Abstract
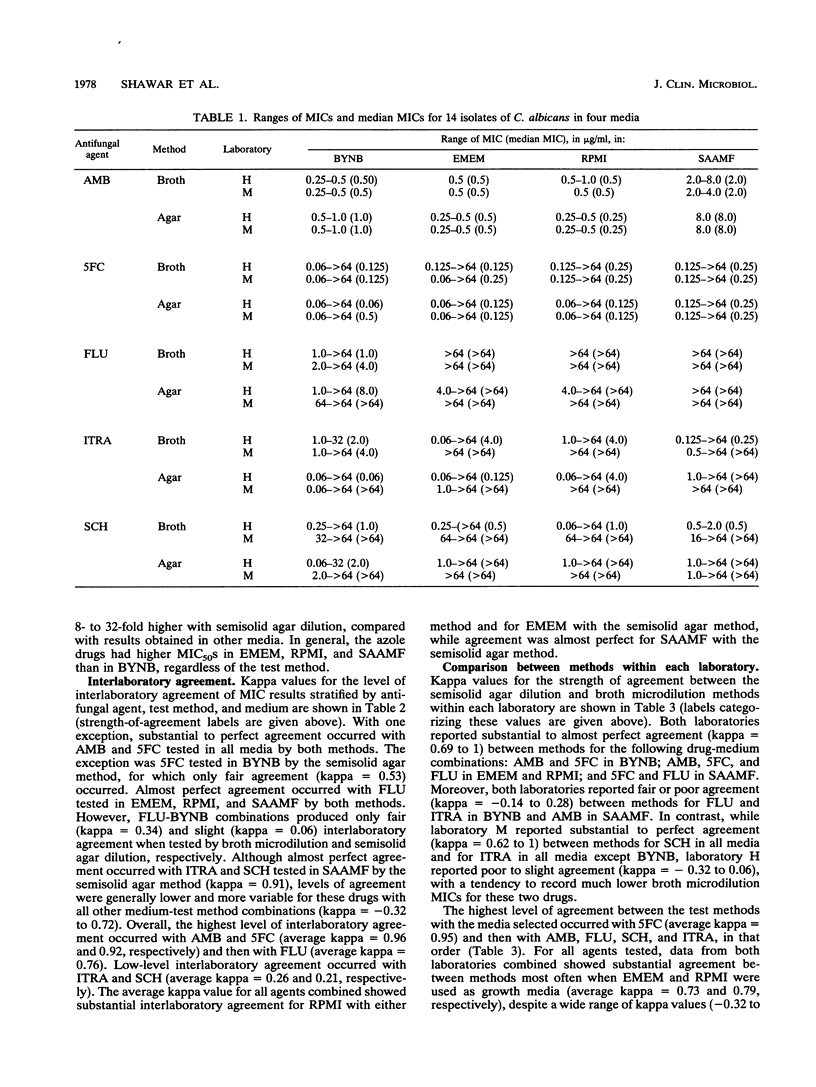
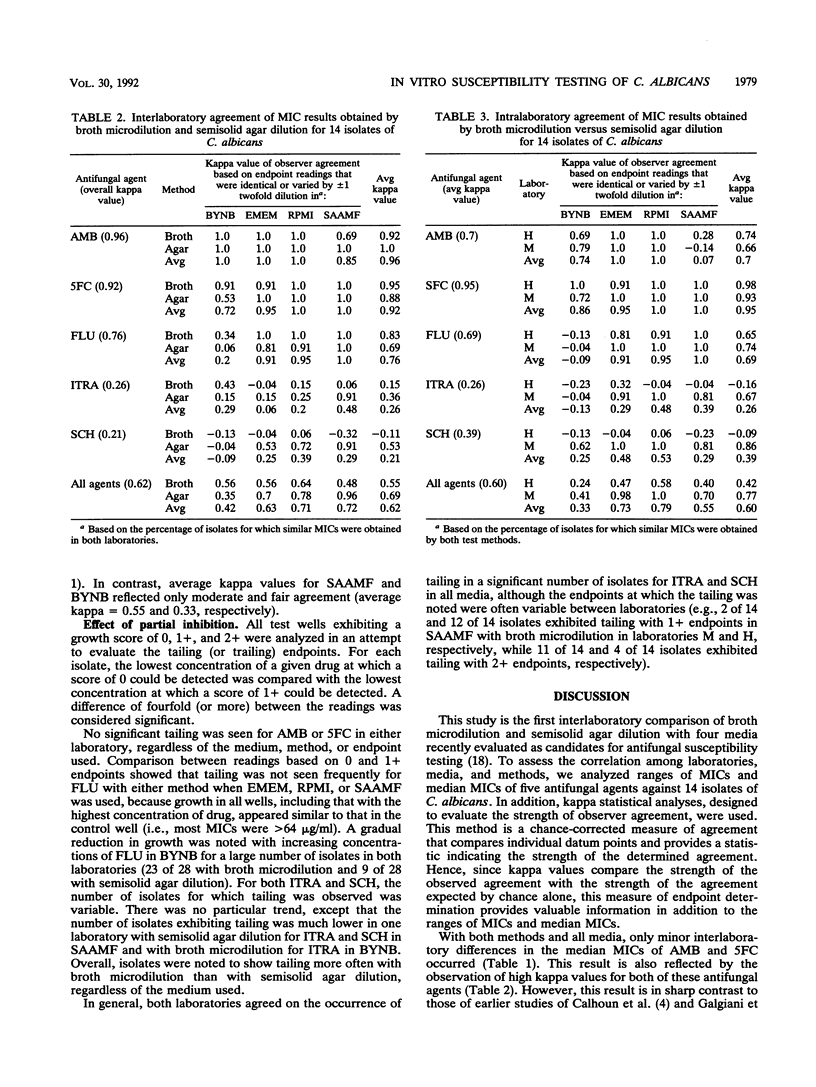
A study was performed in two laboratories to evaluate the effect of growth medium and test methodology on inter- and intralaboratory variations in the MICs of amphotericin B (AMB), flucytosine (5FC), fluconazole (FLU), itraconazole (ITRA), and the triazole Sch 39304 (SCH) against 14 isolates of Candida albicans. Testing was performed by broth microdilution and semisolid agar dilution with the following media, buffered to pH 7.0 with morpholinepropanesulfonic acid (MOPS): buffered yeast nitrogen base (BYNB), Eagle's minimal essential medium (EMEM), RPMI 1640 medium (RPMI), and synthetic amino acid medium for fungi (SAAMF). Inocula were standardized spectrophotometrically, and endpoints were defined by the complete absence of growth for AMB and by no more than 25% of the growth in the drug-free control for all other agents. Comparative analyses of median MICs, as determined by each test method, were made for all drug-medium combinations. Both methods yielded similar (+/- 1 twofold dilution) median MICs for AMB in EMEM and RPMI, 5FC in all media, and FLU in EMEM, RPMI, and SAAMF. In contrast, substantial between-method variations in median MICs were seen for AMB in BYNB and SAAMF, FLU In BYNB, and ITRA and SCH in all media. Interlaboratory concordance of median MICs was good for AMB, 5FC, and FLU but poor for ITRA and SCH in all media. Endpoint determinations were analyzed by use of kappa statistical analyses for evaluating the strength of observer agreement. Moderate to almost perfect interlaboratory agreement occurred with AMB and 5FC in all media and with FLU in EMEM, RPMI, and SAAMF, irrespective of the test method. Slight to almost perfect interlaboratory agreement occurred with ITRA and SCH in EMEM, RPMI, and SAAMF when tested by semisolid agar dilution but not broth microdilution. Kappa values assessing intralaboratory agreement between methods were high for 5FC in all media, for AMB in BYNB, ENEM, and RPMI, and for FLU in EMEM, RPMI, and SAAMF. One laboratory, but not the other, reported substantial to almost perfect agreement between methods for ITRA, and SCH in EMEM, RPMI, and SAAMF. Both laboratories