Abstract
Untreated cystathionine β-synthase (CBS) deficiency in humans is characterized by extremely elevated plasma total homocysteine (tHcy>200 μM), with thrombosis as the major cause of morbidity. Treatment with vitamins and diet leads to a dramatic reduction in thrombotic events, even though patients often still have severe elevations in tHcy (>80 μM). To understand the difference between extreme and severe hyperhomocysteinemia, we have examined two mouse models of CBS deficiency: Tg-hCBS Cbs−/− mice, with a mean serum tHcy of 169 μM, and Tg-I278T Cbs−/− mice, with a mean tHcy of 296 μM. Only Tg-I278T Cbs−/− animals exhibited strong biological phenotypes, including facial alopecia, osteoporosis, endoplasmic reticulum (ER) stress in the liver and kidney, and a 20% reduction in mean survival time. Metabolic profiling of serum and liver reveals that Tg-I278T Cbs−/− mice have significantly elevated levels of free oxidized homocysteine but not protein-bound homocysteine in serum and elevation of all forms of homocysteine and S-adenosylhomocysteine in the liver compared to Tg-hCBS Cbs−/− mice. RNA profiling of livers indicate that Tg-I278T Cbs−/− and Tg-hCBS Cbs−/− mice have unique gene signatures, with minimal overlap. Our results indicate that there is a clear pathogenic threshold effect for tHcy and bring into question the idea that mild elevations in tHcy are directly pathogenic. Gupta, S., Kühnisch, J., Mustafa, A., Lhotak, S., Schlachterman, A., Slifker, M. J., Klein-Szanto, A., High, K. A., Austin, R. C., Kruger, W. D. Mouse models of cystathionine β-synthase deficiency reveal significant threshold effects of hyperhomocysteinemia.
Keywords: osteoporosis, ER stress, inborn error of metabolism, sulfur metabolism
Cystathionine β-synthase (CBS) deficiency is the most common inborn error of sulfur metabolism and is the cause of classical homocystinuria, a condition characterized by very high levels of plasma total homocysteine (tHcy). Moreover, large-scale epidemiological studies have shown that a mild increase in tHcy in the general population is associated with increased risk of several age-related diseases including coronary artery disease, stroke, osteoporosis, and dementia (1,2,3,4). Homocysteine is a thiol containing amino acid derived from the metabolism of methionine and is a metabolic precursor of both methionine and cysteine (5). In plasma or serum from normal individuals, most homocysteine is disulfide bonded with other sulfur-containing amino acids (10–20%) or to cysteine present in protein (80–90%) (6). The sum total of all forms of homocysteine present is tHcy. In healthy adults, tHcy concentration in plasma ranges from 5 to 15 μM, but untreated patients with CBS deficiency often have tHcy in excess of 200 μM (7).
CBS-deficient patients suffer at an early age from various pathologies, including thrombosis, osteoporosis, mental retardation, and dislocated lenses (8). The major cause of mortality and morbidity in these patients is thrombosis. Treatment with a combination of B vitamins, dietary methionine restriction, and betaine supplementation can significantly reduce the incidences of vascular events despite the fact that post-treatment homocysteine levels are still several times higher than levels found in the normal population (9,10,11). These findings suggest that there may be a threshold effect for tHcy.
To understand the pathogenic mechanism of elevated tHcy, Watanabe et al. (12) created a mouse that contained a CBS knockout allele (Cbs−). It was found that Cbs−/− homozygotes had a neonatal lethal phenotype due to liver dysfunction. To circumvent this problem, our lab created a transgenic mouse (Tg-hCBS), in which the human CBS cDNA is under control of the zinc inducible metallothionein promoter (13). By supplying zinc in the drinking water during pregnancy and lactation, we were able to rescue the neonatal lethal phenotype and generate Tg-hCBS Cbs−/− animals. At weaning, zinc could be removed causing the animals to develop severe hyperhomocysteinemia (>150 μM). A second mouse model developed by our lab utilized a mutant human CBS transgene (Tg-I278T), which expressed human CBS containing the I278T mutation, the most common allele found in CBS-deficient patients (14). Surprisingly, Tg-I278T Cbs−/− animals also survive the neonatal period, even though the I278T CBS enzyme has less than 3% of the activity of wild-type CBS (15). These animals exhibit extreme hyperhomocysteinemia (>250 μM), even with zinc water supplementation.
In the present study, we compare and contrast the phenotypes of these two models of CBS deficiency, thus differentiating between extreme and severe hyperhomocysteinemia. Our results show that there is a clear threshold effect of tHcy on a variety of phenotypes in the mouse. These studies shed light as to why even moderate lowering of tHcy in CBS-deficient patients, without achieving complete normalization, can result in dramatic clinical improvements.
MATERIALS AND METHODS
Mouse models
All mice are in a C57BL6 strain background. The Cbs−, Tg-hCBS, and Tg-I278T alleles were originally created as described previously (12, 13, 15). Tg-hCBS Cbs−/− and Tg-I278T Cbs−/− mice were generated either by mating a transgene-positive Cbs−/− male with a transgene-positive Cbs−/+ female or a transgene positive Cbs−/+ male with a transgene positive Cbs−/+ female, in a cage with 25 mM ZnCl2 added to the drinking water. Control animals for all experiments were sibling transgene-positive animals that were either Cbs−/+ or Cbs+/+. Pups were genotyped at day 10 for both the transgene and the Cbs knockout allele, as described previously (13). Pups were generally weaned between 30 and 40 days and then placed in cages with non-ZnCl2-supplemented water. For aging studies, the animals were monitored weekly for overall health and survival. Animals were fed standard rodent chow (Teklad 2018SX; Harlan Teklad, Madison, WI, USA) ad libitum. Survival data were analyzed with a log rank test using GraphPad Prism 4.0 (GraphPad Software, San Diego, CA, USA).
Bone studies
For microcomputer tomography (CT) analysis, mice were sacrificed, and femur and lumbar vertebrae were dissected and fixed in phosphate-buffered 4% paraformaldehyde. After fixation, tissue samples were dehydrated in ethanol and finally embedded in methyl methacrylate. Plastic-embedded bone samples were measured in a vivaCT 40 (Scanco Medical, Bassersdorf, Switzerland; www.scanco.ch). Evaluation and imaging of micro-CT scans occurred using Amira software (Carlsbad, CA, USA; www.amiravis.com).
Clotting studies and histopathology of the heart and aorta
Prothrombin time (PT) and activated partial thromboplastin time (APTT) clotting assays were performed as described previously (16). For histopathology, the heart and aortic tree were excised, fixed overnight in 10% buffered formaldehyde, embedded in paraffin, and sectioned. Longitudinal serial sections (6 μm thick) were collected from the entire arterial tree. The sections included thoracic and abdominal aorta, including the bifurcation, as well as carotid and renal arteries. Usually, two consecutive sections were on one slide. The base of the heart containing the aortic root was processed similarly. All sections were stained with hematoxylin and eosin and evaluated for endothelial and medial lesions by an experienced mouse pathologist (A.K.S.).
