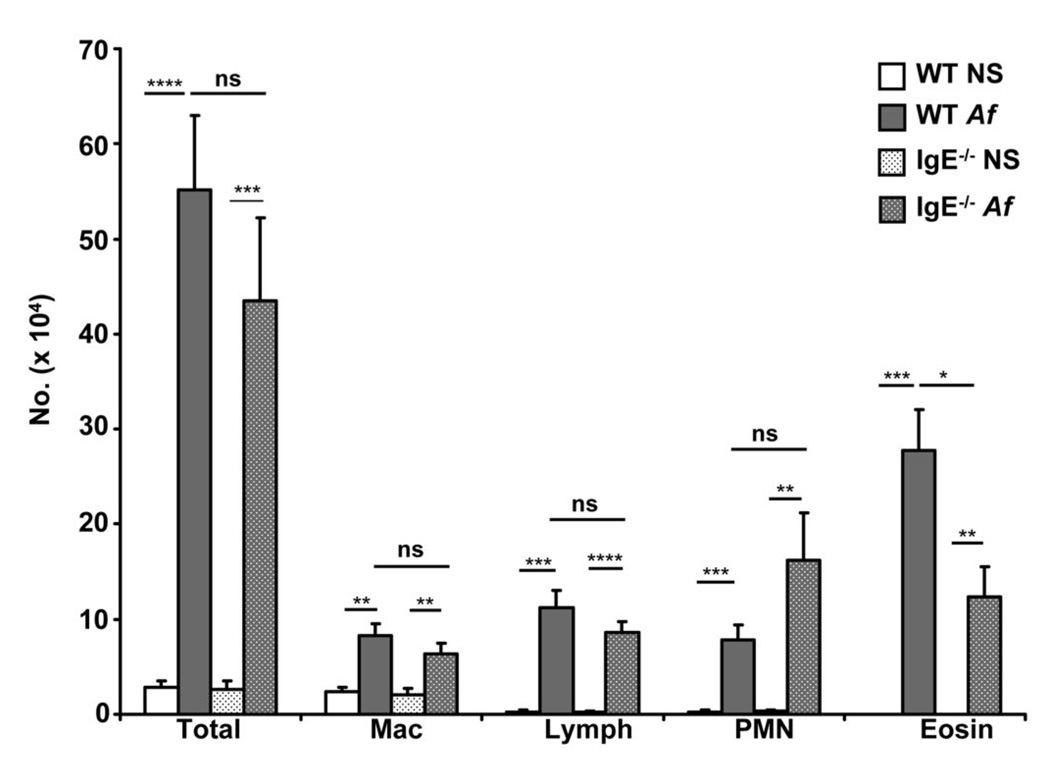
IgE-induced mast cell expansion and IL-5 production are accompanied by increased airway eosinophilia in WT mice
Activated mast cells produce a number of cytokines and chemokines capable of acting upon vascular endothelium and trafficking leukocytes to orchestrate an inflammatory cell infiltrate. Given the increase in mast cells in the airways of allergen treated animals as well as our observation of IgE effects on mast cell IL-5 production, we considered the possibility that cellular infiltration into the allergen exposed airway might be modulated by IgE. BAL fluid of Af-treated WT and IgE−/− mice was subjected to cytologic analysis to characterize infiltrating cell populations. A very dramatic increase in airway cells was induced by Af treatment in both WT and IgE−/− mice (Fig. 7) consistent with our previous observation that allergen-induced airway inflammation can arise independently of IgE antibodies (21). As expected, the cellular profile included macrophages, lymphocytes, neutrophils and eosinophils. Notably, WT and IgE−/− mice differed with respect to recruitment of eosinophils to the airway. Although a significant eosinophil influx occurred both in the presence and absence of IgE, the BAL of IgE−/− mice contained less than 50% the number of eosinophils as did WT animals (Fig. 7). There appeared to be no other statistically-significant differences in the cellular composition of WT vs. IgE−/− BAL. An apparent trend toward increased numbers of neutrophils was observed in the BAL of IgE−/− mice, although not reaching statistical significance, suggesting that the decrease in eosinophils was accompanied by a parallel increase in neutrophils in the absence of IgE.
Figure 7.
IgE antibodies regulate the pulmonary cellular infiltrate elicited by allergen inhalation. Differential cell counts were performed on Wright-Geimsa-stained cytocentrifuged BAL specimens from wild-type (WT) and IgE−/− mice treated with Af or normal saline (NS). Percentages of macrophages (Mac), lymphocytes (lymph), polymorphonuclear neutrophils (PMN) and eosinophils (eosin) were enumerated in individual samples and are depicted as a fraction of the total cell count. n=5 mice per group. ns = not significant * = p<0.05; ** = p<0.01; *** = p<0.001; ****=p<0.0001.
