Abstract
The possible impact upon human visual cortex from saccades to remembered target locations was investigated using fMRI. A specific location in the upper-right or upper-left visual quadrant served as the saccadic target. After a delay of 2400 msecs, an auditory signal indicated whether to execute a saccade to that location (go trial) or to cancel the saccade and remain centrally fixated (no-go). Group fMRI analysis revealed activation specific to the remembered target location for executed saccades, in contralateral lingual gyrus. No-go trials produced similar, albeit significantly reduced effects. Individual retinotopic mapping confirmed that on go trials, quadrant-specific activations arose in those parts of ventral V1, V2, and V3 that coded the target location for the saccade, whereas on no-go trials only the corresponding parts of V2 and V3 were significantly activated. These results indicate that a spatial-motor saccadic task (i.e. making an eye-movement to a remembered location) is sufficient to activate retinotopic visual cortex spatially corresponding to the target location, and that this activation is also present (though reduced) when no saccade is executed. We discuss the implications of finding that saccades to remembered locations can affect early visual cortex, not just those structures conventionally associated with eye-movements, in relation to recent ideas about attention, spatial working memory, and the notion that recently activated representations can be ‘refreshed’ when needed.
Introduction
The neural networks underlying saccadic eye-movements in parietal and frontal cortex, as well as in subcortical structures such as the superior colliculus (SC), are increasingly well characterized (e.g. see Schall and Hanes, 1993; Colby et al., 1995; Kustov and Robinson, 1995; Robinson and Kertzman, 1995; Kustov and Robinson, 1996; Corbetta, 1998; Corbetta et al., 1998; Kimmig et al., 2001; Sereno et al., 2001; McPeek and Keller, 2002; Astafiev et al., 2003; Merriam et al., 2003; Moore and Armstrong, 2003; Moore et al., 2003; Orban et al., 2005; Ipata et al., 2006; Ozyurt et al., 2006; Schluppeck et al., 2006). For example, the frontal eye fields (FEF) has been found to reflect saccade goals and be predictive of subsequent saccade direction and reaction time (e.g. Connolly et al., 2005; Curtis and D’Esposito, 2006), whereas activation in parietal areas may reflect more visual properties related to the saccade target (Merriam et al., 2003; Medendorp et al., 2006).
Here we focus instead on the possible impact of saccadic tasks upon visual cortex (other than those trivially due to retinal changes caused by an actual eye-movement). Because most saccadic research in neuroscience has focused on eye-movement structures in fronto-parietal regions, or in the SC, but much less is known about the possible impacts upon visual cortex. There is now increasing interest in this, particularly in animal work (e.g. Ross et al., 2001; Moore and Armstrong, 2003; Moore et al., 2003). For example, recent neurophysiological work in monkeys (Super et al., 2004; Super and Lamme, 2007) examined pre-saccadic activity in area V1, and found increases in firing rate for neurons whose receptive fields corresponded to the target location for the upcoming saccade.
In humans, previous PET or fMRI studies of saccade-induced effects on visual cortex have typically focused instead on ‘global’ (i.e., spatially unselective) changes found extensively across all of visual cortex, when subtracting static gaze from active gaze-shifts executed either in the presence of visual stimulation, or in darkness (e.g. Paus et al., 1995; Kleiser et al., 2004; Sylvester et al., 2005). It thus currently remains unknown whether saccades can have spatially-specific effects on activity in early human visual cortex, that depend on the target location for a planned and/or executed saccade (though see Eimer et al., 2006; Konen et al., 2007; Macaluso et al., 2007).
In contrast, numerous human studies have now shown that retinotopic visual cortex can be affected in a spatially-specific manner by the direction of ‘attention’, in perceptual tasks that explicitly require visual discriminations at one location or another without any eye-movements (e.g. Kastner et al., 1998; Brefczynski and DeYoe, 1999; Gandhi et al., 1999; Kastner et al., 1999; Somers et al., 1999; Hopfinger et al., 2000; Noesselt et al., 2002; McMains and Somers, 2004; Geng et al., 2006; Ruff and Driver, 2006; Bestmann et al., 2007; Serences and Yantis, 2007). Moreoever, psychophysical studies (Hoffman and Subramaniam, 1995; Kowler et al., 1995; Deubel and Schneider, 1996; Castet et al., 2006) have reported some enhancements of visual judgments at the target location of an upcoming saccade. Such findings have been taken as evidence for possible links between mechanisms for saccade planning and spatial attention. Indeed, it is increasingly argued that overlapping neural networks may be involved in attention effects and saccade plans (Rizzolatti et al., 1987; Weber and Fischer, 1995; Tolias et al., 2001; Moore et al., 2003; Awh et al., 2006a; Eimer et al., 2006 for ERP data). On the other hand, while there have now been many human fMRI studies showing covert ‘attentional’ effects on early visual cortex during perceptual discriminations at one or another peripheral location, without any eye-movements, there has by contrast surprisingly not been any human fMRI study (to our knowledge) specifically assessing whether a saccadic task can influence human visual cortex, in a potentially analogous fashion.
Accordingly, here we used a saccade paradigm during human fMRI, that required saccades to be executed to one or another remembered location on ‘go-trials’, but saccades to be with-held on “no-go” trials. This spatial-motor task was the only requirement and thus was quite unlike conventional ‘attentional’ paradigms, that instead require explicit discrimination of (usually anticipated) peripheral stimuli, without any eye-movements (e.g. Wurtz et al., 1982; Colby et al., 1996; Kastner et al., 1998; Brefczynski and DeYoe, 1999; Gandhi et al., 1999; Silver et al., 2005). Here we examined the spatially-specific impact of the saccade task on human visual cortex, with fMRI. Our saccade task was similar in some respects to the paradigm often used in neurophysiological studies of monkey saccade planning (e.g. Andersen, 1997; Thompson et al., 1997; Basso and Wurtz, 1998; Nakamura and Colby, 2000; Sereno et al., 2001; see also e.g. Schluppeck et al., 2005). A specific target location in a particular quadrant was indicated via placemarkers and a symbolic central cue, which were then extinguished. Participants maintained central fixation (as confirmed with an eye-tracker inside the scanner) until 2400 ms later when an auditory signal indicated symbolically (via pitch, over headphones) whether to execute the saccade (go trials) or to cancel any saccade and maintain central fixation instead (no-go). Thus, go- and no-go trials were exactly equivalent up until the auditory imperative signal.
Some recent authors have suggested that maintaining a visual location across a delay may be equivalent to, or overlap neurally with, spatially attending to that location in the absence of current visual input (e.g. see Awh et al., 1999; Awh and Jonides, 2001; Awh et al., 2006b; Awh and Vogel, 2008). Such processes might arguably be potentially involved in a saccade task requiring eye-movements to a remembered visual location, as here. But to date, Awh and colleagues’ proposal about possible overlap between spatial attention and spatial working memory has, to our knowledge, only been assessed in nonsaccadic tasks. Moreover, as noted above the go- and no-go trials in the present saccadic task were strictly equivalent up to the auditory signal indicating whether to execute or withhold the saccade, so when comparing go and no-go trials (as implemented here), any common attentional and/or spatial working memory aspects should be subtracted out.
Nevertheless, to assess the potential spatial-attention issue, we did present visual probes (bilateral checkerboards irrelevant to our saccadic task) on a random half of trials, during the delay period prior to the auditory signal. If the impact upon visual cortex of planning/remembering a saccade to a given location is equivalent to conventional ‘attention’ effects upon visual cortex, then presumably the visual response to the probe checkerboard should be enhanced at the location currently relevant for the saccade on a given trial.
Finally, we note also that a recent idea emerging in the fMRI literature on various aspects (or extensions) of ‘working memory’ may also have potential ramifications for our saccade paradigm, namely the new concept of ‘refreshing’ (see Johnson et al., 2005; Johnson et al., 2007; Raye et al., 2007; Yi et al., in press). Yi et al. (in press, their p4) propose that “refreshing is a mechanisms by which an active representation is briefly sustained or foregrounded- as in a brief though directed at a just vanished image”; while Raye et al. (2007, their p. 1) suggest that “the result of refreshing presumably is to briefly augment ... activity associated with a recently activated representation”. One might speculate that saccading to a remembered (but no longer stimulated) location might conceivably involve such “refreshing” of the corresponding visual location in early visual cortex. But this requires demonstration, and to out knowledge no previous human study has even examined whether the motor task of saccading to remembered locations can have spatially-specific effects upon human visual cortex. Moreover, prior demonstrations of possible ‘refreshing’ effects (e.g. Johnson et al, 2005, 2007; Yi et al, in press) did not reveal effects in very early visual cortex (instead higher-level areas were affected in more cognitive tasks, although Johnson et al., 2005, their Experiments 2 and 3 did examine refreshing of spatial location in higher areas).
Thus, while there were several potential reasons to anticipate that saccades to remembered locations might potentially influence early visual cortex (see above), this had not been put to decisive empirical test hitherto. Accordingly, here we used human fMRI to assess directly whether activity in regions of visual cortex (including retinotopically mapped areas) would be enhanced at the location of the saccade target. We also tested whether any such effects on visual cortex were similar, or more pronounced, for a saccade that was executed on go-trials, as compared with no-go trial, where saccades had to be withheld or inhibited. Note once again that go- and no-go trials were unpredictably intermixed and identical in all aspects until the auditory tone indicating whether this was a saccadic go or no-go trial. By contrasting executed and cancelled saccades, we could assess the impact of executing contralateral saccades when any processes common to both go and no-go trials (such as retaining the target location) are subtracted out.
The data were analyzed with two complementary fMRI analysis approaches. Initially we performed a random-effects group-SPM analysis in normalized stereotactic space to allow for inference to the population (Friston et al., 1999). We supplemented this with individual analyses of specific retinotopically mapped visual areas (V1 - V3) to identify which particular visual areas were affected, and whether any effects on go or no-go trials were truly specific to the location of the saccade target. To anticipate, our group-SPM results revealed BOLD increases in contralateral visual cortex specific to the direction (upper-left or upper-right) of the saccade target, in regions appropriate for the corresponding quadrant. This effect was significantly greater for go than no-go trials, but nevertheless reliable for the latter also. Retinotopic analyses of individual subjects confirmed spatially-specific effects that corresponded to the remembered saccade target location, in areas V1-V3 for go trials, and in areas V2 and V3 for no-go trials.
