Abstract
Objectives
To test the hypothesis that duration of breast feeding is related to changes in vascular function relevant to the development of cardiovascular disease.
Design
Population based observational study.
Setting
Cambridge.
Participants
331 adults (171 women, 160 men) aged between 20 and 28 years, born in Cambridge Maternity Hospital.
Main outcome measures
Distensibility of brachial artery, type and duration of infant feeding, current lipid profile, and other cardiovascular risk factors.
Results
The longer the period of breast feeding the less distensible the artery wall in early adult life, with no sex differences (regression coefficient = −3.93 μm/month, 95% confidence interval −7.29 to −0.57, P=0.02). However, in those breast fed for less than four months, arterial distensibility was not significantly reduced compared with an exclusively formula fed group. The vascular changes observed were not explained by alterations in plasma cholesterol concentration in adult life.
Conclusions
Breast feeding in infancy is related to reduced arterial function 20 years later. These data should not alter current recommendations in favour of breast feeding, which has several benefits for infant health. Further work is needed, however, to explore the optimal duration of breast feeding in relation to cardiovascular outcomes.
Introduction
Nutrition in early postnatal life may have major, long term “programming” effects on the physiology, metabolism, and clinical outcome of animals and humans.1–3 Coronary artery disease is one outcome in humans linked to early growth and nutrition. Although breast feeding has been associated with a lower risk of cardiovascular disease, men born earlier this century who had still been breast fed aged 1 year had higher rates of ischaemic heart disease 60-70 years later compared with the expected rate for men of that age.2 If this observation were causal, it would raise an important question about the optimal duration of breast feeding.
Although observational evidence linking early nutrition to later cardiovascular disease might be subject to confounding, experimental evidence also exists linking atherosclerosis and breast feeding in non-human primates. Studies in baboons that were breast fed or formula fed throughout infancy and then placed on a Western style diet, high in saturated fats, showed that those previously breast fed had an abnormal lipid profile and more arterial fatty streaks in adulthood.1 One hypothesis was that breast feeding programmed baboons to be conservative with cholesterol—perhaps appropriately for their natural diet—but when they consumed a Western diet this programming led to arterial disease. Were these findings supported in humans, it would have implications for infants weaned on to a Western diet.
We studied a UK population to test further the hypothesis that duration of breast feeding might influence later emergence of vascular disease. To minimise potential influences of other lifestyle factors throughout adulthood we studied a young cohort. This was feasible because early pathophysiological changes in the artery, relevant to the development of atherosclerosis, can now be measured early in life with non-invasive imaging techniques.4,5 Our group has already shown links between vascular dysfunction and growth and nutrition during childhood.6,7 In this study, we measured arterial distensibility, a measure of vessel wall stiffness, in a large cohort of adults in their 20s and related it to duration of breast feeding.
Participants and methods
Study design
We studied 331 subjects (160 men; 171 women) aged 20 to 28 years born in Cambridge Maternity Hospital. We invited random samples of those born between 1969 and 1975 to attend a clinic for evaluation of cardiovascular risk profile by venepuncture, questionnaire, physical measurements, and vascular studies. Invitations were sent until the planned sample size was reached. Ethical approval and informed consent were obtained. To determine possible biases in the study sample, we compared the distribution of risk factors in this group to that in the United Kingdom and to data obtained in our questionnaire from a subgroup of people who initially declined to participate.
Measurement of cardiovascular risk factors
We analysed a fasting venous blood sample for insulin, glucose, and lipid profile using routine methods. Weight, height, blood pressure, and smoking history were recorded. We assessed social influences by attained education level and socioeconomic groupings.8 Before attending, participants collected information from their mothers on how they had been fed as an infant. We grouped them as either only bottle fed or having received breast milk. For those who had been breast fed, we recorded how long breast feeding had continued.9
Vascular study measurements
Distension of the brachial artery during the cardiac cycle was measured as described previously.7,10 Briefly, the artery was imaged with an Acuson 128XP/10 and movement of the arterial walls recorded with an automated wall tracking system (Ingenious Medical Systems, Arnhem, Netherlands). By relating change in diameter over the cardiac cycle to pulse pressure, “stiffness” of the vessel wall can be quantified.
Statistical analysis
We assessed continuous relations between explanatory variables and arterial distensibility using multiple regression. Variables were related to absolute distension with pulse pressure, age, sex, and vessel size as independent variables.7 Further models examined adjustment for other factors. To investigate the effect of duration of breast feeding further, the group was dichotomised around the median duration of breast feeding. We then assessed differences between these divisions, as well as other groups (such as breast fed compared with bottle fed), using t tests. Distensibility was represented as a coefficient (change in arterial cross sectional area between diastole and systole relative to the area at diastole, divided by pulse pressure).5,7
Results
We sent invitations to 1526 people, of whom 420 (28%) agreed to participate, 229 (15%) declined, and 877 (57%) did not reply. In all, 344 (23%) subjects were able to attend for vascular investigations. Full details of vascular function, risk factors, and infant feeding practice were available for 331 (96%) of these (table 1). The study group had similar body size, body mass index, blood pressure, and smoking rates to young adults generally in the United Kingdom.11
Table 1.