reported poor agreement between methods for the azoles in BYNB. Discrepancies noted in azole-BYNB combinations were largely due to the greater inhibitory effect of these agents in BYNB than in other media. These results indicate that the semisolid agar dilution and broth microdilution methods with EMEM or RPMI yield equivalent and reproducible MICs for AMB, 5FC, and FLU but not ITRA and SCH.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anaissie E., Paetznick V., Bodey G. P. Fluconazole susceptibility testing of Candida albicans: microtiter method that is independent of inoculum size, temperature, and time of reading. Antimicrob Agents Chemother. 1991 Aug;35(8):1641–1646. doi: 10.1128/aac.35.8.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armelles M. Azole sensitivity in Candida albicans. FEMS Microbiol Lett. 1989 Jul 15;51(1):153–157. doi: 10.1016/0378-1097(89)90499-0. [DOI] [PubMed] [Google Scholar]
- Calhoun D. L., Galgiani J. N. Analysis of pH and buffer effects on flucytosine activity in broth dilution susceptibility testing of Candida albicans in two synthetic media. Antimicrob Agents Chemother. 1984 Sep;26(3):364–367. doi: 10.1128/aac.26.3.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun D. L., Roberts G. D., Galgiani J. N., Bennett J. E., Feingold D. S., Jorgensen J., Kobayashi G. S., Shadomy S. Results of a survey of antifungal susceptibility tests in the United States and interlaboratory comparison of broth dilution testing of flucytosine and amphotericin B. J Clin Microbiol. 1986 Feb;23(2):298–301. doi: 10.1128/jcm.23.2.298-301.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doern G. V., Tubert T. A., Chapin K., Rinaldi M. G. Effect of medium composition on results of macrobroth dilution antifungal susceptibility testing of yeasts. J Clin Microbiol. 1986 Oct;24(4):507–511. doi: 10.1128/jcm.24.4.507-511.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinel-Ingroff A., Kerkering T. M., Goldson P. R., Shadomy S. Comparison study of broth macrodilution and microdilution antifungal susceptibility tests. J Clin Microbiol. 1991 Jun;29(6):1089–1094. doi: 10.1128/jcm.29.6.1089-1094.