Methods
Participants
Seventeen healthy volunteers participated. Data from one were discarded due to a failure to record eye-position adequately. The remaining sixteen subjects (eight females, fifteen right-handed) ranged from 18 to 32 years in age. All were screened for MRI compatibility and gave written informed consent in accord with local ethics. All had normal or corrected visual acuity by self-report.
Experimental design and stimuli
The experiment had a factorial 2 × 2 × 2 design. The factors were saccade executed or withheld (go, no-go); target location (upper-left, upper-right); and bilateral task-irrelevant visual stimulation with the checkerboard probes (present, absent). Every trial (see Figure 1 for a schematic sequence) began with a change in color of the fixation dot, which indicated whether the saccade goal was to the upper right or left. The association of the fixation color (red or green) with saccade-location was counterbalanced across subjects. Simultaneous with the onset of the fixation cue, black dots appeared indicating the exact to-be-remembered saccade location and a non-target location in the opposite hemifield (to avoid unilateral stimulation, see Figure 1). The location of these bilateral dots always appeared within an imaginary 1.4° × 1.4° square, of which the nearest corner was 5° and the furthest corner was 6.4° from the vertical meridian, and an equivalent distance from the horizontal meridian. Dot location was randomly assigned to one of four corners of each imaginary square so as to require subjects to prepare a spatially-specific saccade for each trial. The locations of the target and non-target dots were independent of each other and randomly determined for each trial. This resulted in two possible ‘inner’ and two ‘outer’ saccade target eccentricities which were used for analyzing the spatial specificity of saccades to remembered endpoints (see below).
Figure 1.
Example trial procedure. Each trial began with a colored fixation dot (illustrated in gray here) at bottom-center, plus two black dots, jittered in location, in the upper left and right quadrants. The color of the fixation dot indicated the direction of saccade plan for that trial (upper left or right) and the exact position of the black dots indicated the location of the saccade target on the corresponding side for that trial (the dot on the other side was irrelevant). The peripheral dots disappeared after 1200 ms and were followed by a 1200 ms interval during which either only the fixation dot was visible, or else during which bilateral task-irrelevant checkerboard stimuli could appear for 215 ms at a random point during the interval (as shown in left ‘branch’ of third panels from top). Subjects were informed that any checkerboards were task-irrelevant. Next, an auditory tone signaled to the subject whether to make a saccade to the target (go trial) or to cancel the saccade and hold central fixation (no-go trial). Finally, a 2000-6000 ms blank interval followed before the start of the next trial.
The central cue and peripheral dots were visible for 1200 ms, after which the fixation turned black and the peripheral dots were removed. During the next 1200 ms period, the subject was required only to keep the saccade target in memory while maintaining central fixation. On a random 50% of trials, two contrast-reversing checkerboards (Michelson contrast value = .5) that flickered at 10 Hz for 215 ms (approximately 2 cycles of reversals) were presented during the delay period (see Figure 1, alternative third panels in the sequence shown). The checkerboards were 1.4° × 1.4° visual angle and fully covered the ‘virtual’ square containing all possible saccade-target locations. These brief checkerboards served two purposes. They provided a check for stimulus-responses in regions of visual cortex corresponding to the saccade-target location, within either upper quadrant. Second, they allowed us to test whether there would be any attention-like modulation of the visual response to these checkerboard probes, enhancing the visual response at the location that currently served as the saccade target (in fact no such effect was found, see below). Subjects were informed during behavioral training outside of the scanner that these checkerboards were entirely irrelevant to their saccadic task. The onset of the checkerboards occurred randomly between the offset of the cues and 800 ms later.
At the end of this second 1200 ms period (i.e. 2400 ms since trial onset), a low (300 Hz) or high (600 Hz) auditory tone was played over headphones for 300 ms, indicating whether the subject should make a saccade to the remembered target location as rapidly as possible (go trial) or whether to maintain central fixation instead (no-go), thus cancelling any planned saccade. On go trials, the receptive fields of regions of visual cortex representing the target and non-target locations (as they had been located retinotopically during initial central fixation) would be shifted off the display screen due to the executed saccade. But any such change in visual input during a saccade should not be spatially specific to the retinotopic location of the original saccade target, but rather would arise across much of the retina. Perhaps more importantly, the no-go trials did not involve any actual eye-movement (only retention of the target location), and thus no retinal shifts as confirmed by eye-tracking (see below). The association between tone pitch and go / no-go trials was counterbalanced across subjects. At the end of the trial, a blank inter-trial interval (ISI) was jittered between 2000 - 6000 ms, during which only the black fixation dot remained visible for subjects to reacquire. The duration of the ISI varied randomly to allow for a uniform sampling of event-related BOLD responses across the whole TR.
Any visual stimuli were presented on a constant light gray background (luminance = 4.2 cd/m2) by means of a video projector and a rear projection screen mounted at the back of the magnet bore. The screen was viewed via a mirror system attached to the head coil. Auditory stimuli were presented by MR-compatible headphones. All stimuli were generated and presented by means of the custom toolbox Cogent (http://www.vislab.ucl.ac.uk/Cogent/index.html) running in MATLAB (The Mathworks, Nantick, MA) on a conventional PC.
Retinotopic mapping of four of the subjects (eight hemispheres) was accomplished using a meridian mapping scan of 205 volumes during which standard “bow-tie” checkerboard stimuli were viewed to map the horizontal or vertical meridians. These subjects also completed an additional “stimulus-localizer” run that consisted of peripheral checkerboard squares, identical to the task-irrelevant stimuli in the main experiment, but now presented for longer durations, with higher contrast (Michelson contrast value = 1), and in only one visual quadrant during each block to allow a direct comparison of stimulus responses in opposite upper quadrants (i.e. left minus right, and vice-versa). Each 1.4° × 1.4° checkerboard was presented in either just the upper-left quadrant, with the nearest (bottom-right) corner at 7° from central fixation diagonally (5° horizontally and 5° vertically), or at the corresponding location in the upper-right quadrant instead. In this stimulus-localizer run, 10-second blocks of passive fixation on the central dot alternated with blocks of equal duration during which the checkerboard stimulus was presented either on the left or right (determined randomly for each block, each side presented ten times), while central fixation was maintained. We then compared activation elicited by upper-left and upper-right checkerboards, to localize sectors of retinotopic cortex responding to possible saccade target locations.
Eye movement classification
Eye tracking was performed at 60Hz by means of a long-distance remote infrared eye tracker (ASL 504a, Applied Science Laboratories). We focus on horizontal eye-position, given that we had left or right saccade targets, and that the eye-tracker used has best resolution in the horizontal dimension. Eye traces obtained during scanning for each trial were initially classified as including a saccade if the eye trace exceeded 2.5° of visual angle from the trialwise median position for a period of 480ms or longer, following the auditory tone that signaled whether the prepared saccade should be executed (‘go’) or withheld / cancelled (‘no-go’). Saccades were then classified as leftward or rightward based on the eye-position values that immediately followed saccade onset (the time at which the eye trace first exceeded the fixation point by 2.5° of visual angle). Note that this classification scheme was also checked against a velocity-based criterion (velocity of temporally smoothed [5 frame moving average] eye trace > 25°/sec for a continuous 64ms period (Fischer et al., 1993a; Fischer et al., 1993b; Weber and Fischer, 1995). The two criteria yielded closely corresponding classification (average correlation between the two classification schemes per subject, r = .9, p < .00005, with less than 7% of all trials discrepant) so this was not considered further. Blinks were defined as loss of pupil data for 3 or more timepoints in combination with deviations greater than 13° visual angle, and were replaced with the median eye-position value for that trial. Finally, saccade behavior for each trial was classified as correct or incorrect based on correspondence with instructed trial condition. Saccade error trials and blinks were modeled separately in the fMRI analyses.
Image and Data processing
Functional images were collected on an Allegra 3 Tesla Siemens MR system with standard head coil (Siemens, Erlangen, Germany), as T2*-weighted echoplanar image (EPI) whole-brain volumes (TR 2080 ms). Each functional volume consisted of 32 tilted axial slices (2mm slice thickness, 75% gap, 3 × 3 mm in-plane resolution). Each subject participated in two experimental runs lasting 16.3 minutes each. In addition, standard retinotopic-mapping procedures were run during a separate session for four participants (and thus 8 hemispheres), together with a blocked stimulus-localizer to identify spatial representations corresponding to saccade target positions in retinotopic visual cortex. All imaging parameters for individual mapping were as for the main experiment, with the exception that each functional volume consisted of only 24 axial slices (TR 1560 ms), tilted to ensure coverage of the entire occipital cortex.
Imaging data were analyzed with SPM2 (http://www.fil.ion.ucl.ac.uk/spm2.html). Image preprocessing included realignment and unwarping (Andersson et al., 2001); spatial normalization to the Montreal Neurological Institute (MNI) standard space; and spatial smoothing using a 9 mm FWHM Gaussian kernel for the group random-effects analysis, in accord with the standard SPM approach. For retinotopic analyses in those subjects that were individually mapped, the realigned and unwarped data were coregistered to their individual T1-weighted anatomical images and spatially smoothed with a smaller kernel (6 mm FWHM Gaussian).
Hemodynamic responses to targets in the eight experimental conditions (in the 2 (go, no-go) x 2 (upper-left, upper-right) x 2 (with or without bilateral visual stimulation) design) were modeled by delta functions placed at the beginning of each trial, and convolved with a canonical hemodynamic response function (HRF) and its temporal derivative. All regressors (8 for the conventional HRF, plus a corresponding 8 for its temporal derivatives) covered the full duration of the trial. Note that it was not necessary to model the delay period separately from subsequent saccadic execution or inhibition in our design, because go and no-go trials were fully equivalent up to the point where execution or inhibition were signaled by the auditory imperative stimulus.
In addition to the experimental conditions of interest, the model also contained a regressor representing behavioral errors (see eye movement classification above); a separate one for blinks; a temporal high-pass filter (128 sec cut-off); and an AR(1) process to account for temporal autocorrelations (Friston et al., 2002). Parameter estimates for all regressors were obtained by maximum-likelihood estimation.