Immunohistochemistry of liver and kidney
Tissue sections (4 μM thick) were deparaffinized, and the endogenous peroxidase activity was blocked with 0.5% H2O2 in methanol for 10 min. For KDEL staining, sections were blocked with 5% normal goat serum and incubated with anti-KDEL antibody (Stressgen, Plymouth Meeting, PA, USA) diluted 1:500, followed by anti-mouse Envision-HRP (Dako Cytomation, Copenhagen, Denmark) diluted 1:3, and Streptavidin-peroxidase (Zymed, Burlingame, CA, USA). For the GADD153 staining, the antigen was heat-retrieved in a rice steamer in Retrieve-all-2 solution (Signet Laboratories, Dedham, MA, USA) for 30 min, and sections were blocked with 5% normal goat serum and incubated with rabbit anti-GADD153 (Santa Cruz Biotechnology, Santa Cruz, CA, USA) antibody at 1:40 dilution, followed by anti-rabbit Envision-HRP (Dako Cytomation) diluted 1:3. Sections were developed in Nova Red peroxidase substrate (Vector Laboratories, Burlingame, CA, USA) and counterstained with hematoxylin. Pictures were taken with a Laborlux S microscope (Leitz; Leica Microsystems, Wetzlar, Germany) using an Olympus DP70 digital camera (Olympus, Tokyo, Japan).
Gene expression analysis of liver by microarray
RNA was extracted from livers using TRIzol Reagent (Invitrogen, Carlsbad, CA, USA) followed by cleaning and removal of contaminating DNA using the RNeasy Mini Kit (Qiagen, Valencia, CA, USA). Quality and quantity of RNA were determined using an Agilent 2100 bioanalyzer (Agilent Technologies, Santa Clara, CA, USA; www.chem.agilent. com). Probe preparation and microarray analysis were performed as described previously (17), except that 500 ng of RNA was labeled according to manufacturer’s instructions (Agilent Technologies). Microarray was done using mouse 44K oligoarray (Agilent Technologies), where control samples were labeled with Cy3 and Tg-hCBS Cbs−/− and Tg-I278T Cbs−/− (4 each) samples were labeled with Cy5 fluorescent dye. Eight hybridizations were performed. The microarray images were quantified, and preprocessing was performed by Agilent’s feature extraction software using default settings (which incorporate outlier flagging, spatial detrend background correction, and lowest normalization to correct for dye bias). A sequence of nonspecific filters was applied to reduce the number of probes in the analysis (removal of uniformly low expressers, probes lacking adequate annotation, and low-quality probes). In cases in which multiple probes mapped to the same Entrez gene, the probe with maximum coefficient of variation across all channels was retained. Additional statistical analyses were performed using the limma (18) package in Bioconductor (19) to identify probes that were differentially expressed. A list of differentially regulated genes for Tg-hCBS Cbs−/− and Tg-I278T Cbs−/− mice was selected on the basis of logFC ≥ 1 or ≤ −1 and P < 0.05. This list was then used for functional pathway analysis employing the WebGestalt data mining system (http://genereg.ornl.gov/webgestalt).
Taqman quantitative reverse transcriptase-polymerase chain reaction (qRT-PCR)
RNA from the same pool as described above was used for one-step qRT-PCR employing Taq Man Gene expression assays (Applied Biosystems, Foster City, CA, USA) for asparagine synthase (Asns; Mm00803785_m1), serum amyloid P-component (Apcs; Mm00488099_g1), and growth arrest and DNA-damage-inducible 45 β (Gadd45b; Mm00435123_m1). Mouse β-actin (Mm00607939_s1) was used as endogenous control. Quantification of signal was achieved by using Applied Biosystems 7900 HT version 2.2.2 sequence detection system. Each sample was assayed in triplicate (100 ng/well) for each gene, and relative signal strength was calculated using the ΔΔCt method.
Measurement of metabolites in serum and liver tissues
Serum and liver metabolite measurements were performed using a Biochrom 30 amino acid analyzer (Biochrom, Cambridge, UK). Extraction and processing of serum were done as described previously (15). For determination of free (nonprotein bound) forms of homocysteine and cysteine, duplicate samples were processed in the absence of dithiothreitol (DTT). Liver tissues were processed by two different methods to determine free and total homocysteine. To determine free homocysteine and other amino acids, livers were carefully weighed, homogenized in 2.5% sulfosalicylic acid using a dounce homogenizer, and centrifuged at 10,000 rpm to obtain the supernatant for metabolite measurement. Processing of liver tissues for tHcy and total cysteine (tCys) quantification was done in the presence of DTT as described previously (13), and values were converted from nanomoles per milligram soluble protein to nanomoles per gram wet weight for comparison with the free forms obtained from sulfosalicylic acid extracts. The amount of homocysteine, homocystine, cysteine, and cystine present in the sample was determined by comparing peak areas with purified standards purchased from Sigma-Aldrich (St. Louis, MO, USA). The standard for Hcy-Cys was formed by overnight incubation of known amount of DL-Hcy with L-Cys in Tris-HCl buffer (50 mM; pH 7.3) containing final concentration of 25 μΜ CuSO4 at room temperature. Amounts of homocystine (Hcy-Hcy) and cystine (Cys-Cys) formed after incubation were subtracted from the amounts of homocysteine and cysteine initially added, to quantify the amount of Hcy-Cys represented by the formed peak area. Total free homocysteine (tfHcy) was calculated by adding the following molar concentrations: tfHcy = Hcy + 2 ∗ (Hcy-Hcy) + Hcy-Cys. Protein bound homocysteine (bHcy) was calculated by subtracting the molar concentration of tfHcy from tHcy. Similarly, total free cysteine (tfCys) was calculated by adding the molar concentration of all the free forms of cysteine: tfCys = Cys + 2 ∗ (Cys-Cys) + Hcy-Cys. Protein-bound cysteine (bCys) was calculated by subtracting the molar concentration of tfCys from tCys.
RESULTS
Tg-I278T Cbs−/− mice have reduced life span
Cohorts of Tg-I278T Cbs−/−, Tg-hCBS Cbs−/−, and sibling controls were aged for up to 900 days. These animals were all born to mothers drinking zinc water to induce transgene expression, but the water was removed at the time of weaning. In adult animals between 6 and 9 mo of age, the mean serum tHcy for Tg-I278T Cbs−/− was 296 ± 28 μM (n=7) compared to 169 ± 25 μM (n=6) for Tg-hCBS Cbs−/− and 5.5 ± 2.3 μM (n=7) for the combined controls. The difference in tHcy between the Tg-hCBS Cbs−/− and Tg-I278T Cbs−/− animals is probably due to slight expression of the transgene in the absence of zinc, resulting in the mice containing Tg-hCBS having a very slight amount of CBS activity. However, even in Tg-hCBS Cbs−/− animals in the absence of zinc, the amount of residual CBS activity was below our limit of detection in both immunoblot and enzyme activity analysis (data not shown).