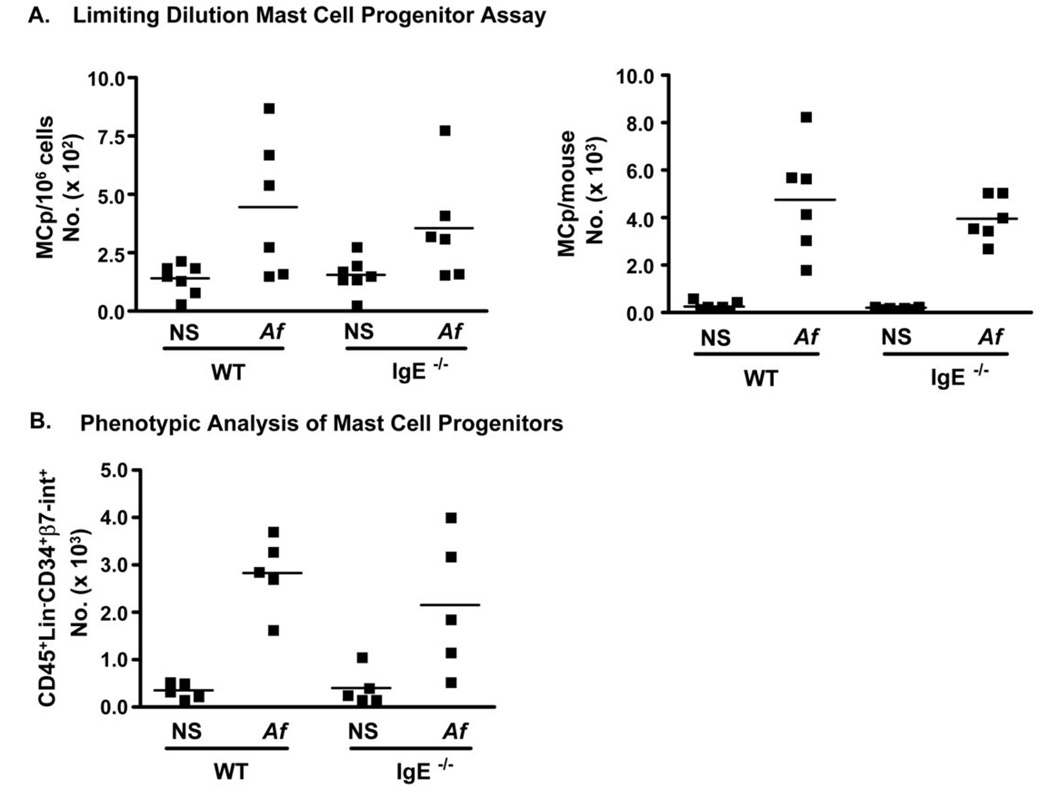
IgE is not required for the recruitment of mast cell progenitors to the lungs of Af-challenged mice
Tissue mast cell expansion during immune responses to parasites and allergens is thought to depend on the recruitment of mast cell precursors (MCp) to sites of inflammation (24, 32–34). To examine whether the expansion of mast cells in our model was a consequence of increased recruitment of MCps to the airways following allergen exposure, we performed limiting dilution analysis for lung MCp (Fig. 8 A). Using this approach, we found that the lungs of saline-treated mice had fewer than 250 MCp per million cells and less than 1000 total MCp per mouse. However, Af treatment induced robust recruitment of MCp to the lungs of both WT (4,772 total ) and IgE−/− (3,976 total) mice, indicating that the recruitment of mast cell precursors during allergic inflammation occurs by IgE-independent mechanisms.
Figure 8.
Mast cell progenitor recruitment to the airways of Af –treated mice occurs independently of ambient IgE levels. Lung mononuclear cells were isolated from naïve or Af-treated WT and IgE−/− mice and mast cell progenitors (MCp) enumerated by limiting dilution culture of IL-3/SCF cultures of lung mononuclear cells as described in Methods. (A) MCp/million lung mononuclear cells and total numbers of lung MCp are shown. (B) CD45+Lin (CD3, CD4, CD8, B220, CD11b, Gr-1)−CD34+β-7integrin+ lung MCps as assessed by flow cytometry.
To confirm this observation, we used a flow cytometric approach which we have previously shown to enumerate tissue MCps (24). Tissue MCps are identified as CD45+, Lineage (CD3, CD4, CD8, B220, CD11b, Gr-1)−, CD34+ and β7-integrin+. Consistent with the limiting dilution assay, examination of lung mononuclear cells for the presence of cells with the antigenic phenotype of MCp showed that allergen exposure led to a strong recruitment of mast cell progenitors and that comparable numbers of MCps were present in the lungs of Af-treated WT and IgE−/− mice (Fig. 8B). The functional and flow cytometric methods for MCp enumeration were quite consistent with each other, indicating similar numbers of cells. These findings therefore suggest that IgE antibodies are not required for the recruitment of mast cell precursors to the airways leading us to speculate that the IgE effect likely occurs at the level of the mature, FcεRI-expressing mast cell.