The analysis in stereotactic space was performed as random-effects group SPM with 16 participants, using one-sample t-tests on contrast images of HRF parameter estimates. Results from the whole-brain analysis of go minus no-go trials are reported at p < .05, FWE-corrected across the whole brain. Contrasts of left minus right go trials (and vice-versa) involved prior anatomical hypotheses (i.e. ventral contralateral visual cortex, given the location of saccade targets in upper visual quadrants), and are therefore reported at p < .001 uncorrected, with a cluster size threshhold (k > 16) determined as the number of voxels per cluster expected from random field theory (see Table 1 and Worsley et al., 1992; Friston et al., 1996). All group SPM results were projected onto a mean structural image created from T1-weighted high resolution anatomical scans that were available for 14/16 of our participants.
Table 1. Contrasts of right versus left go trials.
| Anatomical location | Cluster size | Z value | XYZ (mm) |
|---|---|---|---|
| Go right minus left | |||
| L ventral occipital | 189 | 4.29 | -4 -82 -4 |
| L dorsal occipital* | 443 | 4.46 | -24 -84 22 |
| L middle temporal | 18 | 3.35 | -44 -74 -10 |
| Go left minus right | |||
| R ventral occipital | 249 | 4.34 | 12 -82 -8 |
| L dorsal occipital* | 150 | 4.07 | -22 -104 6 |
| L dorsal occipital* | 54 | 3.85 | -8 -92 18 |
The left dorsal occipital activations most likely reflect the presence of the visible light associated with the light-source for the near-infrared eye-tracker (ASL 504a, Applied Science Laboratories), which always appeared in the bottom right visual field and therefore shifted position along the retina in different directions for upper-left and upper-right executed saccades (while always remaining in the bottom-right quadrant, due to its eccentricity of approximately 14 degrees). Note that such retinal changes due to saccades did not arise on no-go trials, which provide a further critical test of our hypotheses, see main text.
We also conducted Volume-of-interest (VOI) analyses of responses in the group lingual-gyri clusters initially identified as showing effects of contralateral (minus ipsilateral) saccade direction in the go-conditions. For these analyses, we extracted the mean signal from all voxels within the functionally defined VOI, for further comparisons that were orthogonal to the initial VOI-defining contrast and that directly compared the extracted mean signal data by paired t-tests.
To determine borders between retinotopic visual areas V1, V2 and V3, in individually mapped subjects, we used standard mapping procedures with alternating 10-second blocks of checkerboard patterns covering either the horizontal or vertical meridian (see Sereno et al., 1995; Kastner et al., 1998; Haynes and Rees, 2005; Ruff et al., 2006). Segmentation and flattening were performed with mrGray and mrFlatmesh software, http://white.stanford.edu/~brian/mri/segmentUnfold.htm, (Teo et al., 1997; Wandell et al., 2000). Translation of SPM2 analyze image-space into mrGray functional overlay space was done with in-house software (see Haynes and Rees, 2005; Ruff et al., 2006). Functional data from our blocked stimulus-localizer (see above) in individuals were used to locate a saccade-target region of interest (ROI) within each of the individually defined cortical visual areas V1-V3. Data extracted from individual retinotopically defined ROIs were then analyzed using nonparametric sign-test due to the smaller N (total of 8 hemispheres) in the retinotopic approach, for which such a sample-size is fairly standard (e.g.Schluppeck et al., 2005; Silver et al., 2006).
Results
Oculomotor behavior
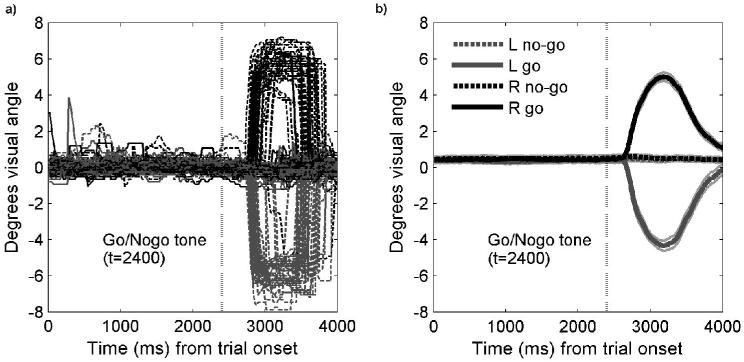
Subjects were instructed to saccade to the remembered target location as quickly and accurately as possible, only when the auditory signal after the delay indicated a go rather than no-go trial. Trials were discarded from the summary eye-position data if there was a blink during the delay period between the dot display and the auditory signal, or if an error occurred (i.e., a saccade on a no-go trial, as on 10% of such trials; or a saccade in the wrong direction on a go trial, as on 1% of such trials). Figure 2a plots for a single representative subject the trial-wise horizontal eye-position data traces grouped by condition of interest (i.e., go trials with saccade executed to upper-left or upper-right quadrant, or no-go trials where a saccade was not executed). The corresponding group average (+/- 1 SE) for all trials and participants included in the fMRI analysis is plotted in Figure 2b, grouped again by conditions of interest. Note that the smoothly curved group data in Figure 2b reflects averaging across trials and subjects, each of which had slightly different saccade-onset latencies (cf. the less smooth single-trial data in Figure 2a). The pattern in Figure 2 clearly indicates that saccades were correctly executed to the right or left in the go trials, and that central fixation was maintained in the retained no-go trials that were included for fMRI data analysis.
Figure 2.
a) Individual eye position traces from all trials in one participant for left and right go and no-go conditions. b) Mean (and hence smooth) eye-position traces, with standard error of the mean, for left and right go and no-go conditions across all participants. The gray vertical dashed line represents the time at which the auditory go or no-go tone occurred. Note the maintenance of central fixation on the no-go trials.
As expected, there was no difference in mean saccadic reaction times for left or right go trials following the auditory signal to execute the prepared saccade (left = 174 msec, right = 175 msec, t(15) < 0.5, n.s.). We further analyzed the horizontal amplitude of saccades for the go trials. The actual position of the dot target (first panel in Figure 1) in a given quadrant had been randomly assigned to one of the four corners of an imaginary 1.4° × 1.4° square, the lower bottom corner of which was 5° horizontal visual angle from the central fixation point (see Methods). We varied this exact position so as to encourage participants to plan a spatially-specific saccade on each trial, rather than simply using a single default saccade amplitude to one side or the other. Analyses of the eye data confirmed that participants did indeed adjust their saccades on go trials according to the specific target-eccentricity on a trial-by-trial basis, as our task demanded. When comparing the horizontal amplitude of saccades for a target dot that had been located at an ‘outer’ or ‘inner’ corner of the imaginary square on one side or the other, we found that the mean horizontal amplitude for the observed saccades on go trials were as follows: outer left = -5.4°; inner left = -4.4°; outer right = 5.4 °; inner right = 4.5 ° (negative values represent leftward and positive values represent rightward saccades from fixation). The differences in amplitude between outer and inner targets were reliable (t(15) = 6.8, p < .001, for the left; t(15) = 4.9, p < .001, for the right), confirming that the saccade task was performed with significant spatial precision.
Group fMRI Results: Visual cortex activity specific to the target quadrant for the saccade
Our main question of interest concerned the presence of activation in visual cortex that related specifically to the direction (left minus right, or vice-versa) of the instructed saccade. Although we later turn to individual retinotopic analyses of visual areas V1-V3, we begin with a group-SPM random-effects analysis in stereotactic space that allows for inference at the population level (Friston et al., 1999). For this analysis, we anticipated effects in ventral visual cortex, known to contain representations of the contralateral upper visual field (e.g. DeYoe et al., 1996; Tootell et al., 1998; van Essen, 2003; Wandell et al., 2005), to which saccades were directed in our task. For the go trials on which saccades were executed, we contrasted upper-left saccades to upper-right saccades (former minus the latter, or vice-versa). These two separate comparisons revealed symmetric regions in the contralateral left lingual gyrus for right minus left saccades, and in the contralateral right lingual gyrus for left minus right saccades (Figure 3ab; see also Table 1 for whole brain results, included only for completeness). The activations within ventral occipital lingual gyri for trials with executed saccades were thus specific to the visual quadrant containing the saccade target (since ventral lingual gyri represent the upper contralateral quadrant; see also retinotopic analyses below). Note also that the two symmetric patterns in either occipital hemisphere can be considered independent replications of each other.
Figure 3.

a) Activations for right minus left (blue) and left minus right (red) go trials in the contralateral lingual gyrus. These clusters reflect quadrant-appropriate activations associated with the saccade target location. b) Average percent signal change and standard error of the differences for the relevant contrast from the left and right lingual gyrus clusters, showing significant t-test differences (indicated by stars) between contralateral minus ipsilateral target trials in the go conditions (rightmost pairs of bars) and the no-go conditions (leftmost pairs of bars). Note the independent replication of the results for the two lingual gyrus clusters. c) Average percent signal change and s.e.d. from the left and right lingual gyrus clusters, now separated for the random half of trials with visual checkerboard stimulation during the delay period (more saturated colors, ‘Vis’) versus those without (less saturated colors, ‘NoVis). Visual stimulation produced a general increase in activation (‘Vis’ bars in plots give higher values than the comparable (paired) NoVis condition), but this did not interact with the separate effect from the side of the saccade target (i.e. contralateral ‘right’ conditions give higher values than corresponding ipsilateral ‘left’conditions for the left lingual cluster, regardless of the Vis/NoVis manipulation; and likewise contralateral ‘left’ conditions give higher values than ipsilateral ‘right’ for the right lingual gyrus cluster, again regardles of Vis/NoVis).