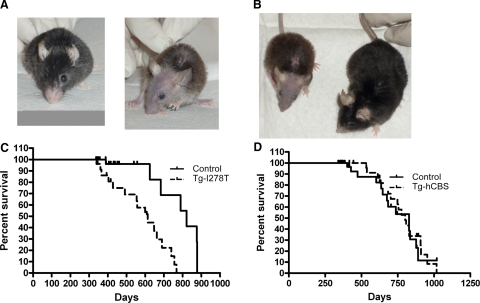
We noticed obvious phenotypic differences in Tg-I278T Cbs−/− compared to Tg-hCBS Cbs−/− and control animals. Tg-I278T Cbs−/− animals exhibited extensive alopecia of the face and head, were smaller in size, and had a hunched appearance (Fig. 1A, B). In addition, Tg-I278T Cbs−/− had a shorter life span compared to sibling controls (Fig. 1C), with a median survival time of 613 days compared to 821 days (P<0.0003, log rank test). In contrast, there was no difference in the median survival times of Tg-hCBS Cbs−/− animals compared to their sibling controls (Fig. 1D). Necropsies on deceased animals were performed when possible, but, in general, we were unable to identify a specific cause of death.
Figure 1.
Comparison of Tg-hCBS Cbs−/− and Tg-I278T Cbs−/− appearance and survival. A) Frontal view of 913-day-old male Tg-hCBS Cbs−/− (left panel) and 686-day-old male Tg-I278T Cbs−/− (right panel). B) Side-by-side size comparison of same two animals. C) Survival curves of Tg-I278T Cbs−/− compared to sibling controls. D) Survival curve of Tg-hCBS Cbs−/− animals compared to controls. Tick marks show censored animals.
Tg-I278T Cbs−/− mice develop osteoporosis
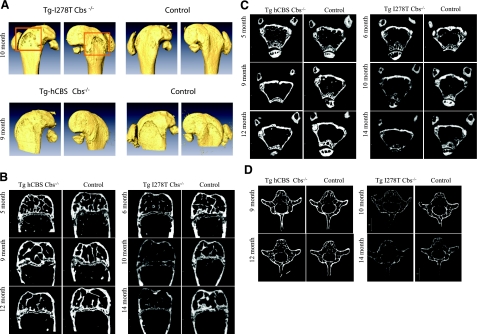
Osteoporosis is a common clinical symptom of CBS deficiency in humans. Therefore, we examined Tg-I278T Cbs−/−, Tg-hCBS Cbs−/−, and control animals for osteoporosis by micro-CT scan. Mice from each group were investigated for any changes in their femur and fifth lumbar vertebrae at various time intervals from 5 to 14 mo. Shape and size of femur were unaffected in both models, indicating normal final development, but at 10 and 14 mo Tg-I278T Cbs−/− mice displayed a rough periosteal surface and small holes (Fig. 2A). Longitudinal sections of femur epiphysis of both Cbs-deficient mouse models were evaluated for any changes in trabecular and cortical bone (Fig. 2B). Trabecular bone in the secondary spongiosa and in the secondary ossification center appears to be unaffected in Tg-hCBS Cbs−/− mice, whereas Tg-I278T Cbs−/− mice showed reduced trabecular bone mass, particularly at 10 and 14 mo time points. In Tg-I278T Cbs−/−, animals reduced trabecular bone volume is accompanied by an increased number of trabeculae, presumably indicating amplified bone resorption by osteoclasts. Moreover, the bone mineral density (BMD), corresponding to the X-ray attenuation, and thus image gray values, of Tg-I278T Cbs−/− femora was reduced, illustrating diminished mineral content of the bone tissue. Transverse sections at the secondary spongiosa level showed reduction of the cortical bone width in Tg-I278T Cbs−/− mice at the same time points (Fig. 2B, C). As individual bones of the skeletal system are differentially affected by systemic metabolic changes and mechanical loading, we also examined the fifth lumbar vertebrae by micro CT analysis. As represented in Fig. 2D, the spine of Tg-hCBS Cbs−/− mice did not show changes in bone volume and BMD. In contrast, Tg-I278T Cbs−/− mice showed decrease bone mass and BMD at indicated time points. These results imply that Tg-I278T Cbs−/− mice exhibit osteoporosis that is evident by 6 mo of age.
Figure 2.
Comparison of femora and fifth lumbar vertebrae from Tg-hCBS Cbs−/− and Tg-I278T Cbs−/− mice with their age-matched controls illustrated by micro-CT analysis. A) Femur epiphysis view showing bone size, shape, and surface. Orange box shows obvious areas of osteoporosis. B) Longitudinal sections of epiphyseal femur, illustrating the amount of trabecular bone in the secondary spongiosa and secondary ossification center, as well as the cortical width and BMD. C) Transverse sections through the entire femur proximal from the growth plate represents changes in the secondary spongiosa trabecular bone and cortical bone width. D) Fifth lumbar vertebrae presented in transverse sections. All investigated sample sections were imaged at comparable positions..
Tg-I278T Cbs−/− animals do not have thrombotic or vascular defects
The major cause of morbidity and mortality in CBS-deficient patients is thromboembolism, and it has been hypothesized that this may be related to a clotting defect (20). Therefore, we examined clotting behavior of plasma in Tg-I278T Cbs−/− and sibling control animals by measuring prothrombin time (PT) and activated partial thromboplastin time (APTT). We did not observe any difference between control and Cbs−/− animals in either assay (Supplemental Fig. 1). In addition, histopathologic examination of the aorta and heart muscle of older Tg-I278T Cbs−/− animals did not reveal any evidence of atherosclerosis or abnormalities in vessel structure (data not shown). These studies show that the thrombotic phenotypes found in human CBS-deficient patients are not observed even in our most extreme mouse model of CBS deficiency.
Tg-I278T Cbs−/− and Tg-hCBS Cbs−/− animals have unique liver gene expression profiles
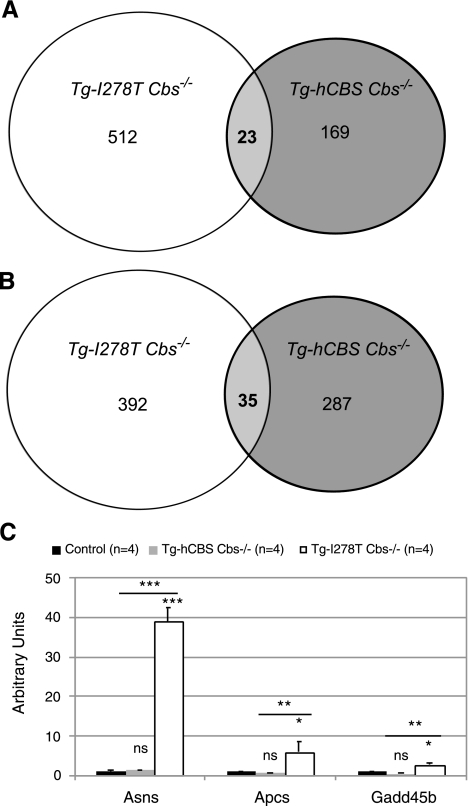
Microarray studies were conducted to differentiate between severe and extreme hyperhomocysteinemia on the basis of gene expression in liver. We performed dual-labeling experiments, in which Cy5-labeled liver RNA from either Tg-hCBS Cbs−/− or Tg-I278T Cbs−/− animals were cohybridized with Cy3-labeled RNA from sibling controls. Four biological replicates were examined for each array. Using the criteria of at least a 2-fold change of average expression and P < 0.05 (see Materials and Methods), we found that 512 known genes were up-regulated in Tg-I278T Cbs−/− mice compared to only 169 in Tg-hCBS Cbs−/− mice. Surprisingly, only 23 of these genes were in common between the two sets. With regard to down-regulated genes, we found 392 genes in Tg-I278T Cbs−/− mice compared to 287 in Tg-hCBS Cbs−/− mice (35 were common) (Fig. 3A, B and Supplemental Tables 5 and 6).
