Abstract
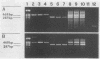
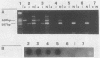
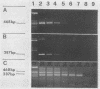
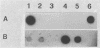
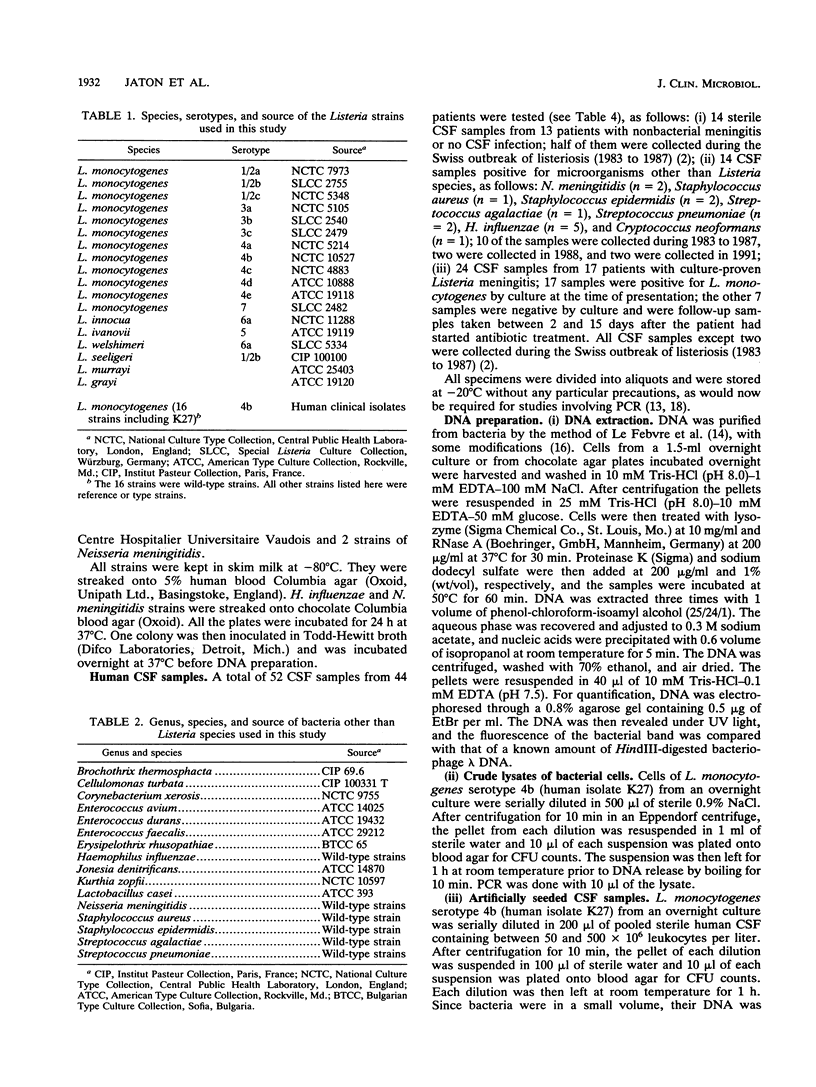
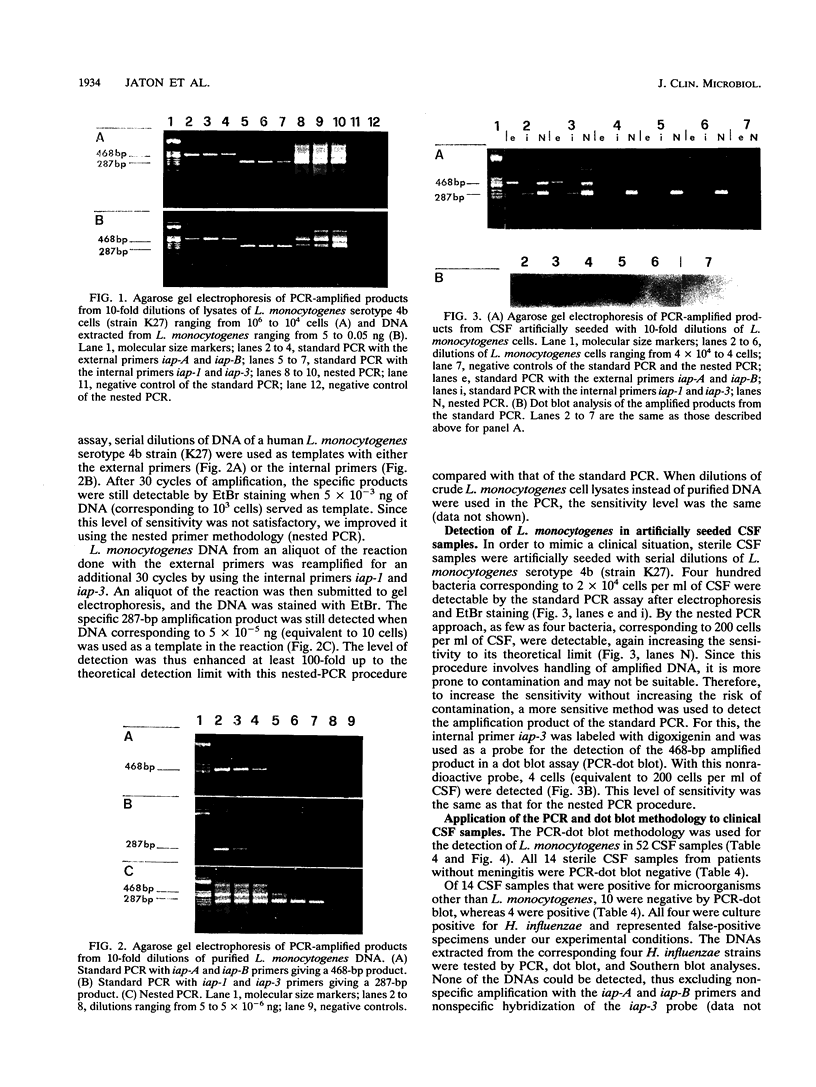
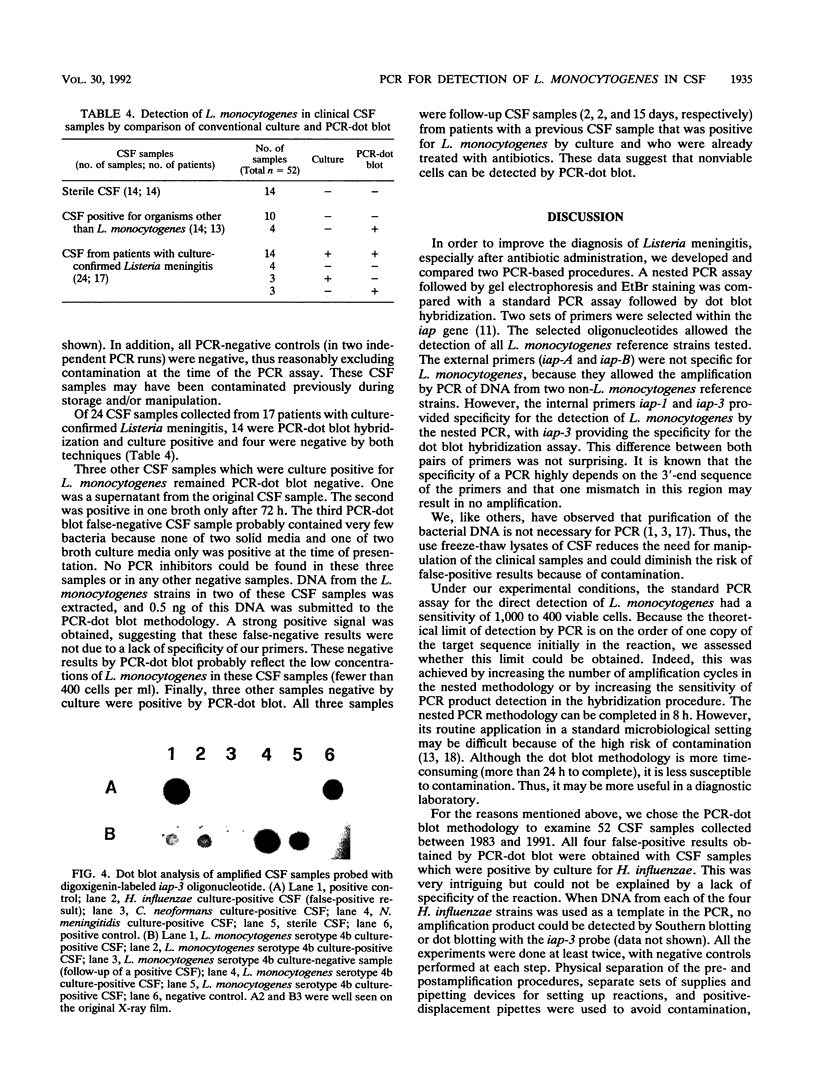
In order to improve the diagnosis of Listeria meningitis or meningoencephalitis, especially in patients who have received antibiotics before their cerebrospinal fluid (CSF) has been examined, two assays for the detection of Listeria monocytogenes based on the polymerase chain reaction (PCR) were evaluated. After a standard PCR, the amplified DNA was detected either by a second round of PCR with internal primers followed by gel electrophoresis and ethidium bromide staining (nested PCR) or by dot blot hybridization to an internal digoxigenin-labeled probe (PCR-dot blot). For PCR, two sets of primers within the invasion-associated protein gene (iap gene) were chosen. They allowed for the highly specific detection of all L. monocytogenes reference strains tested (serotypes 1/2a, 1/2b, 1/2c, 3a, 3b, 3c, 4a, 4b, 4c, 4d, and 7). These primers did not detect amplification products from various other gram-positive or gram-negative bacterial DNAs or human DNA. The sensitivities of both assays were assessed on sterile CSF samples that were artificially seeded with serial dilutions of L. monocytogenes serotype 4b cells. By both methods the limit of detection was less than 10 cells in the initial reaction. Since the nested PCR is more prone to contamination because of manipulation of the amplified products, a standard PCR assay followed by dot blot hybridization was applied to 52 CSF samples in a retrospective study. Of 28 CSF samples which were sterile or positive for bacteria other than Listeria species, 24 were PCR negative. In contrast, from 17 patients with culture-proven Listeria meningitis, 14 of 17 initial