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galgiani J. N., Reiser J., Brass C., Espinel-Ingroff A., Gordon M. A., Kerkering T. M. Comparison of relative susceptibilities of Candida species to three antifungal agents as determined by unstandardized methods. Antimicrob Agents Chemother. 1987 Sep;31(9):1343–1347. doi: 10.1128/aac.31.9.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon M. A., Lapa E. W., Passero P. G. Improved method for azole antifungal susceptibility testing. J Clin Microbiol. 1988 Sep;26(9):1874–1877. doi: 10.1128/jcm.26.9.1874-1877.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes C. E., Bennett R. L., Beggs W. H. Broth dilution testing of Candida albicans susceptibility to ketoconazole. Antimicrob Agents Chemother. 1987 Apr;31(4):643–646. doi: 10.1128/aac.31.4.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landis J. R., Koch G. G. The measurement of observer agreement for categorical data. Biometrics. 1977 Mar;33(1):159–174. [PubMed] [Google Scholar]
- McIntyre K. A., Galgiani J. N. In vitro susceptibilities of yeasts to a new antifungal triazole, SCH 39304: effects of test conditions and relation to in vivo efficacy. Antimicrob Agents Chemother. 1989 Jul;33(7):1095–1100. doi: 10.1128/aac.33.7.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odds F. C. Laboratory evaluation of antifungal agents: a comparative study of five imidazole derivatives of clinical importance. J Antimicrob Chemother. 1980 Nov;6(6):749–761. doi: 10.1093/jac/6.6.749. [DOI] [PubMed] [Google Scholar]
- Pfaller M. A., Burmeister L., Bartlett M. S., Rinaldi M. G. Multicenter evaluation of four methods of yeast inoculum preparation. J Clin Microbiol. 1988 Aug;26(8):1437–1441. doi: 10.1128/jcm.26.8.1437-1441.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaller M. A., Rinaldi M. G., Galgiani J. N., Bartlett M. S., Body B. A., Espinel-Ingroff A., Fromtling R. A., Hall G. S., Hughes C. E., Odds F. C. Collaborative investigation of variables in susceptibility testing of yeasts. Antimicrob Agents Chemother. 1990 Sep;34(9):1648–1654. doi: 10.1128/aac.34.9.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warnock D. W. Antifungal drug susceptibility testing. Curr Top Med Mycol. 1989;3:403–416. doi: 10.1007/978-1-4612-3624-5_12. [DOI] [PubMed] [Google Scholar]