Figure 3.
Venn diagram showing the number of specifically altered genes in liver tissue by microarray. A) Genes that are up-regulated relative to controls with cutoff criteria of logFC ≥ 1 and P < 0.05. B) Down-regulated genes with cutoff criteria of logFC ≤ −1 and P < 0.05 applied. C) Relative levels of mRNA for Asns, Apcs, and Gadd 45b as determined by quantitative real-time RT-PCR. ns, not significant. *P < 0.05, **P < 0.01, ***P < 0.001; Tukey’s multiple comparison test.
The resulting up-regulated and down-regulated gene sets were analyzed for enrichment of particular functional pathways (Supplemental Tables 1–4). This analysis revealed that some pathways are either up- or down-regulated in both models, while others are uniquely affected. The MAPK signaling pathway, PPAR signaling pathway, and the adipocytokine signaling pathway were down-regulated in both Tg-I278T Cbs−/− and Tg-hCBS Cbs−/−, while the cytokine-cytokine receptor and focal adhesion pathways were up-regulated in both models. Nineteen pathways were uniquely up- or down-regulated in Tg-I278T Cbs−/− compared to only 12 for Tg-hCBS Cbs−/− animals. Interestingly, three pathways were differentially regulated in the two models. For example, the androgen and estrogen metabolic pathway was down-regulated in Tg-I278T Cbs−/− but was up-regulated Tg-hCBS Cbs−/−, while the fructose and mannose metabolic pathway had the reverse behavior. These liver microarray results indicate that severe and extreme hyperhomocysteinemia affects the gene expression profiles in a differential manner.
Tg-I278T Cbs−/− mice exhibit signs of endoplasmic reticulum (ER) stress
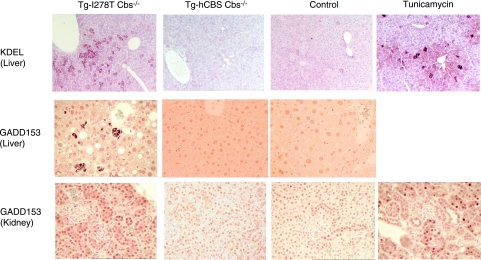
Several studies have shown that homocysteine acts intracellularly to disrupt protein folding in the ER, thereby leading to up-regulation of several ER stress response genes. We examined livers and kidneys of Tg-I278T Cbs−/−, Tg-hCBS Cbs−/−, and sibling control animals for the ER stress markers KDEL and GADD153 by immunohistochemistry. KDEL is the ER retention signal (Lys-Asp-Glu-Leu), and staining with anti-KDEL assesses GRP78 and GRP94 levels (21). We found that 7 of the 8 livers examined from Tg-I278T Cbs−/− mice exhibited increased KDEL staining, often in a mosaic pattern (Fig. 4). This pattern was similar to that observed in mice treated with the known ER stress agent tunicamycin (22). No similar pattern was observed in livers from either Tg-hCBS Cbs−/− (n=3) or control animals (n=5). We did not observe any difference in KDEL staining in the kidney (data not shown). With respect to GADD153 staining, we observed a punctate pattern of staining, in which a few cells highly expressed GADD153 only in livers from Tg-I278T Cbs−/− mice (Fig. 4). In the kidney, we found that 2 of the 6 livers from Tg-I278T Cbs−/− exhibited increased GADD153 staining similar to that observed in the tunicamycin control.
Figure 4.
Examination of ER stress in tissues from Tg-I278T Cbs−/−, Tg-hCBS Cbs−/−, and Cbs+/−controls. Sections of liver and kidney (×40) stained with antibody directed against GADD153 or KDEL. Mouse genotypes are indicated at top. Right-most column shows staining for a control mouse treated with the known ER stress agent tunicamycin.
Additional evidence for ER stress comes from the liver microarray analysis. Examination of the 904 genes that were either up- or down-regulated in Tg-I278T Cbs−/− mice revealed 8 genes that have been associated with ER stress in the literature (Table 1). Seven of these genes were up-regulated, while one was down-regulated. To confirm the microarray results, we examined expression of Asns, Apcs, and Gadd45b by Taqman qRT-PCR (Fig. 3C). All three genes showed an increased expression only in Tg-I278T Cbs−/− mice. Taken together, these results demonstrate that Tg-I278T Cbs−/− mice, but not Tg-hCBS Cbs−/− mice, exhibit ER stress.
TABLE 1.
ER stress-responsive genes in Tg-I278T Cbs−/− mice
| Gene | Description | Reference |
|---|---|---|
| Up-regulated | ||
| Asns | Asparagine synthetase | 36, 37 |
| Apcs | Serum amyloid P-component | 38 |
| Gadd45b, Gadd45g | Growth arrest and DNA-damage-inducible 45 β | 39 |
| Growth arrest and DNA-damage-inducible 45 γ | ||
| Mthfd2 | Methylenetetrahydrofolate dehydrogenase (NAD+ dependent), methenyltetrahydrofolate cyclohydrolase | 40 |
| Phlda2 | Pleckstrin homology-like domain, family A, member 2 | Unpublished results |
| Srebf2 | Sterol regulatory element binding factor 2 | 41 |
| Down-regulated | ||
| Park2 | Parkin | 42 |
Metabolic profiling of serum
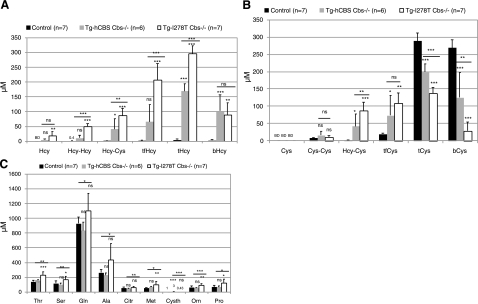
To begin to understand the cause of the differences between the severe and extreme hyperhomocysteinemic mouse models, we performed metabolic profiling of the various forms of homocysteine, cysteine, and other amino acids present in serum of these mice. Homocysteine can exist in serum in three chemical forms: free reduced homocysteine, as an oxidized disulfide with itself or with cysteine, or it can be bound to cysteine in serum proteins such as albumin. To determine the amount of homocysteine in these different forms, we performed amino acid analysis on serum in either neutral or reducing conditions (see Materials and Methods). Serum concentrations of free reduced homocysteine (Hcy), homocystine (Hcy-Hcy), homocysteine-cysteine mixed disulfide (Hcy-Cys), total free homocysteine (tfHcy; the sum total of all of the previous species), total homocysteine (tHcy), and protein-bound homocysteine (bHcy) were measured in Tg-I278T Cbs−/−, Tg-hCBS Cbs−/−, and combined control animals (Fig. 5A). Hcy, Hcy-Hcy, Hcy-Cys, tfHcy, and tHcy were all elevated in Tg-hCBS Cbs−/− mice compared with controls and were further elevated in Tg-I278T Cbs−/− mice. A different pattern emerged in regard to bHcy. Here, we found a large elevation in bHcy in both Tg-hCBS Cbs−/− and Tg-I278T Cbs−/− compared with control, but unlike the free disulfides, we did not observe an increase in Tg-I278T Cbs−/− compared with Tg-hCBS Cbs−/− mice. Our findings suggest that once the carrying capacity of homocysteine in serum has been exceeded, oxidized homocysteine disulfides begin to accumulate. These results show that the difference in tHcy between Tg-hCBS Cbs−/− and Tg-I278T Cbs−/− is mostly due to the differences in the levels of free oxidized homocysteine species found in the serum.
