Abstract
Enterococcus faecalis is a frequent cause of urinary tract infection in hospitalized patients. Recent reports have suggested that the organism may frequently be acquired by cross-infection from other patients. In this study, we used total DNA restriction patterns to type 135 urine isolates of E. faecalis from four sets of patients. Isolates were placed into types (all bands identical) and into groups (most bands identical). Most isolates were discriminated by the typing method, and the results suggested that direct cross-infection occurred rarely if at all. However, two groups of clonally related isolates occurred frequently in the urine specimens and also in feces from hospital-associated patients and were often associated with antibiotic resistance. Isolates from these two groups were found less frequently in feces from people not associated with the hospital.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axelrod P., Talbot G. H. Risk factors for acquisition of gentamicin-resistant enterococci. A multivariate analysis. Arch Intern Med. 1989 Jun;149(6):1397–1401. [PubMed] [Google Scholar]
- Bingen E. H., Denamur E., Lambert-Zechovsky N. Y., Elion J. Evidence for the genetic unrelatedness of nosocomial vancomycin-resistant Enterococcus faecium strains in a pediatric hospital. J Clin Microbiol. 1991 Sep;29(9):1888–1892. doi: 10.1128/jcm.29.9.1888-1892.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chenoweth C., Schaberg D. The epidemiology of enterococci. Eur J Clin Microbiol Infect Dis. 1990 Feb;9(2):80–89. doi: 10.1007/BF01963631. [DOI] [PubMed] [Google Scholar]
- Clewell D. B. Movable genetic elements and antibiotic resistance in enterococci. Eur J Clin Microbiol Infect Dis. 1990 Feb;9(2):90–102. doi: 10.1007/BF01963632. [DOI] [PubMed] [Google Scholar]
- Ferretti J. J., Gilmore K. S., Courvalin P. Nucleotide sequence analysis of the gene specifying the bifunctional 6'-aminoglycoside acetyltransferase 2"-aminoglycoside phosphotransferase enzyme in Streptococcus faecalis and identification and cloning of gene regions specifying the two activities. J Bacteriol. 1986 Aug;167(2):631–638. doi: 10.1128/jb.167.2.631-638.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross P. A., Harkavy L. M., Barden G. E., Flower M. F. The epidemiology of nosocomial enterococcal urinary tract infection. Am J Med Sci. 1976 Jul-Aug;272(1):75–81. doi: 10.1097/00000441-197607000-00009. [DOI] [PubMed] [Google Scholar]
- Hall L. M., Duke B., Guiney M., Williams R. Typing of Enterococcus species by DNA restriction fragment analysis. J Clin Microbiol. 1992 Apr;30(4):915–919. doi: 10.1128/jcm.30.4.915-919.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodel-Christian S. L., Murray B. E. Characterization of the gentamicin resistance transposon Tn5281 from Enterococcus faecalis and comparison to staphylococcal transposons Tn4001 and Tn4031. Antimicrob Agents Chemother. 1991 Jun;35(6):1147–1152. doi: 10.1128/aac.35.6.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain Z., Kuhn M., Lannigan R., Austin T. W. Microbiological investigation of an outbreak of bacteraemia due to Streptococcus faecalis in an intensive care unit. J Hosp Infect. 1988 Nov;12(4):263–271. doi: 10.1016/0195-6701(88)90068-0. [DOI] [PubMed] [Google Scholar]
- Huycke M. M., Spiegel C. A., Gilmore M. S. Bacteremia caused by hemolytic, high-level gentamicin-resistant Enterococcus faecalis. Antimicrob Agents Chemother. 1991 Aug;35(8):1626–1634. doi: 10.1128/aac.35.8.1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordens J. Z., Hall L. M. Chromosomally-encoded gentamicin resistance in 'epidemic' methicillin-resistant Staphylococcus aureus: detection with a synthetic oligonucleotide probe. J Antimicrob Chemother. 1989 Mar;23(3):327–334. doi: 10.1093/jac/23.3.327. [DOI] [PubMed] [Google Scholar]
