Abstract
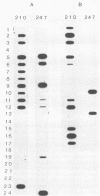
The prevalence and global distribution of two circumsporozoite (CS) genotypes of Plasmodium vivax (VK210 and VK247) were determined by genetic analysis of isolates from 234 malaria-infected patients. Whole blood specimens were collected on filter paper from patients infected with malaria in Thailand, Mexico, Papua New Guinea, Peru, Afghanistan (Pakistan), India, and western Africa and from 50 asymptomatic smear-negative controls. Following extraction of DNA from the filter paper samples, the CS gene was amplified by the polymerase chain reaction and genotyped by using oligoprobes specific for the VK210 and VK247 repeat epitopes. The sensitivity of genotyping from a single blood dot was 95.2%. The VK247 CS genotype was identified in the blood of patients from all seven study areas and was the predominant form present in samples from Thailand (83%) and Papua New Guinea (90%). In contrast, VK247 DNA was present in only 9% of isolates from Mexico. Individuals infected with both genotypes simultaneously were identified in all study areas except Mexico and were particularly common in Thailand (58%) and Papua New Guinea (60%). These findings indicate that the VK247 genotype of P. vivax is widely distributed but that its prevalence varies geographically. In addition, we conclude that use of samples of whole blood on filter paper is a practical and sensitive method for determining the genotypes of large numbers of malaria isolates collected in field settings.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnot D. E., Stewart M. J., Barnwell J. W. Antigenic diversity in Thai Plasmodium vivax circumsporozoite proteins. Mol Biochem Parasitol. 1990 Nov;43(1):147–149. doi: 10.1016/0166-6851(90)90140-h. [DOI] [PubMed] [Google Scholar]
- Brown A. E., Webster H. K., Krinchai K., Gordon D. M., Wirtz R. A., Permpanich B. Characteristics of natural antibody responses to the circumsporozoite protein of Plasmodium vivax. Am J Trop Med Hyg. 1991 Jan;44(1):21–27. doi: 10.4269/ajtmh.1991.44.21. [DOI] [PubMed] [Google Scholar]
- Cassol S., Salas T., Arella M., Neumann P., Schechter M. T., O'Shaughnessy M. Use of dried blood spot specimens in the detection of human immunodeficiency virus type 1 by the polymerase chain reaction. J Clin Microbiol. 1991 Apr;29(4):667–671. doi: 10.1128/jcm.29.4.667-671.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochrane A. H., Nardin E. H., de Arruda M., Maracic M., Clavijo P., Collins W. E., Nussenzweig R. S. Widespread reactivity of human sera with a variant repeat of the circumsporozoite protein of Plasmodium vivax. Am J Trop Med Hyg. 1990 Nov;43(5):446–451. doi: 10.4269/ajtmh.1990.43.446. [DOI] [PubMed] [Google Scholar]
- Coutlée F., Saint-Antoine P., Olivier C., Vessous-Elbaz A., Voyer H., Berrada F., Bégin P., Giroux L., Viscidi R. Discordance between primer pairs in the polymerase chain reaction for detection of human immunodeficiency virus type 1: a role for taq polymerase inhibitors. J Infect Dis. 1991 Oct;164(4):817–818. doi: 10.1093/infdis/164.4.817. [DOI] [PubMed] [Google Scholar]
- Cox F. E. Malaria. Variation and vaccination. Nature. 1991 Jan 17;349(6306):193–193. doi: 10.1038/349193a0. [DOI] [PubMed] [Google Scholar]
- Gordon D. M., Cosgriff T. M., Schneider I., Wasserman G. F., Majarian W. R., Hollingdale M. R., Chulay J. D. Safety and immunogenicity of a Plasmodium vivax sporozoite vaccine. Am J Trop Med Hyg. 1990 Jun;42(6):527–531. doi: 10.4269/ajtmh.1990.42.527. [DOI] [PubMed] [Google Scholar]
- Kain K. C., Keystone J., Franke E. D., Lanar D. E. Global distribution of a variant of the circumsporozoite gene of Plasmodium vivax. J Infect Dis. 1991 Jul;164(1):208–210. doi: 10.1093/infdis/164.1.208. [DOI] [PubMed] [Google Scholar]
- Kain K. C., Lanar D. E. Determination of genetic variation within Plasmodium falciparum by using enzymatically amplified DNA from filter paper disks impregnated with whole blood. J Clin Microbiol. 1991 Jun;29(6):1171–1174. doi: 10.1128/jcm.29.6.1171-1174.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kain K. C., Wirtz R. A., Fernandez I., Franke E. D., Rodriguez M. H., Lanar D. E. Serologic and genetic characterization of Plasmodium vivax from whole blood-impregnated filter paper discs. Am J Trop Med Hyg. 1992 Apr;46(4):473–479. doi: 10.4269/ajtmh.1992.46.473. [DOI] [PubMed] [Google Scholar]
- Qari S. H., Goldman I. F., Povoa M. M., Oliveira S., Alpers M. P., Lal A. A. Wide distribution of the variant form of the human malaria parasite Plasmodium vivax. J Biol Chem. 1991 Sep 5;266(25):16297–16300. [PubMed] [Google Scholar]
- Rosenberg R., Wirtz R. A., Lanar D. E., Sattabongkot J., Hall T., Waters A. P., Prasittisuk C. Circumsporozoite protein heterogeneity in the human malaria parasite Plasmodium vivax. Science. 1989 Sep 1;245(4921):973–976. doi: 10.1126/science.2672336. [DOI] [PubMed] [Google Scholar]
- Zavala F., Masuda A., Graves P. M., Nussenzweig V., Nussenzweig R. S. Ubiquity of the repetitive epitope of the CS protein in different isolates of human malaria parasites. J Immunol. 1985 Oct;135(4):2790–2793. [PubMed] [Google Scholar]