Figure 5.
Metabolic profiling of serum. A) Concentration of various forms of homocysteine in Tg-I278T Cbs−/−, Tg-hCBS Cbs−/−, and control animals. Values are presented as means ± sd (μM). Significance in comparison to control is presented directly above each column; significance between Tg-I278T Cbs−/− and Tg-hCBS Cbs−/− mice is shown above horizontal line. B) Concentration of various forms of cysteine in the indicated strains. C) Concentration of amino acids, which differed significantly in at least one of the three groups. BD, below detection; ns, not significant. *P < 0.05, **P < 0.01, ***P < 0.001; Tukey’s multiple comparison test.
Because CBS is critical for cysteine biosynthesis, we also examined serum cysteine levels. We found that serum tCys was significantly decreased in Tg-hCBS Cbs−/− and Tg-I278T Cbs−/− animals, with Tg-I278T Cbs−/− having nearly a 50% reduction compared to controls (Fig. 5B). Interestingly, this reduction was especially dramatic with regard to protein-bound cysteine (bCys). The percentage of protein-bound cysteine to total cysteine is 88.9%, 59.1%, and 4.3% in control, Tg-hCBS Cbs−/− and Tg-I278T Cbs−/− animals, respectively. Despite a drop in tCys in Tg-hCBS Cbs−/− and Tg-I278T Cbs−/− animals, we observed a significant increase in homocysteine-cysteine disulfide. This implies that the reduced level of bCys observed in the mutant animals is because the cysteine that normally binds to protein is binding to free serum homocysteine instead. Thus the decrease in the protein bound cysteine ratio is driven by elevated serum homocysteine.
We also examined the serum levels of 28 other amino acids in Tg-I278T Cbs−/−, Tg-hCBS Cbs−/−, and control animals (Supplemental Table 7). The levels of threonine, serine, glutamine, alanine, citrulline, methionine, ornithine, and proline were significantly elevated between Tg-I278T Cbs−/− and Tg-hCBS Cbs−/− mice (Fig. 5C). In contrast, cystathionine was significantly decreased in Tg-I278T Cbs−/−, as compared to Tg-hCBS Cbs−/−mice. These results indicate that extreme elevations in tHcy can lead to alteration in the extracellular homeostasis of cysteine and other amino acids.
Metabolic profiling of liver
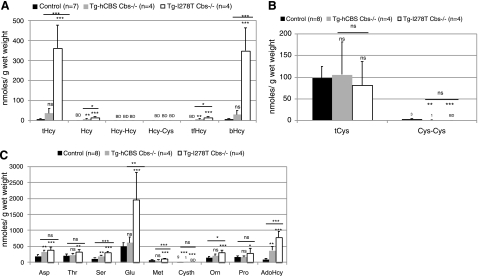
We next examined the effect of extreme vs. severe hyperhomocysteinemia on homocysteine and other amino acids present in liver homogenates. Tg-I278T Cbs−/− mice showed significant elevation in tHcy, bHcy, and tfHcy compared to both control and Tg-hCBS Cbs−/− mice (Fig. 6A). The difference in the concentrations of the various homocysteine species between Tg-I278T Cbs−/− and Tg-hCBS Cbs−/− mice was consistently greater in the liver compared to the serum. For example, in the serum, Tg-hCBS Cbs−/− had tHcy that was 57% that of Tg-I278T Cbs−/−, while in the liver homogenates, it is only 11% of the Tg-I278T Cbs−/− value. Unlike the serum, we did not observe a statistically significant difference in tCys present in the liver, although we did find that both Tg-I278T Cbs−/− and Tg-hCBS Cbs−/− homogenates had lower levels of cystine (Fig. 6B). In liver homogenates, we quantitated the amount of 25 other amine-containing compounds (Supplemental Table 8), and between Tg-hCBS Cbs−/− and Tg-I278T Cbs−/− mice, significant elevations were identified in the levels of serine, glutamate, methionine, ornithine, and AdoHcy (Fig. 6C). Interestingly, serine, methionine, and ornithine were also elevated in serum, whereas elevation in glutamate and AdoHcy was exclusive to the liver.
Figure 6.
Metabolic profiling of liver. A) Concentration of various forms of homocysteine in Tg-I278T Cbs−/−, Tg-hCBS Cbs−/−, and control animals. Values are presented as means ± sd (nmol/g wet weight). Significance in comparison to control is presented directly above each column; significance between Tg-I278T Cbs−/− and Tg-hCBS Cbs−/− mice is shown above horizontal line. B) Concentration of various forms of cysteine in the indicated strains. C) Concentration of amino acids, which differed significantly in at least one of the three groups. BD, below detection; ns, not significant. *P < 0.05, **P < 0.01, ***P < 0.001; Tukey’s multiple comparison test.
DISCUSSION
In this study, we have employed two CBS-deficient mouse models to examine the biological effects of severely elevated tHcy. In Tg-I278T Cbs−/− animals, having a mean tHcy at least 54 times higher than normal mice, we observed several phenotypes, including facial alopecia, osteoporosis, ER stress in the liver and kidney, and a 20% reduction in mean survival. However, Tg-hCBS Cbs−/− mice, despite having tHcy at least 30-fold greater than normal, failed to exhibit any of these phenotypes. These findings suggest that there may be biological buffering mechanisms that can protect mice from elevated tHcy up to a point, above which the toxic effects of homocysteine accumulation become manifest. These results are quite consistent with observation of CBS deficiency in human patients, in which treated patients have a much lower incidence of morbidity and mortality despite having tHcy values that are still 5–10 times higher than normal (11).