IgE is required for the survival of differentiated mast cells in vivo
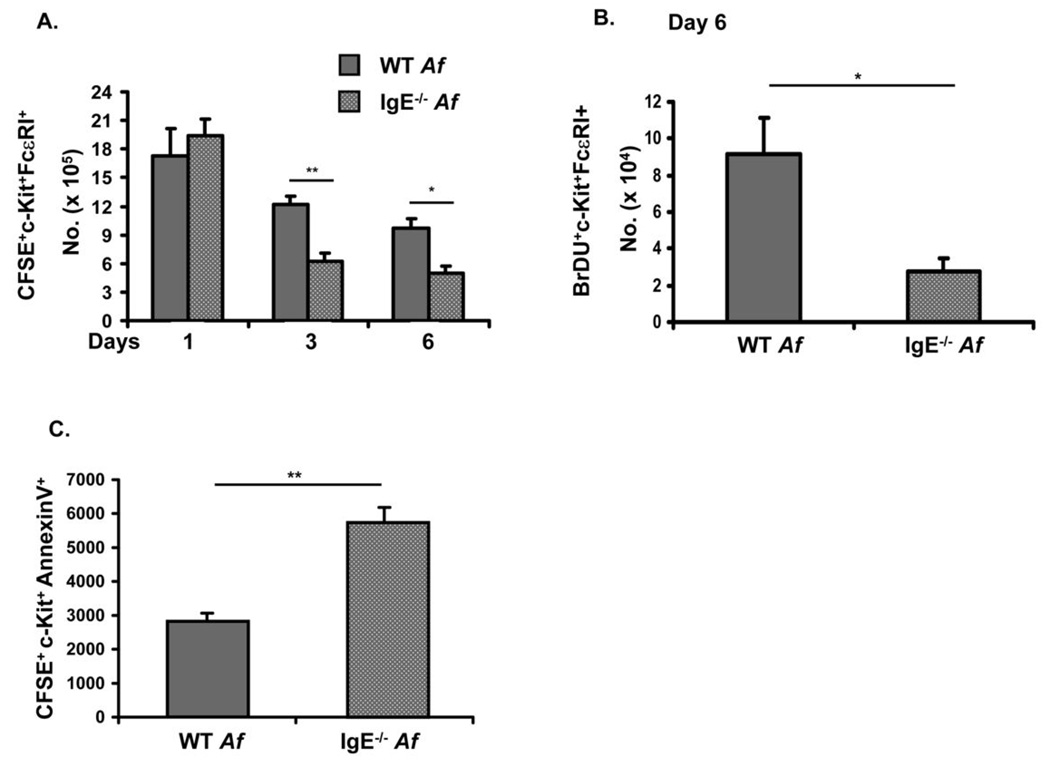
As IgE antibodies appeared not to be necessary for the recruitment of mast cell progenitors during Af-induced allergic inflammation we tested the hypothesis that they might instead enhance the survival of differentiated mast cells in vivo just as they have previously been shown to support survival of cultured cells (15, 16). BMMC were labeled with CFSE and adoptively transferred into the peritoneum of WT and IgE−/− mice that had been treated with Af in order to elicit IgE responses. Enumeration of transferred CFSE+c-Kit+IgE (FcεRI)+ mast cells in the peritoneal fluid of recipient mice showed a gradual decline over a period of 6 days in both WT and IgE−/−mice (Fig. 9A). While initial mast cell recovery at day one was similar in WT and IgE−/− animals, consistent with comparable levels of reconstitution, mast cell numbers were significantly lower in IgE−/− mice on both days 3 and 6 after adoptive transfer, suggesting that IgE antibodies enhanced survival of mast cells in vivo (Fig. 9A). No dilution of the CFSE signal was evident during the six day period of analysis (data not shown), indicating that IgE sustains mast cell survival rather than inducing mast cell proliferation.
Figure 9.
IgE supports the survival of differentiated mast cells in vivo and provides an anti-apoptotic signal. (A). WT and IgE−/− mice were treated with Af. CFSE-labeled BMMC were injected (2 × 106 cells) into the peritoneal cavities of individual mice. Peritoneal cells were recovered 1, 3 and 6 days later and CFSE+c-Kit+FcεRI+ mast cells enumerated. Mice that received unlabeled BMMC served as controls. n=5 mice per group. *=p<0.05. (B). BrdU labeled BMMC (1 × 106 cells) were injected into the peritoneum of Af-treated, WT and IgE−/− mice. 6 days after transfer, c-Kit+BrdU+ and FcεRI+ cells were enumerated by flow cytometry. n=4 mice per group. (C). CFSE-labeled BMMC were injected into the peritoneal cavities of Af-treated WT and IgE− − mice. Six days later, transferred BMMC were recovered and apoptosis assessed by determining surface levels of AnnexinV by flow cytometry. Total numbers of CFSE+c-Kit+AnnexinV+ cells in the peritoneum are shown. *p = <0.05; **p = <0.01.
In order to confirm the effects of IgE on cell survival suggested by the CFSE experiments, we similarly examined the recovery of BrdU-labeled mast cells after adoptive transfer into the peritoneum of Af-treated WT and IgE−/− mice. Six days after transfer, about 3-fold more BrdU+ mast cells were recovered from the peritoneum of Af-treated WT mice than from their IgE−/− counterparts (Fig. 9B). The recovered mast cells expressed both c-Kit and FcεRI as expected.
The relative paucity of transferred mast cells recovered from the peritoneal cavities of IgE−/− vs. WT mice suggested the possibility of IgE delivering anti-apoptotic signals as has been observed in cultured mast cells (15, 16). The possibility of a similar anti-apoptotic effect of IgE in vivo was examined by assessing surface levels of Annexin V on transferred mast cells, the same method which had been used by Asai and colleagues to assess apoptosis levels in cultured mast cells exposed to IgE (15). Significantly higher levels of Annexin V were detected in CFSE+ transferred mast cells recovered from Af-treated IgE−/− mice as compared to WT animals (Fig. 9C), mirroring the survival data shown in Fig. 9A&B. Taken together, these data indicate that IgE antibodies regulate the survival of mature mast cells in the setting of in vivo allergic responses.
Discussion
Mast cells armed with specific IgE antibodies and residing in the mucosa serve as airway sentinels, sensing and responding to inhaled antigens. In patients with asthma, the IgE-mediated activation of these cells following aeroallergen exposure induces release of vasoactive and smooth muscle-constricting mediators which trigger acute airflow obstruction, as well as the production of bioactive lipids, cytokines and chemokines which act in concert to set the stage for eventual cellular influx and allergic airway inflammation. Our results provide strong evidence that IgE antibodies not only serve as allergen sensors for airway mast cells but also function as critical regulators of their survival and phenotype. Thus the cell-sustaining properties of IgE antibodies for cultured mast cells recently reported by a number of groups are important during IgE-inducing allergic responses in vivo as well.
