Abstract
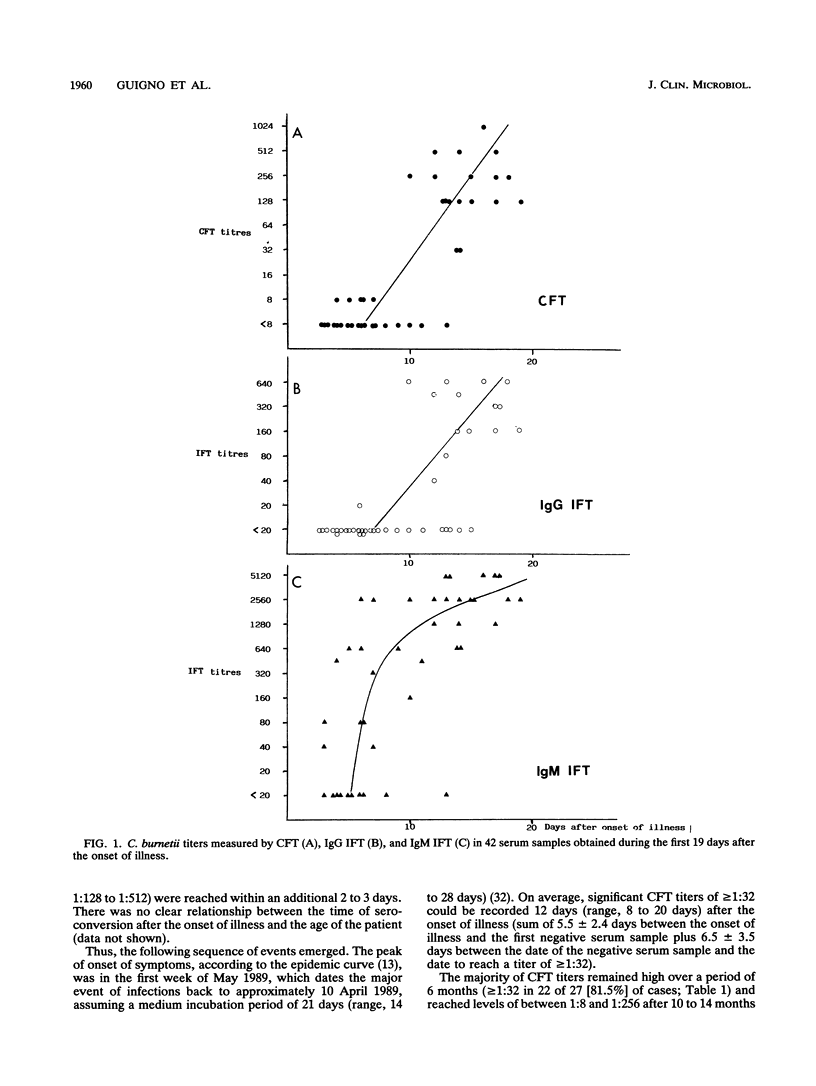
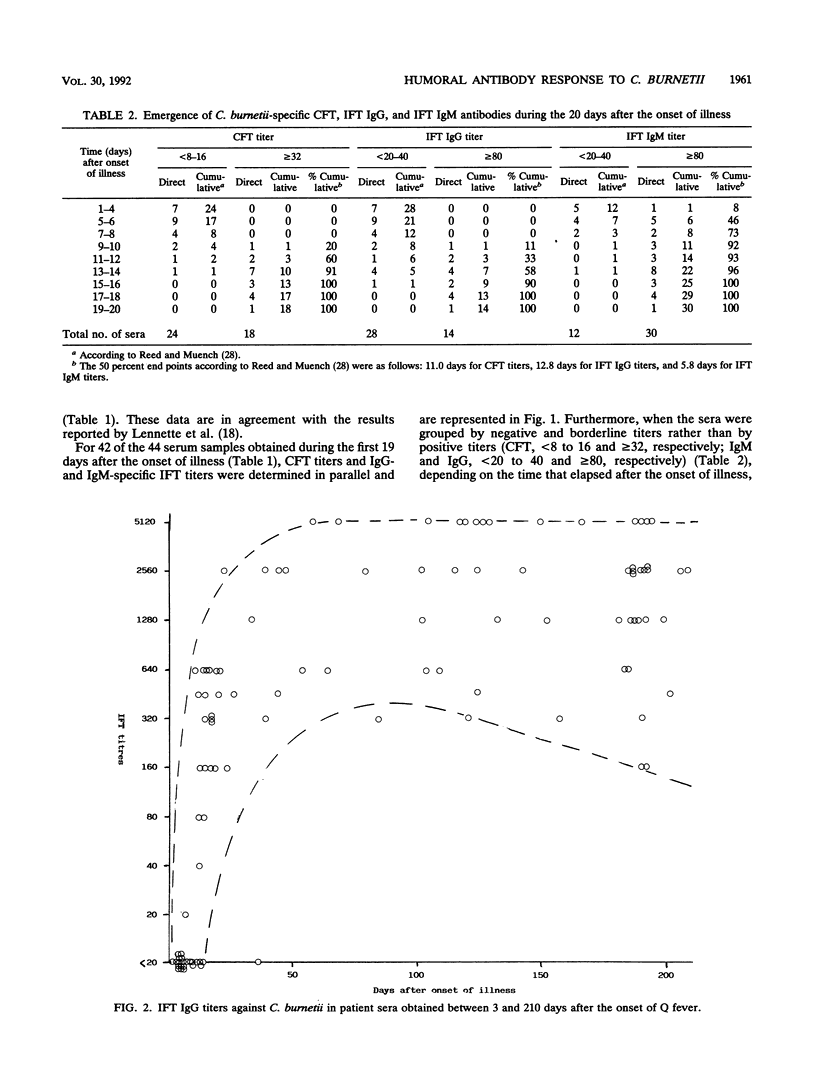
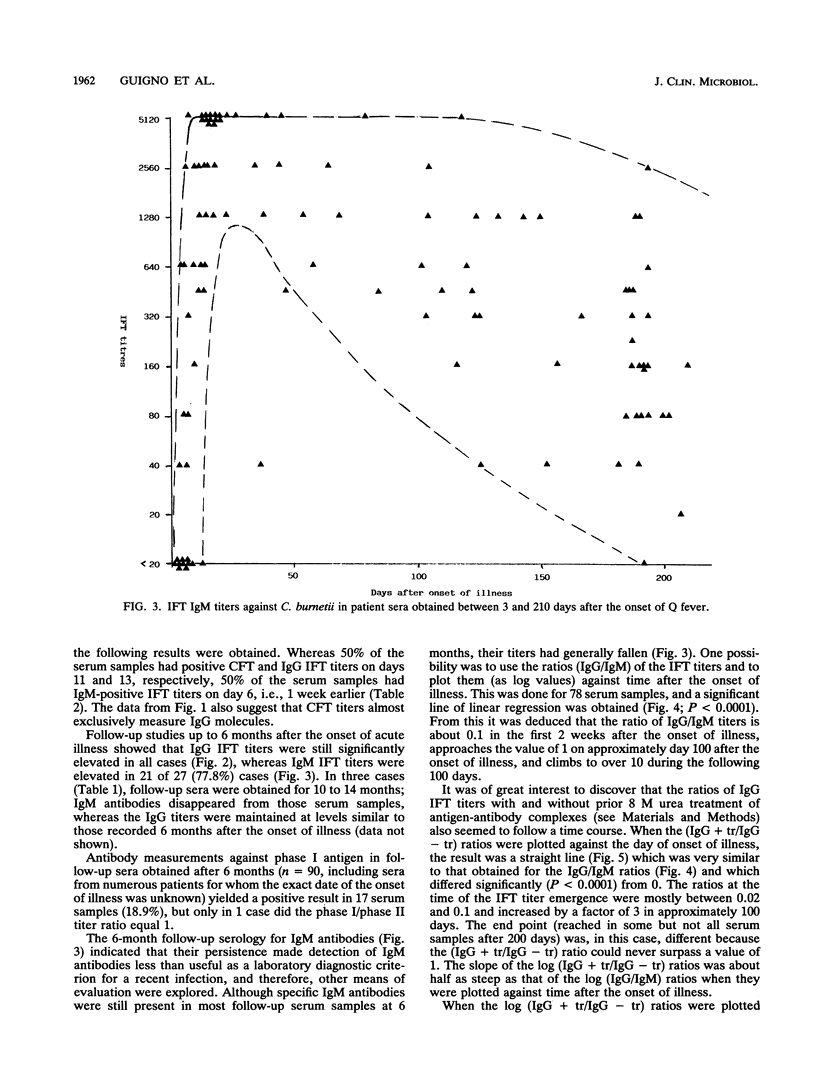
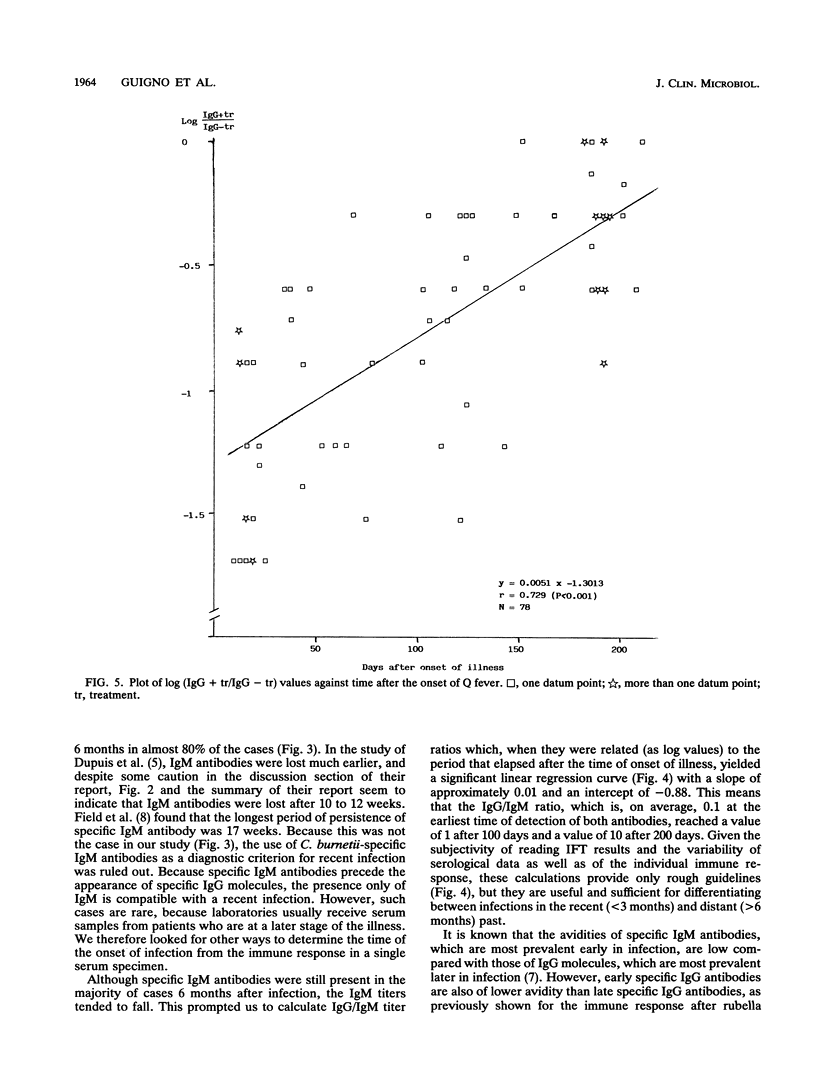
Of 147 patients with acute Q fever diagnosed during a major outbreak in Birmingham, England, in early summer 1989, 41 provided sets of sera which allowed us to make a detailed analysis of the primary humoral immune response. Antibody titers specific for Coxiella burnetii were measured by the complement fixation test and by an immunoglobulin M (IgM)- and IgG-specific indirect immunofluorescence test. The relative avidity of specific IgGs was determined by the indirect immunofluorescence test with and without treatment of antigen-antibody complexes with 8 M urea. The IgG subclass responses after primary infection and their avidities were also determined for a limited number of paired serum specimens. Specific IgM titers persisted for more than 6 months in the majority of cases and were therefore not a sufficient criterion for the diagnosis of recent infection. However, for serial samples the antibody titer ratios (IgG/IgM) and the ratios (IgG titer with treatment/IgG titer without treatment) that indicated relative avidity changed significantly, depending on the time postinfection. Within the IgG class, the C. burnetii-specific antibody response over time was almost exclusively represented by subclass 1 molecules, which thus showed affinity maturation.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Behymer D., Riemann H. P. Coxiella burnetii infection (Q fever). J Am Vet Med Assoc. 1989 Mar 15;194(6):764–767. [PubMed] [Google Scholar]