There are several reasons to believe that the phenotypic differences between Tg-I278T Cbs−/− and Tg-hCBS Cbs−/− mice are due to the differences in tHcy and related homocysteine moieties and not due to some other difference between the strains, such as the site of transgene integration. First, the phenotypes associated with the Tg-I278T Cbs−/− mice only occur when the animals are homozygous for the Cbs− allele and are not present in Tg-I278T Cbs+/+ or Tg-I278T Cbs+/− animals. Second, the facial alopecia and runted phenotypes have also been noted to occur in an independently derived transgene line, Tg-I278T/T424N Cbs−/− (15), and has been noted to occur in nontransgenic Cbs−/− mice that survive the neonatal lethality (23). Both of these lines have tHcy in excess of 200 μM. Finally, Tg-S466L Cbs−/− mice, which have a mean tHcy of 142 μM, resemble the Tg-hCBS Cbs−/− animals in outward appearance and survival characteristics (24).
Osteoporosis in Tg-I278T Cbs−/− mice is a phenotype shared with human CBS-deficient patients. Radiological spinal osteoporosis was observed in 50% of untreated CBS-deficient patients by 16 yr of age (8). Moreover, X-ray studies of surviving Cbs−/− mice by Roberts et al. (25) showed these mice had scoliosis. In this study, we show direct evidence of loss of trabecular, as well as cortical bone mass of the femur and in the fifth lumbar vertebrae in Tg-I278T Cbs−/− mice. However, unlike human CBS-deficient patients, we did not observe any dolichostenomelia (thinning and lengthening of the long bones) responsible for the marfanoid appearance of many CBS-deficient patients. The lack of osteoporosis in Tg-hCBS Cbs−/− mice indicates that homocysteine elevation is only pathogenic when present above a certain threshold level. Interestingly, even in Tg-I278T Cbs−/− mice, we did not observe any evidence of a thrombotic phenotype, indicating that there may be subtle differences in thrombotic behavior between mice and humans.
A threshold effect was also observed with respect to ER stress phenotype. Only Tg-I278T Cbs−/− animals had evidence of ER stress in the liver and kidney, as assessed by immunohistochemistry with ER stress markers. Cell culture studies have shown that the addition of exogenous homocysteine can disrupt protein folding in ER, thereby leading to the activation of the unfolded protein response (UPR) and increased expression of several ER stress response genes (26, 27). In addition, it has been reported that GRP78 and PDI undergo S-homocysteinylation in vitro and in situ in rat liver microsomes (28). Our data indicate that ER stress can also occur in vivo when homocysteine is extremely elevated. A possible mechanism for ER stress induction would be misfolding of ER proteins due to homocysteinylation of cysteine residues normally involved in intramolecular or intermolecular protein crosslinks. Consistent with this idea is our finding that most of the excess homocysteine found in liver tissue extracts was bound to protein.
Moreover, this study also shows that Tg-I278T Cbs−/− and Tg-hCBS Cbs−/− liver gene expression profiles were very different from each other. Although both mouse strains had hundreds of genes either up-regulated or down-regulated compared to control animals, there was very little overlap in their expression profiles. Examination of the metabolic pathways in which genes functioned revealed that distinct and greater numbers of pathways were affected in Tg-I278T Cbs−/− compared to Tg-hCBS Cbs−/−. These findings confirm that exposure to 296 μM tHcy has very different and drastic effects on the physiological state of liver compared to tHcy of 169 μM.
In serum, we found that although Tg-I278T Cbs−/− mice have significantly higher concentrations of tfHcy compared to Tg-hCBS Cbs−/− mice, the amount of bHcy was not different. This shows that serum proteins have a maximum binding capacity with regard to homocysteine, implying that the difference in phenotypes between Tg-I278T Cbs−/− and Tg-hCBS Cbs−/− mice are not due to increased homocysteinylation of serum or other extracellular proteins. The finding that elevated tfHcy is correlated with disease severity is entirely consistent with what is known about human CBS deficiency. Clinically, biochemical control of homocystinuria is often determined by the reduction of tfHcy or homocysteine below a certain level. For example, a patient is often judged as pyridoxine responsive if their tfHcy is less than 20 μM or their homocysteine is below 10 μM (11). Thus, in both humans and mice, the severity of the disease appears associated most closely with the level of serum tfHcy rather than bHcy.
The overall reduction in serum cysteine observed in both Tg-I278T Cbs−/− and Tg-hCBS Cbs−/− mice was not surprising given the observation that as much as 50% of cysteine in mammals is thought to be derived from the transsulfuration pathway (29). Somewhat more surprising was the relationship between tfCys and bCys in the different strains. In control animals, nearly 90% of the cysteine was bound to protein, while in Tg-I278T Cbs−/− animals, only 11% of the cysteine was bound. The reduction of protein-bound cysteine was accompanied by a large increase in homocysteine-cysteine disulfide. These results show that homocysteine in the serum effectively competes with protein-incorporated cysteine for disulfide formation with free cysteine. This same change in the distribution of bound to free cysteine has also been observed in human homocystinuric patients (30). It is also interesting to note that the amount of protein-incorporated cysteine bound either to free cysteine or homocysteine is vastly different in control, Tg-I278T Cbs−/− and Tg-hCBS Cbs−/− mice. In control animals, bCys+bHcy is 275 μM, in Tg-hCBS Cbs−/−, it is 225 μM, and in Tg-I278T Cbs−/−, it is only 120 μM. This finding implies that in the serum of Tg-I278T Cbs−/− animals, a large quantity of protein-incorporated cysteine exists as either free sulfhydryl or thiolate anion. It is possible that this alteration in disulfide cross-linking status may alter protein function and may help explain the phenotypes observed in Tg-I278T Cbs−/− animals.
Examination of homocysteine in liver cell extracts indicates that there is a much larger difference in intracellular homocysteine in Tg-I278T Cbs−/− vs. Tg-hCBS Cbs−/− mice than in the serum. Most of this homocysteine is bound to protein, and this finding is consistent with the hypothesis that intracellular homocysteinylation of proteins may be part of the pathological mechanism of CBS deficiency. In addition, AdoHcy was also significantly elevated in livers of Tg-I278T Cbs−/− mice. This could be explained by the fact that in liver, S-adenosylhomocysteine hydrolase favors the synthesis of AdoHcy when excess homocysteine is present (31). Because AdoHcy is a strong inhibitor of methyltransferases, this accumulation could also be important in the pathogenesis of the disease phenotypes present in Tg-I278T Cbs−/− mice.
Our findings also bear on the larger question of whether mild elevations in tHcy are pathogenic in the general population. Large epidemiological studies have linked mildly elevated tHcy to a variety of diseases, including stroke, heart attack, osteoporosis, age-related dementia, and neural tube defects (1,2,3,4, 32). In these studies, elevated tHcy is generally defined as having plasma concentrations greater than 15 μM. Recently, large intervention trials using folic acid to lower tHcy have failed to provide strong evidence that lowering these modestly elevated tHcy levels can reduce the incidence of coronary artery disease and stroke, thereby raising the question of whether mild elevation in tHcy is itself pathogenic or is a biomarker for other processes (33,34,35). These results and the lack of pathology associated with Tg-hCBS Cbs−/− mice suggest that mild elevations of tHcy associated with diseases of aging may not be directly pathogenic, but may be a marker of other pathogenic processes.