We further corroborated that these lingual gyri activations corresponded to the locations of the saccade targets, by extracting the mean response of these same regions (now treated as VOIs) to the task-irrelevant visual stimuli that were presented around the location of possible saccade targets during the delay period on a random half of the trials (see alternative third panels from top in Figure 1). This confirmed that the lingual-gyrus regions that had shown a significant effect of saccade direction on go-trials indeed also responded to the bilateral visual stimuli covering the possible locations of saccade targets (for extracted left lingual cluster, t(15) = 3.8, p < .005; right lingual cluster, t(15) = 3.1, p < .005 Moreover, we tested for any differences between visual responses when the bilateral checkerboards (irrelevant to the saccade task) were present versus absent, based on the location of the saccade target. If prioritization of the saccade goal operated like conventional ‘attention’ in peripheral visual-discrimination tasks (without any saccade, e.g. Kastner et al., 1998; Brefczynski and DeYoe, 1999; Gandhi et al., 1999; Kastner et al., 1999), then one would expect greater visual activation contralateral to a checkerboard at the location of the saccade goal. But no such interaction between presence of (bilateral) visual stimulation and side of the saccade target was found (left lingual cluster, F(1,15) = 0.005; right lingual cluster, F(1,15) = 0.28; see Figure 3c, which clearly shows that the visual response to probe checkerboards is strictly additive to the effect from side of the saccade target location).
In order to test whether activations specific to saccade direction were present not only when a saccade was executed (go trials), but also when the saccade to the remembered location was withheld or cancelled (no-go trials), we further interrogated the response of the same lingual clusters during the no-go trials, comparing left minus right or right minus left saccade targets. This confirmed higher activations for trials when the remembered (but cancelled) saccade was contralateral (versus ipsilateral). As with the go trials, this no-go result was independently replicated for each hemisphere (left lingual cluster, t(15) = 2.0, p <.05; for the right lingual gyrus cluster, t(15) = 2.4, p < .05; see Figure 3b). These results demonstrate activation specific to the intended saccade target location in visual cortex, even when saccades were subsequently withheld or cancelled so that no changes in retinal input occurred. Thus, merely preparing a saccade to a remembered location was sufficient to produce direction-specific activation within regions of occipital visual cortex (lingual gyri) that represent the target quadrant.
This difference between contralateral and ipsilateral saccade direction in the lingual gyrus ROIs was greater for saccades that were actually executed, as confirmed by significant interactions between trial type (go or no-go) and saccade direction (contralateral or ipsilateral) for activity in both of the lingual-gyrus regions shown in Figure 3a (interaction on the extracted data for left lingual gyrus cluster, F(1,15) = 8.9, p < .05; for right lingual gyrus cluster, F(1,15) = 5.8, p < .05; see Figure 3b). These ROIs had originally been selected for their go-trial effect (so as then to allow a stringent unbiased test for no-go trials in the same region). Therefore, to preclude any selection artifact when assessing the interaction, we next selected lingual gyrus clusters by the main effect of left-versus-right, or vice-versa, collapsing across go and no-go trial types when selecting. Extracted values from these clusters showed the same pattern as for the original clusters, and indeed the outcomes did not differ significantly between the two methods for selecting clusters. In any case, an analogous interaction pattern (i.e. stronger effects on visual cortex for go than no-go trials, albeit with significant effects in both case) was also found in the individual analyses of retinotopic visual areas (see below), in which retinotopic regions-of-interest were selected based on entirely independent stimulus-localizers (see Methods), so that no selection bias could promote an interaction. The larger effects for go than no-go trials suggests that trials with actual saccades produce additional impact on visual cortex at the saccade target location (see also Super et al., 2004, for related single-cell data; Super and Lamme, 2007).
In addition to the directional effects of interest described above, the main effect of saccades per se (i.e. go minus no-go trials overall, regardless of saccade direction), involved widespread activation of both the ventral and dorsal occipital lobe (see Table 2). This spatially nonspecific outcome, when saccade direction is not taken into account, is similar to previous report of saccadic effects (Paus et al., 1995; Kleiser et al., 2004; Sylvester et al., 2005) that did not consider any directional or spatially-specific factor. For completeness, we also implemented the reverse contrast (of no-go minus go trials), since saccade inhibition or cancellation on the no-go trials might arguably involve some additional neural processes (e.g. Pare and Hanes, 2003; Brown et al., 2006; Brown et al., 2008). This no-go minus go contrast did not reveal any effects on occipital cortex, nor any interactions with the side (left or right) of the visual target, but some effects beyond visual cortex were apparent, and are reported at p<.001 uncorrected for completeness (see Table 3). In addition to regions recently implicated in saccade inhibition (Brown et al. 2006, 2008) or more generally in task-switching (e.g. MacDonald et al., 2000; Johnston et al., 2007), this contrast also revealed some of the midline structures in the putative “default network” (e.g. Gusnard and Raichle, 2001) that has traditionally been associated with less active trials. But our most critical findings from the group SPM approach were that saccades to the upper-left versus upper-right produced specific contralateral activations in the lingual gyri, and that a similar, albeit smaller effect, was also found on no-go trials when the target location was retained up to the point where the auditory signal indicated that the saccade should be cancelled.
Table 2. Overall contrast of go minus no-go.
| Anatomical location | Cluster size | Z value | XYZ (mm) |
|---|---|---|---|
| L and R occipital cortex | 1688 | 5.58 | -32 -74 -6 |
| R cerebellum | 48 | 5.42 | 30 -70 -26 |
| R cuneus | 17 | 5.34 | 10 -100 16 |
| R cerebellum | 22 | 5.26 | 4 -76 -28 |
| R angular gyrus | 47 | 5.22 | 38 -80 24 |
| L angular gyrus | 10 | 5.15 | -22 -72 40 |
| 6 | 5.1 | -32 -88 30 | |
| L middle frontal gyrus | 3 | 5.08 | -50 -6 50 |
| 7 | 5.07 | -54 -2 40 |
Table 3. Contrast of no-go minus go trials.
| Anatomical location | Cluster size | Z value | XYZ (mm) |
|---|---|---|---|
| R anterior middle temporal lobe | 1664 | 4.82 | -46 2 -40 |
| L anterior middle temporal lobe | 2559 | 4.59 | 46 -4 -38 |
| R supramarginal gyrus | 561 | 4.28 | 58 -54 46 |
| L supramarginal gyurs | 375 | 3.89 | -42 -66 40 |
| R Middle frontal gyrus | 103 | 3.60 | 36 18 42 |
| L middle frontal gyrus | 194 | 4.66 | -52 28 10 |
| L middle temporal gyrus | 92 | 4.37 | -42 -42 6 |
| L insula | 426 | 4.18 | -36 -22 22 |
| R posterior cingulate | 105 | 3.90 | 12 -36 18 |
| R anterior cingulate | 61 | 3.69 | 16 58 6 |
| 56 | 3.56 | 2 30 2 | |
| L anterior cingulate | 428 | 4.14 | -16 52 6 |
| 56 | 3.38 | -8 56 32 | |
| Medial precentral gyrus | 195 | 3.88 | -4 -28 64 |
| L postcentral gyrus | 70 | 3.54 | -42 -28 60 |
fMRI analysis of individual retinotopically mapped visual areas, V1-V3
To allow further specificity in our conclusions about human visual cortex, we next retinotopically mapped visual areas V1, V2 and V3 in four participants (8 hemispheres) using standard procedures (see Methods, and also Sereno et al., 1995; Kastner et al., 1998; Haynes and Rees, 2005; Ruff et al., 2006). For these participants, we also mapped the specific retinotopic region of interest (ROI) within V1, V2, and V3 that responded to the target area in the upper-left or upper-right visual quadrant, by means of the separate blocked stimulus-localizer scan that was analyzed for individuals (see Methods). These localizer stimuli consisted of unilateral checkerboard stimuli presented at the locations in the upper-left or upper-right visual quadrant to which saccades had been planned or directed in the main experiment (see Figure 1). Each ROI was defined by the cluster surrounding the peak activation for contralateral minus ipsilateral checkerboards in each visual area (i.e. within V1, V2, V3; see Figure 4ab). The retinotopic ROIs all fell within ventral visual cortex in each area, as expected for retinotopic representations of particular locations within upper contralateral quadrants. These individual retinotopic ROIs showed some overlap (∼ 15-30% of voxels) with the bilateral lingual gyrus clusters from the group analyses. Such incomplete overlap would be expected, since retinotopic mapping captures individual functional anatomy in a different way than smoothed and normalized SPMs, and is arguably more appropriate for assessing specific visual areas (see Aine et al., 1996).
Figure 4.
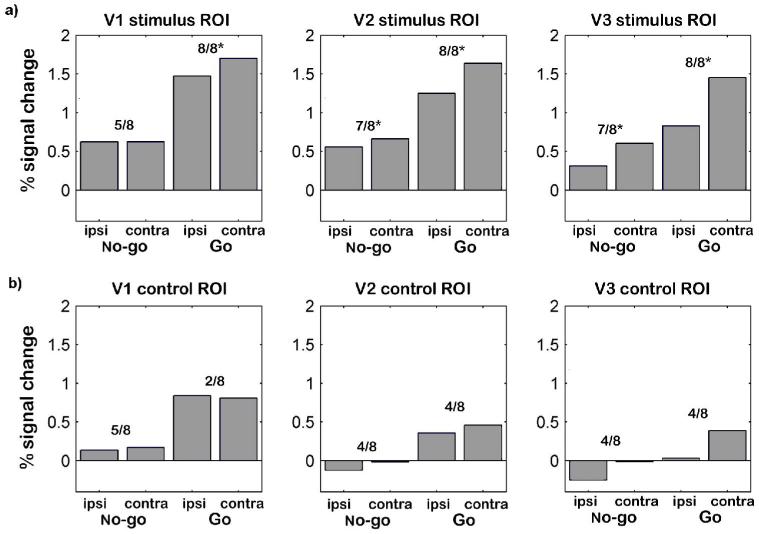
a) Flatmaps from one illustrative subject for each hemisphere, with borders between adjacent retinotopic regions drawn in black, and also showing ROIs defined by the contrast between contralateral and ipsilateral localizer checkerboard stimuli in upper quadrants for ventral V1, V2, and V3 (red-outlined “hot” colored regions). Dorsal ‘control’ ROIs were determined by reflecting the corresponding ventral ROI across the V1 horizontal meridian and then placing it within dorsal visual cortex at a comparable eccentricity and distance from neighboring visual borders (red-outlined ‘cool’ colored regions). b) Top row: Mean percent signal change from ventral retinotopic ROIs defined by response to contralateral localizer checkerboard stimuli presented in the upper quadrants. Ratios correspond to the number of hemispheres (out of the 8) showing greater activation for the contralateral than ipsilateral target localiser stimulus. Visual areas with significant (sign test) number of hemispheres showing this effect are starred (*). Bottom row: Mean percent signal change in dorsal ‘control ROIs’ in response to blocked localizer checkerboard stimuli in the contralateral or ipsilateral upper quadrant.