- Blackburn N. K., Besselaar T. G., Schoub B. D., O'Connell K. F. Differentiation of primary cytomegalovirus infection from reactivation using the urea denaturation test for measuring antibody avidity. J Med Virol. 1991 Jan;33(1):6–9. doi: 10.1002/jmv.1890330103. [DOI] [PubMed] [Google Scholar]
- Devey M. E., Bleasdale-Barr K. M., Bird P., Amlot P. L. Antibodies of different human IgG subclasses show distinct patterns of affinity maturation after immunization with keyhole limpet haemocyanin. Immunology. 1990 Jun;70(2):168–174. [PMC free article] [PubMed] [Google Scholar]
- Devey M. E., Bleasdale K., Lee S., Rath S. Determination of the functional affinity of IgG1 and IgG4 antibodies to tetanus toxoid by isotype-specific solid-phase assays. J Immunol Methods. 1988 Jan 21;106(1):119–125. doi: 10.1016/0022-1759(88)90279-7. [DOI] [PubMed] [Google Scholar]
- Dupuis G., Péter O., Peacock M., Burgdorfer W., Haller E. Immunoglobulin responses in acute Q fever. J Clin Microbiol. 1985 Oct;22(4):484–487. doi: 10.1128/jcm.22.4.484-487.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupuis G., Péter O., Pedroni D., Petite J. Aspects cliniques observés lors d'une épidémie de 415 cas de fièvre Q. Schweiz Med Wochenschr. 1985 Jun 15;115(24):814–818. [PubMed] [Google Scholar]
- Field P. R., Hunt J. G., Murphy A. M. Detection and persistence of specific IgM antibody to Coxiella burnetii by enzyme-linked immunosorbent assay: a comparison with immunofluorescence and complement fixation tests. J Infect Dis. 1983 Sep;148(3):477–487. doi: 10.1093/infdis/148.3.477. [DOI] [PubMed] [Google Scholar]