Supplementary Material
Acknowledgments
We thank the Sequencing, DNA Microarray, Biostatistics and Bioinformatics, Transgenic Mouse, and Laboratory Animal facilities of Fox Chase Cancer Center for their assistance. This work was supported by grants HL16237 and CA06927 from the National Institutes of Health and an appropriation from the Commonwealth of Pennsylvania to the Fox Chase Cancer Center.
References
- Refsum H, Ueland P M, Nygard O, Vollset S E. Homocysteine and cardiovascular disease. Annu Rev Med. 1998;49:31–62. doi: 10.1146/annurev.med.49.1.31. [DOI] [PubMed] [Google Scholar]
- Perry I J, Refsum H, Morris R W, Ebrahim S B, Ueland P M, Shaper A G. Prospective study of serum total homocysteine concentration and risk of stroke in middle-aged British men. Lancet. 1995;346:1395–1398. doi: 10.1016/s0140-6736(95)92407-8. [DOI] [PubMed] [Google Scholar]
- Van Meurs J B, Dhonukshe-Rutten R A, Pluijm S M, van der Klift M, de Jonge R, Lindemans J, de Groot L C, Hofman A, Witteman J C, van Leeuwen J P, Breteler M M, Lips P, Pols H A, Uitterlinden A G. Homocysteine levels and the risk of osteoporotic fracture. N Engl J Med. 2004;350:2033–2041. doi: 10.1056/NEJMoa032546. [DOI] [PubMed] [Google Scholar]
- Seshadri S, Beiser A, Selhub J, Jacques P F, Rosenberg I H, D'Agostino R B, Wilson P W, Wolf P A. Plasma homocysteine as a risk factor for dementia and Alzheimer’s disease. N Engl J Med. 2002;346:476–483. doi: 10.1056/NEJMoa011613. [DOI] [PubMed] [Google Scholar]
- Jhee K H, Kruger W D. The role of cystathionine beta-synthase in homocysteine metabolism. Antioxid Redox Signal. 2005;7:813–822. doi: 10.1089/ars.2005.7.813. [DOI] [PubMed] [Google Scholar]
- Jacobsen D W. Homocysteine and vitamins in cardiovascular disease. Clin Chem. 1998;44:1833–1843. [PubMed] [Google Scholar]
- Kruger W D, Wang L, Jhee K H, Singh R H, Elsas L J., 2nd Cystathionine beta-synthase deficiency in Georgia (USA): correlation of clinical and biochemical phenotype with genotype. Hum Mutat. 2003;22:434–441. doi: 10.1002/humu.10290. [DOI] [PubMed] [Google Scholar]
- Mudd S H, Skovby F, Levy H L, Pettigrew K D, Wilcken B, Pyeritz R E, Andria G, Boers G H, Bromberg I L, Cerone R, Fowler B, Gröbe H, Schmidt H, Schweitzer L. The natural history of homocystinuria due to cystathionine beta-synthase deficiency. Am J Hum Genet. 1985;37:1–31. [PMC free article] [PubMed] [Google Scholar]
- Wilcken D E, Wilcken B. The natural history of vascular disease in homocystinuria and the effects of treatment. J Inher Metab Dis. 1997;20:295–300. doi: 10.1023/a:1005373209964. [DOI] [PubMed] [Google Scholar]
- Yap S, Naughten E R, Wilcken B, Wilcken D E, Boers G H. Vascular complications of severe hyperhomocysteinemia in patients with homocystinuria due to cystathionine beta-synthase deficiency: effects of homocysteine-lowering therapy. Semin Thromb Hemost. 2000;26:335–340. doi: 10.1055/s-2000-8100. [DOI] [PubMed] [Google Scholar]
- Yap S, Boers G H, Wilcken B, Wilcken D E, Brenton D P, Lee P J, Walter J H, Howard P M, Naughten E R. Vascular outcome in patients with homocystinuria due to cystathionine beta-synthase deficiency treated chronically: a multicenter observational study. Arterioscler Thromb Vasc Biol. 2001;21:2080–2085. doi: 10.1161/hq1201.100225. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Osada J, Aratani Y, Kluckman K, Reddick R, Malinow M R, Maeda N. Mice deficient in cystathionine beta-synthase: animal models for mild and severe homocyst(e)inemia. Proc Natl Acad Sci U S A. 1995;92:1585–1589. doi: 10.1073/pnas.92.5.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Jhee K H, Hua X, DiBello P M, Jacobsen D W, Kruger W D. Modulation of cystathionine beta-synthase level regulates total serum homocysteine in mice. Circ Res. 2004;94:1318–1324. doi: 10.1161/01.RES.0000129182.46440.4a. [DOI] [PubMed] [Google Scholar]
- Kraus J P, Janosik M, Kozich V, Mandell R, Shih V, Sperandeo M P, Sebastio G, de Franchis R, Andria G, Kluijtmans L A, Blom H, Boers G H, Gordon R B, Kamoun P, Tsai M Y, Kruger W D, Koch H G, Ohura T, Gaustadnes M. Cystathionine beta-synthase mutations in homocystinuria. Hum Mutat. 1999;13:362–375. doi: 10.1002/(SICI)1098-1004(1999)13:5<362::AID-HUMU4>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Wang L, Chen X, Tang B, Hua X, Klein-Szanto A, Kruger W D. Expression of mutant human cystathionine beta-synthase rescues neonatal lethality but not homocystinuria in a mouse model. Hum Mol Genet. 2005;14:2201–2208. doi: 10.1093/hmg/ddi224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aljamali M N, Margaritis P, Schlachterman A, Tai S J, Roy E, Bunte R, Camire R M, High K A. Long-term expression of murine activated factor VII is safe, but elevated levels cause premature mortality. J Clin Invest. 2008;118:1825–1834. doi: 10.1172/JCI32878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumfelt L L, Zhou Y, Rowley B M, Shinton S A, Hardy R R. Lineage specification and plasticity in CD19- early B cell precursors. J Exp Med. 2006;203:675–687. doi: 10.1084/jem.20052444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth G K. Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Stat Appl Genet Mol Biol. 2004;3:Article3. doi: 10.2202/1544-6115.1027. [DOI] [PubMed] [Google Scholar]
- Gentleman R C, Carey V J, Bates D M, Bolstad B, Dettling M, Dudoit S, Ellis B, Gautier L, Ge Y, Gentry J, Hornik K, Hothorn T, Huber W, Iacus S, Irizarry R, Leisch F, Li C, Maechler M, Rossini A J, Sawitzki G, Smith C, Smyth G, Tierney L, Yang J Y, Zhang J. Bioconductor: open software development for computational biology and bioinformatics. Genome Biol. 2004;5:R80. doi: 10.1186/gb-2004-5-10-r80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudd S H, Levy H L, Kraus J P. Disorders in transsulfuration. Scriver C R, Beaudet A, Sly W, Valle D, editors. New York, NY, USA: McGraw-Hill; The Metabolic Basis of Inherited Disease. 2001:2007–2056. [Google Scholar]