CSF samples were PCR positive, as were 3 of 7 culture-negative followup CSF samples taken after patients received antibiotics. These results support the usefulness of this approach in the diagnosis of Listeria meningitis, in particular, when antibiotic administration precedes culture of CSF.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bessesen M. T., Luo Q. A., Rotbart H. A., Blaser M. J., Ellison R. T., 3rd Detection of Listeria monocytogenes by using the polymerase chain reaction. Appl Environ Microbiol. 1990 Sep;56(9):2930–2932. doi: 10.1128/aem.56.9.2930-2932.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Border P. M., Howard J. J., Plastow G. S., Siggens K. W. Detection of Listeria species and Listeria monocytogenes using polymerase chain reaction. Lett Appl Microbiol. 1990 Sep;11(3):158–162. doi: 10.1111/j.1472-765x.1990.tb00149.x. [DOI] [PubMed] [Google Scholar]
- Datta A. R., Wentz B. A., Shook D., Trucksess M. W. Synthetic oligodeoxyribonucleotide probes for detection of Listeria monocytogenes. Appl Environ Microbiol. 1988 Dec;54(12):2933–2937. doi: 10.1128/aem.54.12.2933-2937.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deneer H. G., Boychuk I. Species-specific detection of Listeria monocytogenes by DNA amplification. Appl Environ Microbiol. 1991 Feb;57(2):606–609. doi: 10.1128/aem.57.2.606-609.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domann E., Chakraborty T. Nucleotide sequence of the listeriolysin gene from a Listeria monocytogenes serotype 1/2a strain. Nucleic Acids Res. 1989 Aug 11;17(15):6406–6406. doi: 10.1093/nar/17.15.6406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flamm R. K., Hinrichs D. J., Thomashow M. F. Cloning of a gene encoding a major secreted polypeptide of Listeria monocytogenes and its potential use as a species-specific probe. Appl Environ Microbiol. 1989 Sep;55(9):2251–2256. doi: 10.1128/aem.55.9.2251-2256.