- Geddes A. M. Q fever. Br Med J (Clin Res Ed) 1983 Oct 1;287(6397):927–928. doi: 10.1136/bmj.287.6397.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths G. M., Berek C., Kaartinen M., Milstein C. Somatic mutation and the maturation of immune response to 2-phenyl oxazolone. Nature. 1984 Nov 15;312(5991):271–275. doi: 10.1038/312271a0. [DOI] [PubMed] [Google Scholar]
- Hall C. J., Richmond S. J., Caul E. O., Pearce N. H., Silver I. A. Laboratory outbreak of Q fever acquired from sheep. Lancet. 1982 May 1;1(8279):1004–1006. doi: 10.1016/s0140-6736(82)92001-3. [DOI] [PubMed] [Google Scholar]
- Hoppe G., Drescher J. Description of a technique for the determination of the concentration and quality of rubella virus antibody molecules and experience with testing sera from humans with recent and past rubella infection. Arch Virol. 1980;65(2):109–121. doi: 10.1007/BF01317322. [DOI] [PubMed] [Google Scholar]
- Inouye S., Hasegawa A., Matsuno S., Katow S. Changes in antibody avidity after virus infections: detection by an immunosorbent assay in which a mild protein-denaturing agent is employed. J Clin Microbiol. 1984 Sep;20(3):525–529. doi: 10.1128/jcm.20.3.525-529.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocks C., Rajewsky K. Stepwise intraclonal maturation of antibody affinity through somatic hypermutation. Proc Natl Acad Sci U S A. 1988 Nov;85(21):8206–8210. doi: 10.1073/pnas.85.21.8206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LENNETTE E. H., CLARK W. H., JENSEN F. W., TOOMB C. J. Q fever studies. XV. Development and persistence in man of complement-fixing and agglutinating antibodies to Coxiella burnetii. J Immunol. 1952 May;68(5):591–598. [PubMed] [Google Scholar]