IgE effects on mast cell homeostasis are not restricted to the allergic state; we have reported that the splenic mastocytosis which occurs during infestation with the parasite, Trichinella spiralis, is IgE dependent (19) and Kitaura and colleagues have shown that IgE can regulate mast cell homeostasis in mice injected with an IgE-secreting hybridoma (20).
The presence of mast cells in bronchial mucosa and smooth muscle is associated with asthma in humans; patients with other forms of bronchial inflammation do not harbor mast cells at these sites (reviewed in (36) and (37)). Mast cell density in the smooth muscle is correlated with responsiveness to methacholine (14). Mast cells also infiltrate mucous glands where they regulate secretion and can additionally be found in the bronchoalveolar space and around airway blood vessels. Chronic asthma is characterized by progressive irreversible airflow obstruction believed to be a consequence of airway “remodeling,” tissue changes in the airway which include fibrosis and angiogenesis. As mast cells are closely associated with airway blood vessels and produce angiogenic factors including histamine, proteases, TGF-β and VEGF, they may provide critical signals for these long term changes. In the present study, we did not observe differences in subepithelial collagen deposition or total lung collagen (data not shown) between IgE−/− vs. WT animals but it is likely that the three week Af protocol does not really recapitulate the years of chronic allergen exposure experienced by patients.
Several groups have employed murine models to assess the role of mast cells and IgE antibodies in allergen-induced airway inflammation. An emerging theme from studies is that, under conditions of strong allergenic stimulation, chronic airway inflammation and BHR can arise completely independently of IgE and mast cells but IgE antibodies are important for acute responses to allergen inhalation. When less intense sensitization protocols are employed, a contributory role for IgE and mast cells can be discerned even in the induction of chronic inflammation. It appears that, as has been observed in other systems, IgE and mast cells may serve as amplifiers of the inflammatory response (17, 38, 39). Our own studies with IgE−/− mice along with the work of others has shown that airway inflammation and BHR can be elicited in allergen challenged mice even in the absence of IgE or other antibodies (21, 40). Similar findings have been reported regarding mast cells. Using a standard protocol in which mice are sensitized to OVA i.p. and then challenged by inhalation, Takeda and colleagues found that airway inflammation and BHR were the same in WT and W/Wv mice (41). Kobayashi and colleagues also observed no defect in eosinophil recruitment and airway inflammation in W/Wv mice but did see a mast cell effect on BHR (42). The groups of Galli and Broide have established conditions under which protocols for OVA sensitization of the airways could be modified, using repetitive exposure, to induce mast cell expansion thus allowing them to define significant contributions of these cells to the allergen-induced eosinophil-predominant inflammation and BHR (43, 44). Studies by Mayr and colleagues using such a chronic OVA protocol, suggested that the mast cell contribution to airway inflammation is IgE-dependent and subsequent experiments using adoptive reconstitution of W/Wv mice with BMMC from WT or FcεRI−/− donors performed by Taube et al. revealed a role for IgE in the airway mast cell response to allergen (45, 46). Additional work by Yu and colleagues, who reconstituted W/Wv mice with BMMC from FcRγ+/+ and FcRγ−/− donors provided evidence for both IgE/IgG-dependent and IgE/IgG-independent mast cell contributions (44). Taken together, these reports suggest that mast cells and IgE antibodies can serve as amplifiers of inflammation in the airway under physiologic allergen-limiting conditions..
The alum-free OVA models employed in the studies reviewed above typically elicit IgE levels around 400 to 500 ng/ml (47). We have previously observed that inhalation alone (without i.p. priming) of aqueous extracts of Af can drive IgE responses that are as much as ten to one hundred times as high (21, 48). As the central goal of our study was to examine the effects of IgE antibody levels on mast cell numbers we chose to use this very robust IgE-inducing protocol. The Af model offered the additional advantage that it might more directly reflect the type of allergen exposure encountered by patients, namely a mixture of protein antigens and non-protein fungal lipids and glycolipids with potential immunostimulatory adjuvant activities for allergic responses. Indeed, we found that repeated Af inhalation, as had been reported by others for repeated OVA inhalation, led to increased airway mast cell numbers in the airways and spleen. These increases were demonstrated using both histology and flow cytometry and corroborated by serum mMCP-1 levels. We have previously published that IgE antibodies are not required for the induction of airway inflammation and BHR in the Af model (21). In contrast, however, our current findings reveal a significant discrepancy between WT and IgE−/− mice in terms of mast cell expansion, providing clear evidence that IgE antibodies specifically regulate the homeostasis of this cell type. Furthermore, our present observations suggest that IgE also regulates eosinophilia in the Af model. In our earlier work, using mice on a 129SvEv background (BALB/c is the strain of the present study) and a different source of Af, we observed that IgE affected neither the recruitment of eosinophils to the BAL nor the induction of BHR in Af-treated mice (21). In that study, the inflammatory response was markedly more intense with IgE levels and total BAL eosinophil counts each about an order of magnitude higher than in the current investigation. We speculate that a combination of factors related to strain differences and Af extract potency may have induced such a strong inflammatory response in the former study as to override the amplifying eosinophil enhancing effect of IgE and mast cells we now observe.
Analyses of mast cell progenitor frequency and mature mast cell survival in the Af-treated mice indicated that there was an intense recruitment of progenitors to the site of allergic airway inflammation regardless of the presence of IgE. As these cells are at most weakly FcεRI-positive, the absence of a requirement for IgE in their recruitment was anticipated (29). In contrast, our studies using cultured mature BMMC labeled with CFSE+ and BrdU+ revealed enhanced survival of differentiated mast cells in the peritoneum of Af-treated WT compared with IgE−/− mice, supporting the hypothesis that IgE antibodies directly regulate the homeostasis of mature mast cells during allergic responses in vivo.