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furrer B., Candrian U., Hoefelein C., Luethy J. Detection and identification of Listeria monocytogenes in cooked sausage products and in milk by in vitro amplification of haemolysin gene fragments. J Appl Bacteriol. 1991 May;70(5):372–379. doi: 10.1111/j.1365-2672.1991.tb02951.x. [DOI] [PubMed] [Google Scholar]
- Göhmann S., Leimeister-Wächter M., Schiltz E., Goebel W., Chakraborty T. Characterization of a Listeria monocytogenes-specific protein capable of inducing delayed hypersensitivity in Listeria-immune mice. Mol Microbiol. 1990 Jul;4(7):1091–1099. doi: 10.1111/j.1365-2958.1990.tb00683.x. [DOI] [PubMed] [Google Scholar]
- Kuhn M., Goebel W. Identification of an extracellular protein of Listeria monocytogenes possibly involved in intracellular uptake by mammalian cells. Infect Immun. 1989 Jan;57(1):55–61. doi: 10.1128/iai.57.1.55-61.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwok S., Higuchi R. Avoiding false positives with PCR. Nature. 1989 May 18;339(6221):237–238. doi: 10.1038/339237a0. [DOI] [PubMed] [Google Scholar]
- Köhler S., Leimeister-Wächter M., Chakraborty T., Lottspeich F., Goebel W. The gene coding for protein p60 of Listeria monocytogenes and its use as a specific probe for Listeria monocytogenes. Infect Immun. 1990 Jun;58(6):1943–1950. doi: 10.1128/iai.58.6.1943-1950.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Febvre R. B., Foley J. W., Thiermann A. B. Rapid and simplified protocol for isolation and characterization of leptospiral chromosomal DNA for taxonomy and diagnosis. J Clin Microbiol. 1985 Oct;22(4):606–608. doi: 10.1128/jcm.22.4.606-608.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mengaud J., Vicente M. F., Cossart P. Transcriptional mapping and nucleotide sequence of the Listeria monocytogenes hlyA region reveal structural features that may be involved in regulation. Infect Immun. 