- Lorenz R. Beschreibung einer modifizierten Technik zur Bestimmung von Qualität und Konzentration von Influenzavirus-Antikörpermolekülen mitteis der Gleichgewichtsfiltration und Vergleich ihrer Aussagefähigkeit mit der konventionellen methode. Zentralbl Bakteriol Orig A. 1973 Oct;225(1):7–18. [PubMed] [Google Scholar]
- Lubach D. Beschreibung einer Methode zur Bestimmung der Anzahl und Qualität von Influenzavirus-Antikörpermolekülen mittels der Gleichgewichtszentrifugation. Zentralbl Bakteriol Orig A. 1975;230(2):141–158. [PubMed] [Google Scholar]
- Marrie T. J., Haldane E. V., Faulkner R. S., Kwan C., Grant B., Cook F. The importance of Coxiella burnetii as a cause of pneumonia in Nova Scotia. Can J Public Health. 1985 Jul-Aug;76(4):233–236. [PubMed] [Google Scholar]
- Marrie T. J., Schlech W. F., 3rd, Williams J. C., Yates L. Q fever pneumonia associated with exposure to wild rabbits. Lancet. 1986 Feb 22;1(8478):427–429. doi: 10.1016/s0140-6736(86)92380-9. [DOI] [PubMed] [Google Scholar]
- Meiklejohn G., Reimer L. G., Graves P. S., Helmick C. Cryptic epidemic of Q fever in a medical school. J Infect Dis. 1981 Aug;144(2):107–113. doi: 10.1093/infdis/144.2.107. [DOI] [PubMed] [Google Scholar]
- Murphy A. M., Field P. R. The persistence of complement-fixing antibodies to Q-fever (Coxiella burneti) after infection. Med J Aust. 1970 Jun 6;1(23):1148–1150. doi: 10.5694/j.1326-5377.1970.tb84481.x. [DOI] [PubMed] [Google Scholar]