- Munro S, Pelham H R. A C-terminal signal prevents secretion of luminal ER proteins. Cell. 1987;48:899–907. doi: 10.1016/0092-8674(87)90086-9. [DOI] [PubMed] [Google Scholar]
- Hossain G S, van Thienen J V, Werstuck G H, Zhou J, Sood S K, Dickhout J G, de Koning A B, Tang D, Wu D, Falk E, Poddar R, Jacobsen D W, Zhang K, Kaufman R J, Austin R C. TDAG51 is induced by homocysteine, promotes detachment-mediated programmed cell death, and contributes to the cevelopment of atherosclerosis in hyperhomocysteinemia. J Biol Chem. 2003;278:30317–30327. doi: 10.1074/jbc.M212897200. [DOI] [PubMed] [Google Scholar]
- Robert K, Maurin N, Ledru A, Delabar J, Janel N. Hyperkeratosis in cystathionine beta synthase-deficient mice: an animal model of hyperhomocysteinemia. Anat Rec A Discov Mol Cell Evol Biol. 2004;280:1072–1076. doi: 10.1002/ar.a.20082. [DOI] [PubMed] [Google Scholar]
- Gupta S, Wang L, Hua X, Krijt J, Kozich V, Kruger W D. Cystathionine beta-synthase p.S466L mutation causes hyperhomocysteinemia in mice. Hum Mutat. 2008;10:48–54. doi: 10.1002/humu.20773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert K, Maurin N, Vayssettes C, Siauve N, Janel N. Cystathionine beta synthase deficiency affects mouse endochondral ossification. Anat Rec A Discov Mol Cell Evol Biol. 2005;282:1–7. doi: 10.1002/ar.a.20145. [DOI] [PubMed] [Google Scholar]
- Ji C, Kaplowitz N. Betaine decreases hyperhomocysteinemia, endoplasmic reticulum stress, and liver injury in alcohol-fed mice. Gastroenterology. 2003;124:1488–1499. doi: 10.1016/s0016-5085(03)00276-2. [DOI] [PubMed] [Google Scholar]
- Werstuck G H, Lentz S R, Dayal S, Hossain G S, Sood S K, Shi Y Y, Zhou J, Maeda N, Krisans S K, Malinow M R, Austin R C. Homocysteine-induced endoplasmic reticulum stress causes dysregulation of the cholesterol and triglyceride biosynthetic pathways. J Clin Invest. 2001;107:1263–1273. doi: 10.1172/JCI11596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glushchenko A V, Jacobsen D W. Molecular targeting of proteins by L-homocysteine: mechanistic implications for vascular disease. Antioxid Redox Signal. 2007;9:1883–1898. doi: 10.1089/ars.2007.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosharov E, Cranford M R, Banerjee R. The quantitatively important relationship between homocysteine metabolism and glutathione synthesis by the transsulfuration pathway and its regulation by redox changes. Biochemistry. 2000;39:13005–13011. doi: 10.1021/bi001088w. [DOI] [PubMed] [Google Scholar]
- Malloy M H, Rassin D K, Gaull G E. Plasma cyst(e)ine in homocyst(e)inemia. Am J Clin Nutr. 1981;34:2619–2621. doi: 10.1093/ajcn/34.12.2619. [DOI] [PubMed] [Google Scholar]
- Ueland P M. Pharmacological and biochemical aspects of S-adenosylhomocysteine and S-adenosylhomocysteine hydrolase. Pharmacol Rev. 1982;34:223–253. [PubMed] [Google Scholar]
- Steegers-Theunissen R P, Boers G H, Trijbels F J, Finkelstein J D, Blom H J, Thomas C M, Borm G F, Wouters M G, Eskes T K. Maternal hyperhomocysteinemia: a risk factor for neural-tube defects? Metab Clin Exp. 1994;43:1475–1480. doi: 10.1016/0026-0495(94)90004-3. [DOI] [PubMed] [Google Scholar]
- Bonaa K H, Njolstad I, Ueland P M, Schirmer H, Tverdal A, Steigen T, Wang H, Nordrehaug J E, Arnesen E, Rasmussen K. Homocysteine lowering and cardiovascular events after acute myocardial infarction. N Engl J Med. 2006;354:1578–1588. doi: 10.1056/NEJMoa055227. [DOI] [PubMed] [Google Scholar]
- Albert C M, Cook N R, Gaziano J M, Zaharris E, MacFadyen J, Danielson E, Buring J E, Manson J E. Effect of folic acid and B vitamins on risk of cardiovascular events and total mortality among women at high risk for cardiovascular disease: a randomized trial. JAMA. 2008;299:2027–2036. doi: 10.1001/jama.299.17.2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonn E, Yusuf S, Arnold M J, Sheridan P, Pogue J, Micks M, McQueen M J, Probstfield J, Fodor G, Held C, Genest J., Jr Homocysteine lowering with folic acid and B vitamins in vascular disease. N Engl J Med. 2006;354:1567–1577. doi: 10.1056/NEJMoa060900. [DOI] [PubMed] [Google Scholar]
- Harding H P, Zhang Y, Zeng H, Novoa I, Lu P D, Calfon M, Sadri N, Yun C, Popko B, Paules R, Stojdl D F, Bell J C, Hettmann T, Leiden J M, Ron D. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol Cell. 2003;11:619–633. doi: 10.1016/s1097-2765(03)00105-9. [DOI] [PubMed] [Google Scholar]
- Barbosa-Tessmann I P, Chen C, Zhong C, Schuster S M, Nick H S, Kilberg M S. Activation of the unfolded protein response pathway induces human asparagine synthetase gene expression. J Biol Chem. 1999;274:31139–31144. doi: 10.1074/jbc.274.44.31139. [DOI] [PubMed] [Google Scholar]
- Zhang K, Shen X, Wu J, Sakaki K, Saunders T, Rutkowski D T, Back S H, Kaufman R J. Endoplasmic reticulum stress activates cleavage of CREBH to induce a systemic inflammatory response. Cell. 2006;124:587–599. doi: 10.1016/j.cell.2005.11.040. [DOI] [PubMed] [Google Scholar]
- Outinen P A, Sood S K, Pfeifer S I, Pamidi S, Podor T J, Li J, Weitz J I, Austin R C. Homocysteine-induced endoplasmic reticulum stress and growth arrest leads to specific changes in gene expression in human vascular endothelial cells. Blood. 1999;94:959–967. [PubMed] [Google Scholar]
- Leclerc D, Rozen R. Endoplasmic reticulum stress increases the expression of methylenetetrahydrofolate reductase through the IRE1 transducer. J Biol Chem. 2008;283:3151–3160. doi: 10.1074/jbc.M708598200. [DOI] [PubMed] [Google Scholar]
- Colgan S M, Tang D, Werstuck G H, Austin R C. Endoplasmic reticulum stress causes the activation of sterol regulatory element binding protein-2. Int J Biochem Cell Biol. 2007;39:1843–1851. doi: 10.1016/j.biocel.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Mengesdorf T, Jensen P H, Mies G, Aufenberg C, Paschen W. Down-regulation of parkin protein in transient focal cerebral ischemia: A link between stroke and degenerative disease? Proc Natl Acad Sci U S A. 2002;99:15042–15047. doi: 10.1073/pnas.232588799. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.