Studies of IgE-mediated growth enhancement of cultured mast cells have not clearly elucidated a mechanism whereby IgE supports survival. In order to determine whether mast cell regulation by IgE might depend on the paracrine effects of other mast-cell growth factors, we assayed the BAL of Af-treated WT and IgE−/− mice for the presence of known mast-cell stimulators including IL-3, IL-4 and IL-9. We observed similar levels of IL-3 in both WT and IgE−/− mice following Af inhalation and levels of IL-9 and IL-4 that were below the limits of detection (not shown). These observations suggested to us that IgE supports mast cell survival in vivo independently of these cytokines. However, a recent report by Kohno and colleagues suggested that IgE regulates the survival of cultured mast cells via production of IL-3 (49). Preliminary studies in our laboratory indicate that mast cell numbers fail to increase in the lungs and BAL of IL-3−/− mice in spite of elevated levels of IgE suggesting that IL-3 may similarly be required for IgE-driven mast cell expansion in vivo (data not shown). Such a requirement for IL-3 has also been seen in the elicitation of mast cell responses during infestation with the nematode parasite, S. Venezuelensis (50).
In order to determine whether, beyond enhancing mast cell numbers, IgE additionally regulates mast cell phenotype in vivo, we examined mast cells for the production of intracellular cytokines and for expression of FcεRI. Our finding that peritoneal mast cells from Af-treated WT mice made more IL-5 than mast cells in the IgE-free environment of IgE−/− mice indicates that IgE can regulate the amplitude of mast cell mediator production. Similarly, the very low levels of FcεRI on mast cells from Af-treated IgE−/− mice point to an important role for IgE antibodies in regulating IgE receptor levels in vivo. There is good evidence that IgE regulation of FcεRI is operative in humans with allergic disease as well. In atopic cohorts, total serum IgE levels are correlated with basophil FcεRI density and treatment with omalizumab has been found to cause diminished FcεRI expression on mast cells, basophils and dendritic cells (51–54). It is tempting to speculate that induction of FcεRI by elevated IgE levels in atopic individuals might provide a positive feedback loop for supporting mast cell survival.
Several groups have reported diminished eosinophilic and mononuclear cell infiltration into the airways of mast cell-deficient mice repeatedly subjected to OVA inhalation (44, 45, 47). We have found that IgE-dependent allergen-induced mast cell expansion is also accompanied by decreased numbers of eosinophils in the airway with an accompanying trend (statistically insignificant in our hands) towards increase in the numbers of neutrophils. Thus the expansion and chronic activation of mast cells in the airways of asthmatic patients along with their capacity to produce IL-5 and eosinophil specific chemokines might serve to amplify the recruitment of eosinophils to the airways.
Production of IgE antibodies is the hallmark of the atopic state. The association of IgE levels with asthma severity and the well-established function of IgE in driving immediate hypersensitivity reactions following allergen encounter have focused attention on this antibody isotype as a therapeutic target. Indeed IgE inhibitors have been developed based on these aspects of IgE biology and are, as expected, very effective in blocking acute allergen-induced airflow obstruction in asthmatics (10). Our findings provide novel insights into the role of IgE antibodies in mast cell homeostasis and asthma pathogenesis and suggest additional potential consequences of IgE blockade. Taken together with human and mouse data on the importance of mast cells in driving the asthmatic response, the results presented here suggest that modulation of IgE responses might provide benefits extending far beyond the inhibition of immediate hypersensitivity.
Acknowledgments
The authors would like to thank Dr. Michiko Oyoshi for help with experiments.
Abbreviations used in this paper
- Af
Aspergillus fumigatus
- WT
Wild-type
- IgE−/−
IgE-deficient
- BHR
Bronchial Hyperresponsiveness
- BMMC
Bone-marrow derived Mast Cells
- MCp
Mast cell progenitor
- Lin
Lineage
Footnotes
This work was supported by National Institutes of Health grants NIAID R01-AI054471 (H.C.O.), NIAID P01 AI 031599 (M.F.G.) and a research grant from Novartis Pharmaceuticals Corporation.