- Persson M. A., Brown S. E., Steward M. W., Hammarström L., Smith C. I., Howard C. R., Wahl M., Rynnel-Dagö B., Lefranc G., Carbonara A. O. IgG subclass-associated affinity differences of specific antibodies in humans. J Immunol. 1988 Jun 1;140(11):3875–3879. [PubMed] [Google Scholar]
- Reilly S., Northwood J. L., Caul E. O. Q fever in Plymouth, 1972-88. A review with particular reference to neurological manifestations. Epidemiol Infect. 1990 Oct;105(2):391–408. doi: 10.1017/s095026880004797x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousseau S., Hedman K. Rubella infection and reinfection distinguished by avidity of IgG. Lancet. 1988 May 14;1(8594):1108–1109. doi: 10.1016/s0140-6736(88)91926-5. [DOI] [PubMed] [Google Scholar]
- Salmon M. M., Howells B., Glencross E. J., Evans A. D., Palmer S. R. Q fever in an urban area. Lancet. 1982 May 1;1(8279):1002–1004. doi: 10.1016/s0140-6736(82)92000-1. [DOI] [PubMed] [Google Scholar]
- Sawyer L. A., Fishbein D. B., McDade J. E. Q fever: current concepts. Rev Infect Dis. 1987 Sep-Oct;9(5):935–946. doi: 10.1093/clinids/9.5.935. [DOI] [PubMed] [Google Scholar]
- Thomas H. I., Morgan-Capner P. Rubella-specific IgG subclass avidity ELISA and its role in the differentiation between primary rubella and rubella reinfection. Epidemiol Infect. 1988 Dec;101(3):591–598. doi: 10.1017/s0950268800029459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas H. I., Morgan-Capner P. Specific IgG subclass antibody in rubella virus infections. Epidemiol Infect. 1988 Jun;100(3):443–454. doi: 10.1017/s0950268800067182. [DOI] [PMC free article] [PubMed] [Google Scholar]