The mean signal change for contralateral and ipsilateral saccade trials from the main experiment within these retinotopically mapped and stimulus-localized ROIs is plotted in Figure 5a. Activation for these ROIs showed a pattern analogous to those from the stereotactic group analysis presented earlier (cf. Figure 3ab), but adds further specificity concerning the particular retinotopic visual cortical areas affected by saccade direction. For the go trials, the ventral ROIs in areas V1, V2 and V3 showed greater activity for contralateral than ipsilateral saccades to the upper quadrant (Figure 5a, rightmost two bars in each plot). This was true for 8/8 hemispheres (p < .005 by sign test) in all three areas, demonstrating a clear preference within each retinotopic ROI for contralateral saccade trials. For the no-go trials (leftmost two bars in each plot of Figure 5a), a similar pattern of more activation for contralateral than for ipsilateral saccades to the upper quadrant (now concerning only planned but cancelled saccades) was observed for 7/8 hemispheres (p < .05) in ventral V2 and V3, but was not reliable (only 5/8 hemispheres) in ventral V1. These retinotopic results accord with but extend the initial group analysis, in demonstrating quadrant-specific saccadic effects that were stronger for go than no-go trials in V1, V2, and V3 (sign test of interaction pattern, p < .005 in V1 and V2; p < .05 in V3), but that nevertheless remained reliable in V2 and V3 for no-go trials where saccades were not executed.
Figure 5.
a) Percent signal change for stimulus-localized retinotopic saccade-target ROIs in ventral V1, V2, V3, as a function of saccadic condition. Ratios given above pairs of bars represent the number of hemispheres (out of 8) showing greater activation for contralateral than ipsilateral saccade targets. Visual areas with a significant (by sign test) number of hemispheres in the expected direction for a particular effect (go or no-go) are starred (*) correspondingly. Ventral V1, V2 and V3 ROIs all showed significant effects of contralateral versus ipsilateral saccade targets for go trials; while V2 and V3 (but not V1) also showed this for no-go trials. b) Percent signal change from corresponding ‘control’ ROIs in dorsal V1, V2, V3 (see main text). Ratios given above a particular pair of conditions represent the number of hemispheres showing greater activation for contralateral compared to ipsilateral saccade targets.
To assess whether any saccade-related effects were genuinely specific to these ROIs, and thus spatially specific to retinotopic representations of the saccade-target location within visual cortex, we selected analogous ‘control ROIs’ from dorsal visual cortex for V1-V3 (Figure 4ab). Control ROIs in dorsal visual cortex were determined by initial ‘mirroring’ of the corresponding ventral visual area ROI around the horizontal meridian that divides ventral and dorsal V1, followed by minimal displacements within the dorsal visual area on the flattened representation of each hemisphere in order to achieve maximum correspondence in eccentricity and relative distance from neighboring visual borders as for the original ventral ROI (see outlined control ROIs in dorsal V1-V3, Figure 4a). These dorsal ‘control ROIs’ correspond to representations of the lower contralateral visual quadrants, and therefore did not respond visually to the possible saccade target locations (as confirmed by our finding of no activity difference in these regions between contralateral versus ipsilateral blocked checkerboard stimulation in the upper quadrants; see Figure 4b). Hence, these dorsal control-ROIs should not be affected by saccade direction, if those directional effects do indeed concern only retinotopic representations of the upper-quadrant saccade-target locations in ventral visual cortex.
Consistent with our expectation that dorsal ROIs should not show directional effects for saccades to the upper visual quadrants, there were no effects when comparing contralateral versus ipsilateral saccade-direction conditions, neither for go trials nor for no-go trials (see Figure 5b). Instead, the dorsal control ROIs only show the nonspecific general increase in activation whenever saccades were executed (go minus no-go trials), regardless of the saccade direction (see also Paus et al., 1995; Kleiser et al., 2004; Sylvester et al., 2005, for similar global effects), for all 8 hemispheres in V1, V2 and V3 (Figure 5b). This overall global effect may simply reflect retinal changes induced by eye-movements, but as discussed in more detail below, such retinal changes cannot explain our critical findings of quadrant-specific increases in activation corresponding to the saccade target location. These more specific effects were found only within the contralateral ventral regions of retinotopic cortex that represented the upper-quadrant target location for the saccade. Moreover, they arose in V2 and V3 even on no-go trials, where no eye-movement was executed and thus no retinal changes were induced.
Discussion
We found evidence for spatially-specific activation in retinotopic human visual cortex in a task requiring saccades, to a remembered target location, to be executed or withheld on hearing an auditory imperative stimulus. Greater activation was found in ventral visual cortex for saccades to the contralateral upper quadrant (versus the ipsilateral upper quadrant; see Figure 3ab). This outcome was found with SPM-group analysis in the occipital lingual gyri, analogously for both hemispheres. Results from right and left lingual gyrus thus independently replicated each other: Both showed higher activity levels for contralateral than ipsilateral saccades to the upper quadrant. This same pattern was also found (albeit at a reduced level, see Figure 3b) for no-go trials, on which the saccade to the remembered location was withheld or cancelled, as confirmed by eye-tracking (see Figure 2).
Individual retinotopic analyses confirmed that the contralateral activations for go-trials occurred within sectors of ventral V1, V2 and V3 that represent (i.e. respond to visual stimulation at) the specific location of contralateral saccade targets in the upper quadrant (see Figure 5a). A similar outcome was found in V2 and V3 (though not V1) for the no-go trials, on which the saccade was not executed (see Figure 5a). No equivalent saccade-direction effects were found in analogous dorsal ‘control ROIs’ representing the lower quadrants (see Figure 5b), thus further confirming the spatial specificity of our effects to saccade target locations.
In addition to the spatially-specific effects of primary interest, trials with versus without executed saccades (i.e go minus no-go) also produced diffuse increases in visual cortical activation across all visual cortex (see also Paus et al., 1995; Kleiser et al., 2004; Sylvester et al., 2005). In the present paradigm, these spatially nonspecific effects may simply reflect global retinal changes consequent to executed saccades. The opposite global contrast (i.e. of no-go minus go) did not affect occipital cortex, and did not lead to any interactions with target side. Thus, withholding or inhibiting a specific saccade did not specifically affect BOLD signal in visual cortex. Nevertheless, there were some effects well beyond visual cortex for no-go minus go-trials (see Table 3), that may reflect response-inhibition, along with areas in the “default” network commonly associated with less active trial-types (e.g. Gusnard and Raichle, 2001). Some of the regions showing a no-go > go effect overlapped with those from a recent fMRI study of saccade inhibition by Brown et al. (2006; Brown et al, 2008) including the supramarginal gyrus and anterior cingulate, suggesting that here the no-go auditory signal resulted in cancellation of a saccade to the remembered location rather than the lack of a motor response being purely passive on no-go trials. But any such saccade inhibition, specific to no-go trials, did not have an effect on visual cortex.
Returning to the most important novel findings in our study, which all did concern visual cortex, the spatially-specific effects on go trials were found in sectors of visual cortex that clearly represented the saccade target location in particular, as shown by the overlap with stimulus-responsive activations in the group analysis, and by the results for stimulus-localized ROIs (c.f. dorsal control ROIs) in the retinotopic analyses. These spatially-specific effects were also found to arise to some extent even on no-go trials, for which the eye did not shift, as confirmed with on-line tracking during scanning. Although the effects on visual cortex for no-go trials were significantly smaller than those when actually executing a saccade on go-trials (and had the qualitative difference that only V2 and V3 were affected on the no-go trials, not V1 unlike go-trials), the no-go findings indicate that even when the saccade is not ultimately executed, retaining the location of the saccade target can modulate visual cortex corresponding to the location of the planned saccade.
As already noted in our Introduction, some authors have proposed that saccadic-planning might be equivalent to, or show some overlap with, processes of covert spatial attention (e.g. Rizzolatti et al., 1987; Weber and Fischer, 1995; Tolias et al., 2001; Moore et al., 2003; Awh et al., 2006a; Eimer et al., 2006). Furthermore, Awh and colleages (e.g. Awh and Jonides, 2001; Awh et al., 2006b) have proposed that retaining a visual location in spatial working memory may involve maintaining covert spatial attention to that location (although they had not examined this during saccadic tasks). Finally, an emerging view is that recently activated representations may be ‘refreshed’ in a top-down manner when they become needed again (Johnson et al., 2005; Johnson et al., 2007; Raye et al., 2007), a concept that might arguably be extended in a novel manner to saccades to remembered locations, as studied here. Below we discuss how these various, potentially related, cognitive perspectives may shed light on our new findings.
While it has often been suggested that saccade-plans may induce attention-like effects (Hoffman and Subramaniam, 1995; Kowler et al., 1995; Deubel and Schneider, 1996; Castet et al., 2006), surprisingly this had never hitherto been assessed with detailed fMRI of retinotopic visual cortex in humans in a saccade paradigm. While Sereno et al. (2001) did report some “spotty” effect on visual cortex in their particular saccade task, that was mentioned only in a footnote (their note 37), and cannot be considered definitive evidence, as confirmed by Marty Sereno (per. comm.). Moreover, the present effects on visual cortex did not resemble conventional ‘attention’ effects on visual cortex, in several respects. Notably, although retinotopic visual cortex was modulated here by saccades to a remembered location, the contralateral response to probe checkerboard stimuli at the location of the saccade target was not affected. This is unlike typical findings in attentional studies of visual selection for perceptual tasks. We note in any case that our task only required saccades to a remembered location, with no perceptual comparison having to be made there, in contrast (by design) to standard attentional paradigms, in which peripheral perceptual decisions are made without any eye-movements being allowed. Finally, we note also the present difference between go and no-go trials (with the former showing stronger effects on visual cortex, and being the only trials to affect V1 significantly. This does not seem readily explainable by any ‘attentional’ factors common to go and no-go trials.