1989 Dec;57(12):3695–3701. doi: 10.1128/iai.57.12.3695-3701.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nocera D., Bannerman E., Rocourt J., Jaton-Ogay K., Bille J. Characterization by DNA restriction endonuclease analysis of Listeria monocytogenes strains related to the Swiss epidemic of listeriosis. J Clin Microbiol. 1990 Oct;28(10):2259–2263. doi: 10.1128/jcm.28.10.2259-2263.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel R. J., Fries J. W., Piessens W. F., Wirth D. F. Sequence analysis and amplification by polymerase chain reaction of a cloned DNA fragment for identification of Mycobacterium tuberculosis. J Clin Microbiol. 1990 Mar;28(3):513–518. doi: 10.1128/jcm.28.3.513-518.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persing D. H. Polymerase chain reaction: trenches to benches. J Clin Microbiol. 1991 Jul;29(7):1281–1285. doi: 10.1128/jcm.29.7.1281-1285.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Scharf S. J., Horn G. T., Erlich H. A. Direct cloning and sequence analysis of enzymatically amplified genomic sequences. Science. 1986 Sep 5;233(4768):1076–1078. doi: 10.1126/science.3461561. [DOI] [PubMed] [Google Scholar]
- Schuchat A., Swaminathan B., Broome C. V. Epidemiology of human listeriosis. Clin Microbiol Rev. 1991 Apr;4(2):169–183. doi: 10.1128/cmr.4.2.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas E. J., King R. K., Burchak J., Gannon V. P. Sensitive and specific detection of Listeria monocytogenes in milk and ground beef with the polymerase chain reaction. Appl Environ Microbiol. 1991 Sep;57(9):2576–2580. doi: 10.1128/aem.57.9.2576-2580.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wernars K., Heuvelman C. J., Chakraborty T., Notermans S. H. Use of the polymerase chain reaction for direct detection of Listeria monocytogenes in soft cheese. J Appl Bacteriol. 1991 Feb;70(2):121–126. doi: 10.1111/j.1365-2672.1991.tb04437.x. [DOI] [PubMed] [Google Scholar]