References
- 1.Holgate ST. Epithelium dysfunction in asthma. J. Allergy Clin. Immunol. 2007;120:1233–1244. doi: 10.1016/j.jaci.2007.10.025. quiz 1245-1236. [DOI] [PubMed] [Google Scholar]
- 2.Larche M, Robinson DS, Kay AB. The role of T lymphocytes in the pathogenesis of asthma. J. Allergy Clin. Immunol. 2003;111:450–463. doi: 10.1067/mai.2003.169. quiz 464. [DOI] [PubMed] [Google Scholar]
- 3.Meiler F, Zimmermann M, Blaser K, Akdis CA, Akdis M. T-cell subsets in the pathogenesis of human asthma. Curr. Allergy Asthma Rep. 2006;6:91–96. doi: 10.1007/s11882-006-0045-0. [DOI] [PubMed] [Google Scholar]
- 4.Umetsu DT, Dekruyff RH. Immune dysregulation in asthma. Curr. Opin. Immunol. 2006;18:727–732. doi: 10.1016/j.coi.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 5.Sears MR, Burrows B, Flannery EM, Herbison GP, Hewitt CJ, Holdaway MD. Relation between airway responsiveness and serum IgE in children with asthma and in apparently normal children. N. Engl. J. Med. 1991;325:1067–1071. doi: 10.1056/NEJM199110103251504. [DOI] [PubMed] [Google Scholar]
- 6.van Herwerden L, Harrap SB, Wong ZY, Abramson MJ, Kutin JJ, Forbes AB, Raven J, Lanigan A, Walters EH. Linkage of high-affinity IgE receptor gene with bronchial hyperreactivity, even in absence of atopy. Lancet. 1995;346:1262–1265. doi: 10.1016/s0140-6736(95)91863-9. [DOI] [PubMed] [Google Scholar]
- 7.Postma DS, Bleecker ER, Amelung PJ, Holroyd KJ, Xu J, Panhuysen CI, Meyers DA, Levitt RC. Genetic susceptibility to asthma--bronchial hyperresponsiveness coinherited with a major gene for atopy. N. Engl. J. Med. 1995;333:894–900. doi: 10.1056/NEJM199510053331402. [DOI] [PubMed] [Google Scholar]
- 8.Barnes KC, Neely JD, Duffy DL, Freidhoff LR, Breazeale DR, Schou C, Naidu RP, Levett PN, Renault B, Kucherlapati R, Iozzino S, Ehrlich E, Beaty TH, Marsh DG. Linkage of asthma and total serum IgE concentration to markers on chromosome 12q: evidence from Afro-Caribbean and Caucasian populations. Genomics. 1996;37:41–50. doi: 10.1006/geno.1996.0518. [DOI] [PubMed] [Google Scholar]
- 9.Nickel R, Wahn U, Hizawa N, Maestri N, Duffy DL, Barnes KC, Beyer K, Forster J, Bergmann R, Zepp F, Wahn V, Marsh DG. Evidence for linkage of chromosome 12q15–q24.1 markers to high total serum IgE concentrations in children of the German Multicenter Allergy Study. Genomics. 1997;46:159–162. doi: 10.1006/geno.1997.5013. [DOI] [PubMed] [Google Scholar]
- 10.Fahy JV, Cockcroft DW, Boulet LP, Wong HH, Deschesnes F, Davis EE, Ruppel J, Su JQ, Adelman DC. Effect of aerosolized anti-IgE (E25) on airway responses to inhaled allergen in asthmatic subjects. Am. J. Respir. Crit. Care Med. 1999;160:1023–1027. doi: 10.1164/ajrccm.160.3.9810012. [DOI] [PubMed] [Google Scholar]
- 11.Koshino T, Arai Y, Miyamoto Y, Sano Y, Itami M, Teshima S, Hirai K, Takaishi T, Ito K, Morita Y. Airway basophil and mast cell density in patients with bronchial asthma: relationship to bronchial hyperresponsiveness. J. Asthma. 1996;33:89–95. doi: 10.3109/02770909609054536. [DOI] [PubMed] [Google Scholar]
- 12.Koshino T, Arai Y, Miyamoto Y, Sano Y, Takaishi T, Hirai K, Ito K, Morita Y. Mast cell and basophil number in the airway correlate with the bronchial responsiveness of asthmatics. Int. Arch. Allergy Immunol. 1995;107:378–379. doi: 10.1159/000237042. [DOI] [PubMed] [Google Scholar]
- 13.Gibson PG, Saltos N, Borgas T. Airway mast cells and eosinophils correlate with clinical severity and airway hyperresponsiveness in corticosteroid-treated asthma. J. Allergy Clin. Immunol. 2000;105:752–759. doi: 10.1067/mai.2000.105319. [DOI] [PubMed] [Google Scholar]
- 14.Brightling CE, Bradding P, Symon FA, Holgate ST, Wardlaw AJ, Pavord ID. Mast-cell infiltration of airway smooth muscle in asthma. N. Engl. J. Med. 2002;346:1699–1705. doi: 10.1056/NEJMoa012705. [DOI] [PubMed] [Google Scholar]
- 15.Asai K, Kitaura J, Kawakami Y, Yamagata N, Tsai M, Carbone DP, Liu FT, Galli SJ, Kawakami T. Regulation of mast cell survival by IgE. Immunity. 2001;14:791–800. doi: 10.1016/s1074-7613(01)00157-1. [DOI] [PubMed] [Google Scholar]
- 16.Kalesnikoff J, Huber M, Lam V, Damen JE, Zhang J, Siraganian RP, Krystal G. Monomeric IgE stimulates signaling pathways in mast cells that lead to cytokine production and cell survival. Immunity. 2001;14:801–811. doi: 10.1016/s1074-7613(01)00159-5. [DOI] [PubMed] [Google Scholar]
- 17.Kawakami T, Galli SJ. Regulation of mast-cell and basophil function and survival by IgE. Nat. Rev. Immunol. 2002;2:773–786. doi: 10.1038/nri914. [DOI] [PubMed] [Google Scholar]
- 18.Kawakami T, Kitaura J. Mast cell survival and activation by IgE in the absence of antigen: a consideration of the biologic mechanisms and relevance. J. Immunol. 2005;175:4167–4173. doi: 10.4049/jimmunol.175.7.4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gurish MF, Bryce PJ, Tao H, Kisselgof AB, Thornton EM, Miller HR, Friend DS, Oettgen HC. IgE enhances parasite clearance and regulates mast cell responses in mice infected with Trichinella spiralis. J. Immunol. 2004;172:1139–1145. doi: 10.4049/jimmunol.172.2.1139. [DOI] [PubMed] [Google Scholar]