Turning to the proposals of Awh and colleagues, (Awh et al., 1999; Awh and Jonides, 2001; Awh and Vogel, 2008), we are sympathetic to their suggestion that some spatial working-memory tasks involve processes akin to spatial attention (see also Spivey and Geng, 2001). Indeed, the two previously separate fields of attention research on the one hand, and working-memory research on the other hand, now seem to be moving increasingly close together (see Driver and Husain, 2002; D’Esposito, 2007). But we note that to date Awh and colleagues have assessed overlap between possible working-memory and attentional processes only for perceptual tasks, unlike the motoric saccade task used here. We note also that the significant differences of interest here, between go and no-go triasl, cannot readily be explained by putative cognitive processes (such as attention/working memory). These should be common to both types of trial, since both required the saccade target location to be retained up until the auditory imperative signal indicating whether to execute or withhold the saccade. While one might perhaps argue that the go-trials required slightly longer retention of the target location (which could arguably be forgotten fairly quickly on hearing the no-go signal), in fact saccades were also executed fairly quickly (within ∼ 175 msec of the auditory go-signal, some of which time must presumably involve motor processing). It seems implausible that adding such a short time (< 175 msec) to the effective 2400 msec ‘delay’ could have produced such substantial impacts on the visual BOLD signal, as found here.
Finally, we consider the emerging concept that recently activated representations may be briefly “refreshed” when needed in the service of an ongoing task (Johnson et al., 2005; Johnson et al., 2007; Raye et al., 2007). Again, we are sympathetic to this idea, which (as for attention and working memory also) involves top-down modulation of representations, but now envisaged in a highly flexible manner. We think that this new concept might potentially provide a rather parsimonious interpretation for both our no-go and our go data: Both types of trial showed that retaining the saccade target location could ‘reach down’ to modulate early visual cortex in a top-down manner, but there was an increase in the size of visual modulation when the saccade was actually executed on go-trials. This seems reminiscent of Raye et al.’s (2007) proposal that refreshing may further “augment” a recently activated representation when it becomes particularly task-relevant again (as when actually executing the saccade here). While the concept of “refreshing” may thus provide one appealing new interpretation for our data, we note that extending the concept to encompass a motoric saccade task that affects the very earliest level of visual cortex (here, even V1 on go-trials), would be a considerable extension of other recent work on refreshing (Johnson et al. 2005; 2007; Raye et al., 2007; Yi et al, in press), which typically reported effects for higher brain areas during more cognitive tasks, even when specifically studying ‘refreshing’ of spatial locations (e.g. Johnson et al., 2005; Johnson et al., 2007).
From these various related cognitive perspectives, the present new findings that saccades to a remembered location can modulate human retinotopic visual cortex, in a highly spatially-specific manner, seem to make good functional sense. On the other hand, prior to obtaining these new results, there was no guarantee that such a motoric saccade task would affect human visual cortex in the spatially-selective manner, found here. Our new finding of greater effects on go than no-go trials, for human V1, accords well with the invasive single-cell work of Super and colleagues (2004 Super and Lamme, 2007) in monkeys. They found that monkey V1 neurons whose receptive fields overlapped with the target for an upcoming saccade often fired most just before an actual saccade arose, as on the go-trials here. The putative cognitive processes underlying this effect in monkeys have received rather less discussion to date than for attentional, working memory, and refreshing issues in human research.
Nevertheless, many enduring discussions illustrate that exact relation between saccade planning and covert attention is a recurring issue that remains of considerable interest (e.g. Hoffman and Subramaniam, 1995; Kowler et al., 1995; Deubel and Schneider, 1996; Corbetta, 1998; Corbetta et al., 1998; Nobre et al., 2000; Tolias et al., 2001; Moore et al., 2003; Kincade et al., 2005; Madelain et al., 2005; Armstrong et al., 2006; Eimer et al., 2006; Wardak et al., 2006). What our results clearly show is that a saccade task can modulate human visual cortex in a spatially-specific fashion, even when no perceptual comparison task is explicitly required (unlike conventional attention paradigms). We note also that the present differences between go and no-go trials cannot be explained by any processes common to both. Moreover, while visual cortex was modulated by our saccade task, the visual response to probe checkerboards was not, unlike standard ‘attention’ effects. Instead, the activation seen here in visual cortex might potentially contribute to saccadic processing; for example, by providing a high resolution spatial map for guiding saccade endpoints (see Tolias et al., 2001; Moore et al., 2003; Super et al., 2004; Merriam et al., 2007).
In summary, we required saccades to be prepared, and sometimes executed, to particular remembered locations in the upper left or right visual quadrant. On go-trials with an actual saccade, we found that activation was enhanced for ventral sectors of V1, V2 and V3 that corresponded to the contralateral target location, but not for dorsal sectors that would represent the lower quadrants. No-go trials produced analogous albeit reduced effects on V2 and V3, but none in V1. Our new findings thus show that a motor task requiring saccades to remembered locations can have spatially-specific effects on human visual cortex. While most (though not all) previous work on saccade tasks has focused on eye-movement related areas in fronto-parietal cortex or subcortically, saccade tasks clearly have strong implications for visual cortex also. This provides a new illustration of the extent to which task-related factors can ‘reach down’ to influence early visual cortex in a top-down manner, even for motor tasks.
Acknowledgment
This research was supported by a USA Royal Society International Postdoctoral Fellowship and an NEI training fellowship to JJG, and by grants from the Wellcome Trust and Medical Research Council to JD. JD holds a Royal Society - Leverhulme Trust Senior Research Fellowship.
References
- Aine CJ, Supek S, George JS, Ranken D, Lewine J, Sanders J, Best E, Tiee W, Flynn ER, Wood CC. Retinotopic organization of human visual cortex: departures from the classical model. Cereb Cortex. 1996;6:354–361. doi: 10.1093/cercor/6.3.354. [DOI] [PubMed] [Google Scholar]
- Andersen RA. Multimodal integration for the representation of space in the posterior parietal cortex. Philos Trans R Soc Lond B Biol Sci. 1997;352:1421–1428. doi: 10.1098/rstb.1997.0128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson JL, Hutton C, Ashburner J, Turner R, Friston K. Modeling geometric deformations in EPI time series. Neuroimage. 2001;13:903–919. doi: 10.1006/nimg.2001.0746. [DOI] [PubMed] [Google Scholar]
- Armstrong KM, Fitzgerald JK, Moore T. Changes in visual receptive fields with microstimulation of frontal cortex. Neuron. 2006;50:791–798. doi: 10.1016/j.neuron.2006.05.010. [DOI] [PubMed] [Google Scholar]
- Astafiev SV, Shulman GL, Stanley CM, Snyder AZ, Van Essen DC, Corbetta M. Functional organization of human intraparietal and frontal cortex for attending, looking, and pointing. J Neurosci. 2003;23:4689–4699. doi: 10.1523/JNEUROSCI.23-11-04689.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awh E, Jonides J. Overlapping mechanisms of attention and spatial working memory. Trends Cogn Sci. 2001;5:119–126. doi: 10.1016/s1364-6613(00)01593-x. [DOI] [PubMed] [Google Scholar]
- Awh E, Vogel EK. The bouncer in the brain. Nat Neurosci. 2008;11:5–6. doi: 10.1038/nn0108-5. [DOI] [PubMed] [Google Scholar]
- Awh E, Armstrong KM, Moore T. Visual and oculomotor selection: links, causes and implications for spatial attention. Trends Cogn Sci. 2006a;10:124–130. doi: 10.1016/j.tics.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Awh E, Vogel EK, Oh SH. Interactions between attention and working memory. Neuroscience. 2006b;139:201–208. doi: 10.1016/j.neuroscience.2005.08.023. [DOI] [PubMed] [Google Scholar]
- Awh E, Jonides J, Smith EE, Buxton RB, Frank LR, Love T, Wong EC, Gmeindl L. Rehersal in spatial working memory: Evidence from neuroimaging. Psychological Science. 1999;10:433–437. [Google Scholar]
- Basso MA, Wurtz RH. Modulation of neuronal activity in superior colliculus by changes in target probability. J Neurosci. 1998;18:7519–7534. doi: 10.1523/JNEUROSCI.18-18-07519.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bestmann S, Ruff CC, Blakemore C, Driver J, Thilo KV. Spatial attention changes excitability of human visual cortex to direct stimulation. Curr Biol. 2007;17:134–139. doi: 10.1016/j.cub.2006.11.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brefczynski JA, DeYoe EA. A physiological correlate of the ‘spotlight’ of visual attention. Nat Neurosci. 1999;2:370–374. doi: 10.1038/7280. [DOI] [PubMed] [Google Scholar]
- Brown MR, Vilis T, Everling S. Isolation of saccade inhibition processes: Rapid event-related fMRI of saccades and nogo trials. Neuroimage. 2008;39:793–804. doi: 10.1016/j.neuroimage.2007.09.035. [DOI] [PubMed] [Google Scholar]