- Luginbuhl L. M., Rotbart H. A., Facklam R. R., Roe M. H., Elliot J. A. Neonatal enterococcal sepsis: case-control study and description of an outbreak. Pediatr Infect Dis J. 1987 Nov;6(11):1022–1026. [PubMed] [Google Scholar]
- Moellering R. C., Jr The Garrod Lecture. The enterococcus: a classic example of the impact of antimicrobial resistance on therapeutic options. J Antimicrob Chemother. 1991 Jul;28(1):1–12. doi: 10.1093/jac/28.1.1. [DOI] [PubMed] [Google Scholar]
- Murray B. E., Singh K. V., Markowitz S. M., Lopardo H. A., Patterson J. E., Zervos M. J., Rubeglio E., Eliopoulos G. M., Rice L. B., Goldstein F. W. Evidence for clonal spread of a single strain of beta-lactamase-producing Enterococcus (Streptococcus) faecalis to six hospitals in five states. J Infect Dis. 1991 Apr;163(4):780–785. doi: 10.1093/infdis/163.4.780. [DOI] [PubMed] [Google Scholar]
- Murray B. E. The life and times of the Enterococcus. Clin Microbiol Rev. 1990 Jan;3(1):46–65. doi: 10.1128/cmr.3.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson J. E., Singh K. V., Murray B. E. Epidemiology of an endemic strain of beta-lactamase-producing Enterococcus faecalis. J Clin Microbiol. 1991 Nov;29(11):2513–2516. doi: 10.1128/jcm.29.11.2513-2516.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
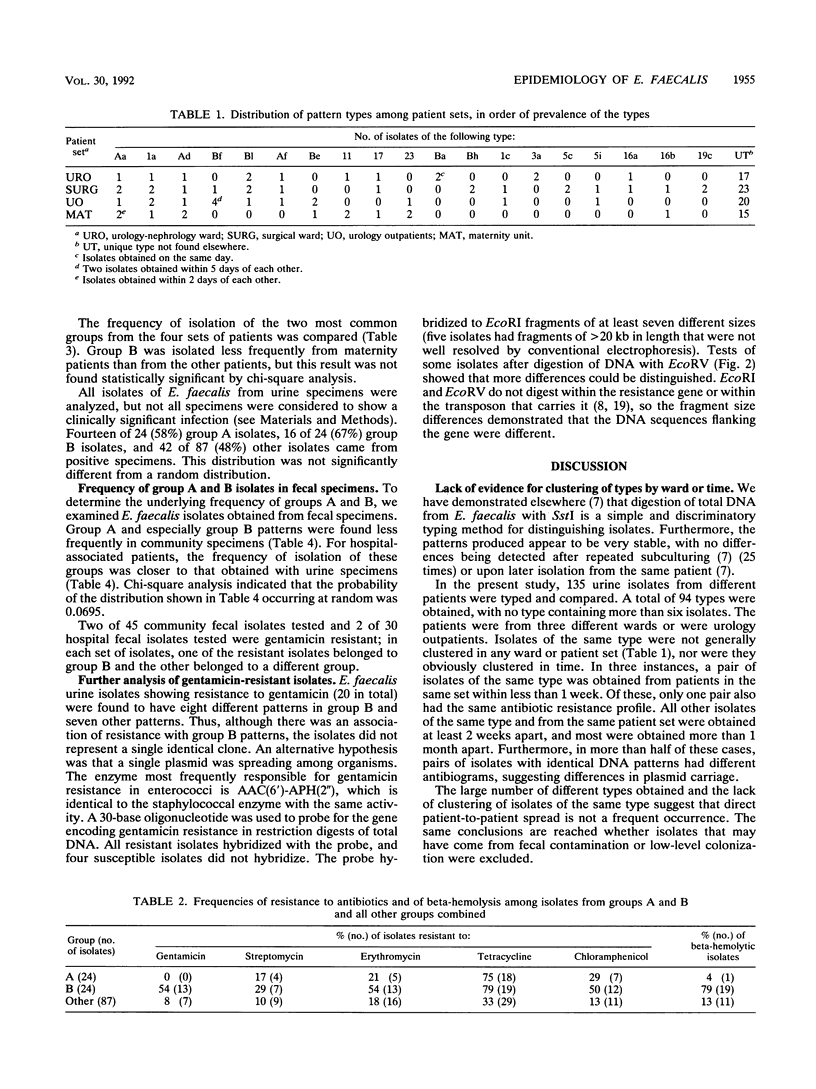
- Patterson J. E., Zervos M. J. High-level gentamicin resistance in Enterococcus: microbiology, genetic basis, and epidemiology. Rev Infect Dis. 1990 Jul-Aug;12(4):644–652. doi: 10.1093/clinids/12.4.644. [DOI] [PubMed] [Google Scholar]
- Rouch D. A., Byrne M. E., Kong Y. C., Skurray R. A. The aacA-aphD gentamicin and kanamycin resistance determinant of Tn4001 from Staphylococcus aureus: expression and nucleotide sequence analysis. J Gen Microbiol. 1987 Nov;133(11):3039–3052. doi: 10.1099/00221287-133-11-3039. [DOI] [PubMed] [Google Scholar]
- Salvi R. J., Ahroon W., Saunders S. S., Arnold S. A. Evoked potentials: computer-automated threshold-tracking procedure using an objective detection criterion. Ear Hear. 1987 Jun;8(3):151–156. [PubMed] [Google Scholar]
- Upholt W. B. Estimation of DNA sequence divergence from comparison of restriction endonuclease digests. Nucleic Acids Res. 1977;4(5):1257–1265. doi: 10.1093/nar/4.5.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanakunakorn C. The prevalence of high-level aminoglycoside resistance among enterococci isolated from blood cultures during 1980-1988. J Antimicrob Chemother. 1989 Jul;24(1):63–68. doi: 10.1093/jac/24.1.63. [DOI] [PubMed] [Google Scholar]
- Weems J. J., Jr, Lowrance J. H., Baddour L. M., Simpson W. A. Molecular epidemiology of nosocomial, multiply aminoglycoside resistant Enterococcus faecalis. J Antimicrob Chemother. 1989 Aug;24(2):121–130. doi: 10.1093/jac/24.2.121. [DOI] [PubMed] [Google Scholar]
- Zervos M. J., Dembinski S., Mikesell T., Schaberg D. R. High-level resistance to gentamicin in Streptococcus faecalis: risk factors and evidence for exogenous acquisition of infection. J Infect Dis. 1986 Jun;153(6):1075–1083. doi: 10.1093/infdis/153.6.1075. [DOI] [PubMed] [Google Scholar]
- Zervos M. J., Kauffman C. A., Therasse P. M., Bergman A. G., Mikesell T. S., Schaberg D. R. Nosocomial infection by gentamicin-resistant Streptococcus faecalis. An epidemiologic study. Ann Intern Med. 1987 May;106(5):687–691. doi: 10.7326/0003-4819-106-5-687. [DOI] [PubMed] [Google Scholar]