- 20.Kitaura J, Song J, Tsai M, Asai K, Maeda-Yamamoto M, Mocsai A, Kawakami Y, Liu FT, Lowell CA, Barisas BG, Galli SJ, Kawakami T. Evidence that IgE molecules mediate a spectrum of effects on mast cell survival and activation via aggregation of the FcepsilonRI. Proc. Natl. Acad. Sci. U S A. 2003;100:12911–12916. doi: 10.1073/pnas.1735525100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mehlhop PD, van de Rijn M, Goldberg AB, Brewer JP, Kurup VP, Martin TR, Oettgen HC. Allergen-induced bronchial hyperreactivity and eosinophilic inflammation occur in the absence of IgE in a mouse model of asthma. Proc. Natl. Acad. Sci. U S A. 1997;94:1344–1349. doi: 10.1073/pnas.94.4.1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oettgen HC, Martin TR, Wynshaw-Boris A, Deng C, Drazen JM, Leder P. Active anaphylaxis in IgE-deficient mice. Nature. 1994;370:367–370. doi: 10.1038/370367a0. [DOI] [PubMed] [Google Scholar]
- 23.Bryce PJ, Miller ML, Miyajima I, Tsai M, Galli SJ, Oettgen HC. Immune sensitization in the skin is enhanced by antigen-independent effects of IgE. Immunity. 2004;20:381–392. doi: 10.1016/s1074-7613(04)00080-9. [DOI] [PubMed] [Google Scholar]
- 24.Abonia JP, Hallgren J, Jones T, Shi T, Xu Y, Koni P, Flavell RA, Boyce JA, Austen KF, Gurish MF. Alpha-4 integrins and VCAM-1, but not MAdCAM-1, are essential for recruitment of mast cell progenitors to the inflamed lung. Blood. 2006;108:1588–1594. doi: 10.1182/blood-2005-12-012781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gurish MF, Tao H, Abonia JP, Arya A, Friend DS, Parker CM, Austen KF. Intestinal mast cell progenitors require CD49dbeta7 (alpha4beta7 integrin) for tissue-specific homing. J. Exp. Med. 2001;194:1243–1252. doi: 10.1084/jem.194.9.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lantz CS, Yamaguchi M, Oettgen HC, Katona IM, Miyajima I, Kinet JP, Galli SJ. IgE regulates mouse basophil Fc epsilon RI expression in vivo. J. Immunol. 1997;158:2517–2521. [PubMed] [Google Scholar]
- 28.Yamaguchi M, Lantz CS, Oettgen HC, Katona IM, Fleming T, Miyajima I, Kinet JP, Galli SJ. IgE enhances mouse mast cell Fc(epsilon)RI expression in vitro and in vivo: evidence for a novel amplification mechanism in IgE-dependent reactions. J. Exp. Med. 1997;185:663–672. doi: 10.1084/jem.185.4.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arinobu Y, Iwasaki H, Gurish MF, Mizuno S, Shigematsu H, Ozawa H, Tenen DG, Austen KF, Akashi K. Developmental checkpoints of the basophil/mast cell lineages in adult murine hematopoiesis. Proc. Natl. Acad. Sci. U S A. 2005;102:18105–18110. doi: 10.1073/pnas.0509148102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang C, Sali A, Stevens RL. Regulation and function of mast cell proteases in inflammation. J. Clin. Immunol. 1998;18:169–183. doi: 10.1023/a:1020574820797. [DOI] [PubMed] [Google Scholar]
- 31.Miller HR, Pemberton AD. Tissue-specific expression of mast cell granule serine proteinases and their role in inflammation in the lung and gut. Immunology. 2002;105:375–390. doi: 10.1046/j.1365-2567.2002.01375.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dillon SB, MacDonald TT. Limit dilution analysis of mast cell precursor frequency in the gut epithelium of normal and Trichinella spiralis infected mice. Parasite Immunol. 1986;8:503–511. doi: 10.1111/j.1365-3024.1986.tb00865.x. [DOI] [PubMed] [Google Scholar]
- 33.Artis D, Humphreys NE, Potten CS, Wagner N, Muller W, McDermott JR, Grencis RK, Else KJ. Beta7 integrin-deficient mice: delayed leukocyte recruitment and attenuated protective immunity in the small intestine during enteric helminth infection. Eur. J. Immunol. 2000;30:1656–1664. doi: 10.1002/1521-4141(200006)30:6<1656::AID-IMMU1656>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 34.Pennock JL, Grencis RK. In vivo exit of c-kit+/CD49d(hi)/beta7+ mucosal mast cell precursors from the bone marrow following infection with the intestinal nematode Trichinella spiralis. Blood. 2004;103:2655–2660. doi: 10.1182/blood-2003-09-3146. [DOI] [PubMed] [Google Scholar]
- 35.Vermes I, Haanen C, Steffens-Nakken H, Reutelingsperger C. A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled Annexin V. J. Immunol. Methods. 1995;184:39–51. doi: 10.1016/0022-1759(95)00072-i. [DOI] [PubMed] [Google Scholar]
- 36.Bradding P, Walls AF, Holgate ST. The role of the mast cell in the pathophysiology of asthma. J. Allergy Clin. Immunol. 2006;117:1277–1284. doi: 10.1016/j.jaci.2006.02.039. [DOI] [PubMed] [Google Scholar]