- Brown MR, Goltz HC, Vilis T, Ford KA, Everling S. Inhibition and generation of saccades: rapid event-related fMRI of prosaccades, antisaccades, and nogo trials. Neuroimage. 2006;33:644–659. doi: 10.1016/j.neuroimage.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Castet E, Jeanjean S, Montagnini A, Laugier D, Masson GS. Dynamics of attentional deployment during saccadic programming. J Vis. 2006;6:196–212. doi: 10.1167/6.3.2. [DOI] [PubMed] [Google Scholar]
- Colby CL, Duhamel JR, Goldberg ME. Oculocentric spatial representation in parietal cortex. Cereb Cortex. 1995;5:470–481. doi: 10.1093/cercor/5.5.470. [DOI] [PubMed] [Google Scholar]
- Colby CL, Duhamel JR, Goldberg ME. Visual, presaccadic, and cognitive activation of single neurons in monkey lateral intraparietal area. J Neurophysiol. 1996;76:2841–2852. doi: 10.1152/jn.1996.76.5.2841. [DOI] [PubMed] [Google Scholar]
- Connolly JD, Goodale MA, Goltz HC, Munoz DP. fMRI activation in the human frontal eye field is correlated with saccadic reaction time. J Neurophysiol. 2005;94:605–611. doi: 10.1152/jn.00830.2004. [DOI] [PubMed] [Google Scholar]
- Corbetta M. Frontoparietal cortical networks for directing attention and the eye to visual locations: identical, independent, or overlapping neural systems? Proc Natl Acad Sci U S A. 1998;95:831–838. doi: 10.1073/pnas.95.3.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Akbudak E, Conturo TE, Snyder AZ, Ollinger JM, Drury HA, Linenweber MR, Petersen SE, Raichle ME, Van Essen DC, Shulman GL. A common network of functional areas for attention and eye movements. Neuron. 1998;21:761–773. doi: 10.1016/s0896-6273(00)80593-0. [DOI] [PubMed] [Google Scholar]
- Curtis CE, D’Esposito M. Selection and maintenance of saccade goals in the human frontal eye fields. J Neurophysiol. 2006;95:3923–3927. doi: 10.1152/jn.01120.2005. [DOI] [PubMed] [Google Scholar]
- D’Esposito M. From cognitive to neural models of working memory. Philos Trans R Soc Lond B Biol Sci. 2007;362:761–772. doi: 10.1098/rstb.2007.2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deubel H, Schneider WX. Saccade target selection and object recognition: evidence for a common attentional mechanism. Vision Res. 1996;36:1827–1837. doi: 10.1016/0042-6989(95)00294-4. [DOI] [PubMed] [Google Scholar]
- DeYoe EA, Carman GJ, Bandettini P, Glickman S, Wieser J, Cox R, Miller D, Neitz J. Mapping striate and extrastriate visual areas in human cerebral cortex. Proc Natl Acad Sci U S A. 1996;93:2382–2386. doi: 10.1073/pnas.93.6.2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driver J, Husain M. The role of spatial working memory deficits in pathological search by neglect patients. In: Karnath H-O, Milner DA, Vallar G, editors. The cognitive and neural bases of spatial neglect. Oxford University Press; Oxford: 2002. pp. 351–362. [Google Scholar]
- Eimer M, Van Velzen J, Gherri E, Press C. Manual response preparation and saccade programming are linked to attention shifts: ERP evidence for covert attentional orienting and spatially specific modulations of visual processing. Brain Res. 2006;1105:7–19. doi: 10.1016/j.brainres.2005.10.060. [DOI] [PubMed] [Google Scholar]
- Fischer B, Biscaldi M, Otto P. Saccadic eye movements of dyslexic adult subjects. Neuropsychologia. 1993a;31:887–906. doi: 10.1016/0028-3932(93)90146-q. [DOI] [PubMed] [Google Scholar]
- Fischer B, Weber H, Biscaldi M, Aiple F, Otto P, Stuhr V. Separate populations of visually guided saccades in humans: reaction times and amplitudes. Experimental Brain Research. 1993b;92:528–541. doi: 10.1007/BF00229043. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ. How many subjects constitute a study? Neuroimage. 1999;10:1–5. doi: 10.1006/nimg.1999.0439. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes A, Poline JB, Price CJ, Frith CD. Detecting activations in PET and fMRI: levels of inference and power. Neuroimage. 1996;4:223–235. doi: 10.1006/nimg.1996.0074. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Penny W, Phillips C, Kiebel S, Hinton G, Ashburner J. Classical and Bayesian inference in neuroimaging: theory. Neuroimage. 2002;16:465–483. doi: 10.1006/nimg.2002.1090. [DOI] [PubMed] [Google Scholar]
- Gandhi SP, Heeger DJ, Boynton GM. Spatial attention affects brain activity in human primary visual cortex. Proc Natl Acad Sci U S A. 1999;96:3314–3319. doi: 10.1073/pnas.96.6.3314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng JJ, Eger E, Ruff CC, Kristjansson A, Rotshtein P, Driver J. On-line attentional selection from competing stimuli in opposite visual fields: Effects on human visual cortex and control processes. J Neurophysiol. 2006 doi: 10.1152/jn.01245.2005. [DOI] [PubMed] [Google Scholar]
- Gusnard DA, Raichle ME. Searching for a baseline: functional imaging and the resting human brain. Nat Rev Neurosci. 2001;2:685–694. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
- Haynes JD, Rees G. Predicting the orientation of invisible stimuli from activity in human primary visual cortex. Nat Neurosci. 2005;8:686–691. doi: 10.1038/nn1445. [DOI] [PubMed] [Google Scholar]
- Hoffman JE, Subramaniam B. The role of visual attention in saccadic eye movements. Percept Psychophys. 1995;57:787–795. doi: 10.3758/bf03206794. [DOI] [PubMed] [Google Scholar]
- Hopfinger JB, Buonocore MH, Mangun GR. The neural mechanisms of top-down attentional control. Nat Neurosci. 2000;3:284–291. doi: 10.1038/72999. [DOI] [PubMed] [Google Scholar]
- Ipata AE, Gee AL, Goldberg ME, Bisley JW. Activity in the lateral intraparietal area predicts the goal and latency of saccades in a free-viewing visual search task. J Neurosci. 2006;26:3656–3661. doi: 10.1523/JNEUROSCI.5074-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MK, Raye CL, Mitchell KJ, Greene EJ, Cunningham WA, Sanislow CA. Using fMRI to investigate a component process of reflection: prefrontal correlates of refreshing a just-activated representation. Cogn Affect Behav Neurosci. 2005;5:339–361. doi: 10.3758/cabn.5.3.339. [DOI] [PubMed] [Google Scholar]
- Johnson MR, Mitchell KJ, Raye CL, D’Esposito M, Johnson MK. A brief thought can modulate activity in extrastriate visual areas: Top-down effects of refreshing just-seen visual stimuli. Neuroimage. 2007;37:290–299. doi: 10.1016/j.neuroimage.2007.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston K, Levin HM, Koval MJ, Everling S. Top-down control-signal dynamics in anterior cingulate and prefrontal cortex neurons following task switching. Neuron. 2007;53:453–462. doi: 10.1016/j.neuron.2006.12.023. [DOI] [PubMed] [Google Scholar]
- Kastner S, De Weerd P, Desimone R, Ungerleider LG. Mechanisms of directed attention in the human extrastriate cortex as revealed by functional MRI. Science. 1998;282:108–111. doi: 10.1126/science.282.5386.108. [DOI] [PubMed] [Google Scholar]
- Kastner S, Pinsk MA, De Weerd P, Desimone R, Ungerleider LG. Increased activity in human visual cortex during directed attention in the absence of visual stimulation. Neuron. 1999;22:751–761. doi: 10.1016/s0896-6273(00)80734-5. [DOI] [PubMed] [Google Scholar]
- Kimmig H, Greenlee MW, Gondan M, Schira M, Kassubek J, Mergner T. Relationship between saccadic eye movements and cortical activity as measured by fMRI: quantitative and qualitative aspects. Exp Brain Res. 2001;141:184–194. doi: 10.1007/s002210100844. [DOI] [PubMed] [Google Scholar]
- Kincade JM, Abrams RA, Astafiev SV, Shulman GL, Corbetta M. An event-related functional magnetic resonance imaging study of voluntary and stimulus-driven orienting of attention. J Neurosci. 2005;25:4593–4604. doi: 10.1523/JNEUROSCI.0236-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleiser R, Seitz RJ, Krekelberg B. Neural correlates of saccadic suppression in humans. Curr Biol. 2004;14:386–390. doi: 10.1016/j.cub.2004.02.036. [DOI] [PubMed] [Google Scholar]
- Konen CS, Kleiser R, Bremmer F, Seitz RJ. Different cortical activations during visuospatial attention and the intention to perform a saccade. Exp Brain Res. 2007 doi: 10.1007/s00221-007-0995-z. [DOI] [PubMed] [Google Scholar]
- Kowler E, Anderson E, Dosher B, Blaser E. The role of attention in the programming of saccades. Vision Res. 1995;35:1897–1916. doi: 10.1016/0042-6989(94)00279-u. [DOI] [PubMed] [Google Scholar]
- Kustov AA, Robinson DL. Modified saccades evoked by stimulation of the macaque superior colliculus account for properties of the resettable integrator. J Neurophysiol. 1995;73:1724–1728. doi: 10.1152/jn.1995.73.4.1724. [DOI] [PubMed] [Google Scholar]
- Kustov AA, Robinson DL. Shared neural control of attentional shifts and eye movements. Nature. 1996;384:74–77. doi: 10.1038/384074a0. [DOI] [PubMed] [Google Scholar]
- Macaluso E, Frith CD, Driver J. Delay activity and sensory-motor translation during planned eye or hand movements to visual or tactile targets. J Neurophysiol. 2007 doi: 10.1152/jn.00192.2007. [DOI] [PubMed] [Google Scholar]
- MacDonald AW, 3rd, Cohen JD, Stenger VA, Carter CS. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000;288:1835–1838. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- Madelain L, Krauzlis RJ, Wallman J. Spatial deployment of attention influences both saccadic and pursuit tracking. Vision Res. 2005;45:2685–2703. doi: 10.1016/j.visres.2005.05.009. [DOI] [PubMed] [Google Scholar]
- McMains SA, Somers DC. Multiple spotlights of attentional selection in human visual cortex. Neuron. 2004;42:677–686. doi: 10.1016/s0896-6273(04)00263-6. [DOI] [PubMed] [Google Scholar]
- McPeek RM, Keller EL. Saccade target selection in the superior colliculus during a visual search task. J Neurophysiol. 2002;88:2019–2034. doi: 10.1152/jn.2002.88.4.2019. [DOI] [PubMed] [Google Scholar]