- 37.Marone G, Triggiani M, de Paulis A. Mast cells and basophils: friends as well as foes in bronchial asthma? Trends Immunol. 2005;26:25–31. doi: 10.1016/j.it.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 38.Galli SJ, Kalesnikoff J, Grimbaldeston MA, Piliponsky AM, Williams CM, Tsai M. Mast cells as "tunable" effector and immunoregulatory cells: recent advances. Annu. Rev. Immunol. 2005;23:749–786. doi: 10.1146/annurev.immunol.21.120601.141025. [DOI] [PubMed] [Google Scholar]
- 39.Galli SJ, Nakae S, Tsai M. Mast cells in the development of adaptive immune responses. Nat. Immunol. 2005;6:135–142. doi: 10.1038/ni1158. [DOI] [PubMed] [Google Scholar]
- 40.Korsgren M, Erjefalt JS, Korsgren O, Sundler F, Persson CG. Allergic eosinophil-rich inflammation develops in lungs and airways of B cell-deficient mice. J. Exp. Med. 1997;185:885–892. doi: 10.1084/jem.185.5.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Takeda K, Hamelmann E, Joetham A, Shultz LD, Larsen GL, Irvin CG, Gelfand EW. Development of eosinophilic airway inflammation and airway hyperresponsiveness in mast cell-deficient mice. J. Exp. Med. 1997;186:449–454. doi: 10.1084/jem.186.3.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kobayashi T, Miura T, Haba T, Sato M, Serizawa I, Nagai H, Ishizaka K. An essential role of mast cells in the development of airway hyperresponsiveness in a murine asthma model. J. Immunol. 2000;164:3855–3861. doi: 10.4049/jimmunol.164.7.3855. [DOI] [PubMed] [Google Scholar]
- 43.Ikeda RK, Miller M, Nayar J, Walker L, Cho JY, McElwain K, McElwain S, Raz E, Broide DH. Accumulation of peribronchial mast cells in a mouse model of ovalbumin allergen induced chronic airway inflammation: modulation by immunostimulatory DNA sequences. J. Immunol. 2003;171:4860–4867. doi: 10.4049/jimmunol.171.9.4860. [DOI] [PubMed] [Google Scholar]
- 44.Yu M, Tsai M, Tam SY, Jones C, Zehnder J, Galli SJ. Mast cells can promote the development of multiple features of chronic asthma in mice. J. Clin. Invest. 2006;116:1633–1641. doi: 10.1172/JCI25702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mayr SI, Zuberi RI, Zhang M, de Sousa-Hitzler J, Ngo K, Kuwabara Y, Yu L, Fung-Leung WP, Liu FT. IgE-dependent mast cell activation potentiates airway responses in murine asthma models. J. Immunol. 2002;169:2061–2068. doi: 10.4049/jimmunol.169.4.2061. [DOI] [PubMed] [Google Scholar]
- 46.Taube C, Wei X, Swasey CH, Joetham A, Zarini S, Lively T, Takeda K, Loader J, Miyahara N, Kodama T, Shultz LD, Donaldson DD, Hamelmann EH, Dakhama A, Gelfand EW. Mast cells, Fc epsilon RI, and IL-13 are required for development of airway hyperresponsiveness after aerosolized allergen exposure in the absence of adjuvant. J. Immunol. 2004;172:6398–6406. doi: 10.4049/jimmunol.172.10.6398. [DOI] [PubMed] [Google Scholar]
- 47.Williams CM, Galli SJ. Mast cells can amplify airway reactivity and features of chronic inflammation in an asthma model in mice. J. Exp. Med. 2000;192:455–462. doi: 10.1084/jem.192.3.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mehlhop PD, van de Rijn M, Brewer JP, Kisselgof AB, Geha RS, Oettgen HC, Martin TR. CD40L, but not CD40, is required for allergen-induced bronchial hyperresponsiveness in mice. Am. J. Respir. Cell Mol. Biol. 2000;23:646–651. doi: 10.1165/ajrcmb.23.5.3954. [DOI] [PubMed] [Google Scholar]
- 49.Kohno M, Yamasaki S, Tybulewicz VL, Saito T. Rapid and large amount of autocrine IL-3 production is responsible for mast cell survival by IgE in the absence of antigen. Blood. 2005;105:2059–2065. doi: 10.1182/blood-2004-07-2639. [DOI] [PubMed] [Google Scholar]
- 50.Lantz CS, Boesiger J, Song CH, Mach N, Kobayashi T, Mulligan RC, Nawa Y, Dranoff G, Galli SJ. Role for interleukin-3 in mast-cell and basophil development and in immunity to parasites. Nature. 1998;392:90–93. doi: 10.1038/32190. [DOI] [PubMed] [Google Scholar]
- 51.Beck LA, Marcotte GV, MacGlashan D, Togias A, Saini S. Omalizumab-induced reductions in mast cell Fce psilon RI expression and function. J. Allergy Clin. Immunol. 2004;114:527–530. doi: 10.1016/j.jaci.2004.06.032. [DOI] [PubMed] [Google Scholar]
- 52.Prussin C, Griffith DT, Boesel KM, Lin H, Foster B, Casale TB. Omalizumab treatment downregulates dendritic cell FcepsilonRI expression. J. Allergy Clin. Immunol. 2003;112:1147–1154. doi: 10.1016/j.jaci.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 53.Saini SS, Klion AD, Holland SM, Hamilton RG, Bochner BS, Macglashan DW., Jr The relationship between serum IgE and surface levels of FcepsilonR on human leukocytes in various diseases: correlation of expression with FcepsilonRI on basophils but not on monocytes or eosinophils. J. Allergy Clin. Immunol. 2000;106:514–520. doi: 10.1067/mai.2000.108431. [DOI] [PubMed] [Google Scholar]
- 54.Saini SS, MacGlashan DW, Jr, Sterbinsky SA, Togias A, Adelman DC, Lichtenstein LM, Bochner BS. Down-regulation of human basophil IgE and FC epsilon RI alpha surface densities and mediator release by anti-IgE-infusions is reversible in vitro and in vivo. J. Immunol. 1999;162:5624–5630. [PubMed] [Google Scholar]