- Medendorp WP, Goltz HC, Vilis T. Directional selectivity of BOLD activity in human posterior parietal cortex for memory-guided double-step saccades. J Neurophysiol. 2006;95:1645–1655. doi: 10.1152/jn.00905.2005. [DOI] [PubMed] [Google Scholar]
- Merriam EP, Genovese CR, Colby CL. Spatial updating in human parietal cortex. Neuron. 2003;39:361–373. doi: 10.1016/s0896-6273(03)00393-3. [DOI] [PubMed] [Google Scholar]
- Merriam EP, Genovese CR, Colby CL. Remapping in human visual cortex. J Neurophysiol. 2007;97:1738–1755. doi: 10.1152/jn.00189.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore T, Armstrong KM. Selective gating of visual signals by microstimulation of frontal cortex. Nature. 2003;421:370–373. doi: 10.1038/nature01341. [DOI] [PubMed] [Google Scholar]
- Moore T, Armstrong KM, Fallah M. Visuomotor origins of covert spatial attention. Neuron. 2003;40:671–683. doi: 10.1016/s0896-6273(03)00716-5. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Colby CL. Visual, saccade-related, and cognitive activation of single neurons in monkey extrastriate area V3A. J Neurophysiol. 2000;84:677–692. doi: 10.1152/jn.2000.84.2.677. [DOI] [PubMed] [Google Scholar]
- Nobre AC, Gitelman DR, Dias EC, Mesulam MM. Covert visual spatial orienting and saccades: overlapping neural systems. Neuroimage. 2000;11:210–216. doi: 10.1006/nimg.2000.0539. [DOI] [PubMed] [Google Scholar]
- Noesselt T, Hillyard SA, Woldorff MG, Schoenfeld A, Hagner T, Jancke L, Tempelmann C, Hinrichs H, Heinze HJ. Delayed striate cortical activation during spatial attention. Neuron. 2002;35:575–587. doi: 10.1016/s0896-6273(02)00781-x. [DOI] [PubMed] [Google Scholar]
- Orban GA, Claeys K, Nelissen K, Smans R, Sunaert S, Todd JT, Wardak C, Durand JB, Vanduffel W. Mapping the parietal cortex of human and non-human primates. Neuropsychologia. 2005 doi: 10.1016/j.neuropsychologia.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Ozyurt J, Rutschmann RM, Greenlee MW. Cortical activation during memory-guided saccades. Neuroreport. 2006;17:1005–1009. doi: 10.1097/01.wnr.0000224765.00078.4e. [DOI] [PubMed] [Google Scholar]
- Pare M, Hanes DP. Controlled movement processing: superior colliculus activity associated with countermanded saccades. J Neurosci. 2003;23:6480–6489. doi: 10.1523/JNEUROSCI.23-16-06480.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T, Marrett S, Worsley KJ, Evans AC. Extraretinal modulation of cerebral blood flow in the human visual cortex: implications for saccadic suppression. J Neurophysiol. 1995;74:2179–2183. doi: 10.1152/jn.1995.74.5.2179. [DOI] [PubMed] [Google Scholar]
- Raye CL, Johnson MK, Mitchell KJ, Greene EJ, Johnson MR. Refreshing: a minimal executive function. Cortex. 2007;43:135–145. doi: 10.1016/s0010-9452(08)70451-9. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Riggio L, Dascola I, Umilta C. Reorienting attention across the horizontal and vertical meridians: evidence in favor of a premotor theory of attention. Neuropsychologia. 1987;25:31–40. doi: 10.1016/0028-3932(87)90041-8. [DOI] [PubMed] [Google Scholar]
- Robinson DL, Kertzman C. Covert orienting of attention in macaques. III. Contributions of the superior colliculus. J Neurophysiol. 1995;74:713–721. doi: 10.1152/jn.1995.74.2.713. [DOI] [PubMed] [Google Scholar]
- Ross J, Morrone MC, Goldberg ME, Burr DC. Changes in visual perception at the time of saccades. Trends Neurosci. 2001;24:113–121. doi: 10.1016/s0166-2236(00)01685-4. [DOI] [PubMed] [Google Scholar]
- Ruff CC, Driver J. Attentional preparation for a lateralized visual distractor: behavioral and fMRI evidence. J Cogn Neurosci. 2006;18:522–538. doi: 10.1162/jocn.2006.18.4.522. [DOI] [PubMed] [Google Scholar]
- Ruff CC, Blankenburg F, Bjoertomt O, Bestmann S, Freeman E, Haynes JD, Rees G, Josephs O, Deichmann R, Driver J. Concurrent TMS-fMRI and psychophysics reveal frontal influences on human retinotopic visual cortex. Curr Biol. 2006;16:1479–1488. doi: 10.1016/j.cub.2006.06.057. [DOI] [PubMed] [Google Scholar]
- Schall JD, Hanes DP. Neural basis of saccade target selection in frontal eye field during visual search. Nature. 1993;366:467–469. doi: 10.1038/366467a0. [DOI] [PubMed] [Google Scholar]
- Schluppeck D, Glimcher P, Heeger DJ. Topographic organization for delayed saccades in human posterior parietal cortex. J Neurophysiol. 2005;94:1372–1384. doi: 10.1152/jn.01290.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schluppeck D, Curtis CE, Glimcher PW, Heeger DJ. Sustained activity in topographic areas of human posterior parietal cortex during memory-guided saccades. J Neurosci. 2006;26:5098–5108. doi: 10.1523/JNEUROSCI.5330-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serences JT, Yantis S. Spatially selective representations of voluntary and stimulus-driven attentional priority in human occipital, parietal, and frontal cortex. Cereb Cortex. 2007;17:284–293. doi: 10.1093/cercor/bhj146. [DOI] [PubMed] [Google Scholar]
- Sereno MI, Pitzalis S, Martinez A. Mapping of contralateral space in retinotopic coordinates by a parietal cortical area in humans. Science. 2001;294:1350–1354. doi: 10.1126/science.1063695. [DOI] [PubMed] [Google Scholar]
- Sereno MI, Dale AM, Reppas JB, Kwong KK, Belliveau JW, Brady TJ, Rosen BR, Tootell RB. Borders of multiple visual areas in humans revealed by functional magnetic resonance imaging. Science. 1995;268:889–893. doi: 10.1126/science.7754376. [DOI] [PubMed] [Google Scholar]
- Silver MA, Ress D, Heeger DJ. Topographic maps of visual spatial attention in human parietal cortex. J Neurophysiol. 2005;94:1358–1371. doi: 10.1152/jn.01316.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver MA, Ress D, Heeger DJ. Neural correlates of sustained spatial attention in human early visual cortex. J Neurophysiol. 2006 doi: 10.1152/jn.00677.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somers DC, Dale AM, Seiffert AE, Tootell RB. Functional MRI reveals spatially specific attentional modulation in human primary visual cortex. Proc Natl Acad Sci U S A. 1999;96:1663–1668. doi: 10.1073/pnas.96.4.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spivey MJ, Geng JJ. Oculomotor mechanisms activated by imagery and memory: eye movements to absent objects. Psychol Res. 2001;65:235–241. doi: 10.1007/s004260100059. [DOI] [PubMed] [Google Scholar]
- Super H, Lamme VA. Strength of figure-ground activity in monkey primary visual cortex predicts saccadic reaction time in a delayed detection task. Cereb Cortex. 2007;17:1468–1475. doi: 10.1093/cercor/bhl058. [DOI] [PubMed] [Google Scholar]
- Super H, van der Togt C, Spekreijse H, Lamme VA. Correspondence of presaccadic activity in the monkey primary visual cortex with saccadic eye movements. Proc Natl Acad Sci U S A. 2004;101:3230–3235. doi: 10.1073/pnas.0400433101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylvester R, Haynes JD, Rees G. Saccades differentially modulate human LGN and V1 responses in the presence and absence of visual stimulation. Curr Biol. 2005;15:37–41. doi: 10.1016/j.cub.2004.12.061. [DOI] [PubMed] [Google Scholar]
- Teo PC, Sapiro G, Wandell BA. Creating connected representations of cortical gray matter for functional MRI visualization. IEEE Trans Med Imaging. 1997;16:852–863. doi: 10.1109/42.650881. [DOI] [PubMed] [Google Scholar]
- Thompson KG, Bichot NP, Schall JD. Dissociation of visual discrimination from saccade programming in macaque frontal eye field. J Neurophysiol. 1997;77:1046–1050. doi: 10.1152/jn.1997.77.2.1046. [DOI] [PubMed] [Google Scholar]
- Tolias AS, Moore T, Smirnakis SM, Tehovnik EJ, Siapas AG, Schiller PH. Eye movements modulate visual receptive fields of V4 neurons. Neuron. 2001;29:757–767. doi: 10.1016/s0896-6273(01)00250-1. [DOI] [PubMed] [Google Scholar]
- Tootell RB, Hadjikhani NK, Vanduffel W, Liu AK, Mendola JD, Sereno MI, Dale AM. Functional analysis of primary visual cortex (V1) in humans. Proc Natl Acad Sci U S A. 1998;95:811–817. doi: 10.1073/pnas.95.3.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Essen DC. Organization of visual areas in macaque and human cerebral cortex. In: Chalupa LM, Werner JS, editors. The visual neurosciences. Bradford Books; Boston: 2003. pp. 507–521. [Google Scholar]
- Wandell B, Chial S, Backus S. Visualization and measurement of the cortical surface. Journal of Cognitive Neruoscience. 2000;12:739–752. doi: 10.1162/089892900562561. [DOI] [PubMed] [Google Scholar]
- Wandell BA, Brewer AA, Dougherty RF. Visual field map clusters in human cortex. Philos Trans R Soc Lond B Biol Sci. 2005;360:693–707. doi: 10.1098/rstb.2005.1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardak C, Ibos G, Duhamel JR, Olivier E. Contribution of the monkey frontal eye field to covert visual attention. J Neurosci. 2006;26:4228–4235. doi: 10.1523/JNEUROSCI.3336-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber H, Fischer B. Gap duration and location of attention focus modulate the occurrence of left/right asymmetries in the saccadic reaction times of human subjects. Vision Res. 1995;35:987–998. doi: 10.1016/0042-6989(94)00186-p. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Marrett S, Neelin P, Evans AC. A three-dimensional statistical analysis for CBF activation studies in human brain. Journal of Cerebral Blood Flow and Metabolism. 1992;12:900–918. doi: 10.1038/jcbfm.1992.127. [DOI] [PubMed] [Google Scholar]
- Wurtz RH, Goldberg ME, Robinson DL. Brain mechanisms of visual attention. Sci Am. 1982;246:124–135. doi: 10.1038/scientificamerican0682-124. [DOI] [PubMed] [Google Scholar]
- Yi DJ, Turk-Brown NB, Chun MM, Johnson MK. When a thought equals a look: Refreshing enhances perceptual memory. Journal of Cognitive Neruoscience. doi: 10.1162/jocn.2008.20094. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]