Main characteristics of study population. Values are mean (SD or range) unless stated otherwise
| Variable | Breast fed | Bottle fed | P value for difference between groups | Participants who only completed questionnaire* |
|---|---|---|---|---|
| No of participants (men/women) | 149 (67/82) | 182 (93/89) | — | 55 (27/28) |
| Age (years) | 23 (20 to 28) | 23 (20 to 27) | 0.07 | 23 (21 to 27) |
| Height (cm) | 170 (10) | 168 (9) | 0.03 | 161 (25) |
| Weight (kg) | 70.4 (14.5) | 68.7 (13.1) | 0.28 | 68.3 (17) |
| Body mass index (kg/m2) | 24.2 (4.1) | 24.3 (3.7) | 0.83 | 26.8 (6.3) |
| Length of breast feeding (months) | 3.33 (0 to 18) | — | — | — |
| Resting arterial diameter (mm) | 3.32 (0.59) | 3.28 (0.59) | 0.45 | — |
| Distensibility coefficient (mm Hg−1) | 0.133 (0.07) | 0.140 (0.08) | 0.38 | — |
| Cholesterol (mmol/l) | 4.43 (0.99) | 4.61 (1.01) | 0.11 | — |
| LDL cholesterol (mmol/l) | 2.71 (0.88) | 2.90 (0.93) | 0.07 | — |
| HDL cholesterol (mmol/l) | 1.18 (0.25) | 1.18 (0.31) | 0.96 | — |
| Systolic blood pressure (mm Hg) | 128 (14) | 128 (14) | 0.93 | — |
| Diastolic blood pressure (mm Hg) | 70 (9) | 71 (8) | 0.31 | — |
| Smoking history (No (%)): | ||||
| Smokers | 49 (33) | 64 (35) | 0.78 | 21 (38) |
| Former smokers | 25 (17) | 22 (12) | 3 (6) | |
| Non-smokers | 75 (50) | 96 (53) | 31 (56) | |
| No (%) in social class: | ||||
| I | 12 (8) | 13 (7) | 0.19 | — |
| II | 36 (24) | 33 (18) | — | |
| IIINM | 51 (34) | 62 (34) | — | |
| IIIM | 24 (16) | 36 (20) | — | |
| IV | 22 (15) | 33 (18) | — | |
| V | 4 (3) | 5 (3) | — |
LDL=low density lipoprotein, HDL= high density lipoprotein. *Data presented for comparison.
Arterial distensibility
The distensibility coefficient was 0.129 mm Hg-1 in men and 0.145 mm Hg-1 in women (95% confidence interval for difference −0.032 to −0.002, P=0.04). There was no variation with age (regression coefficient= −1.6, 95% confidence interval −4.9 to 1.8, P=0.36). As expected, reduced distensibility was related to increased total serum cholesterol concentration and systolic blood pressure (table 2).7 Social class and smoking were unrelated to arterial function. Arterial distensibility was not related to birthweight or weight in infancy.
Table 2.
Linear regression coefficients for associations between main variables and arterial distension (with pulse pressure, age, sex, and resting vessel size included in model). Units are μm per unit change in explanatory variable
| Variable | Regression coefficient (95% CI) | P value |
|---|---|---|
| Total cholesterol (mmol/l) | −8.5 (−14.5 to −2.5) | 0.005 |
| LDL cholesterol (mmol/l) | −8.8 (−15.6 to −1.9) | 0.01 |
| Body mass index (kg/m2) | −21.1 (−36.5 to 5.8) | 0.007 |
| Diastolic blood pressure (mm Hg) | −1.92 (−2.68 to −1.16) | <0.001 |
| Social class | −0.1 (−0.84 to 0.60) | 0.75 |
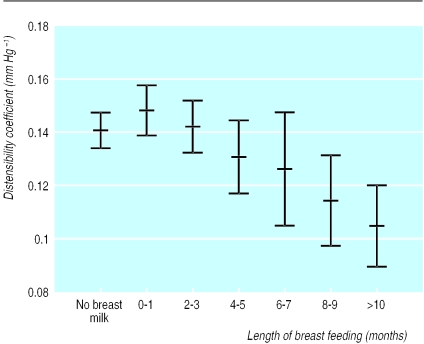
There was an inverse relation between duration of breast feeding and arterial distensibility (regression coefficient = −3.93 μm/month, 95% confidence interval −7.29 to −0.57, P=0.02) (figure). The regression coefficient was negative in men (−2.9) and women (−4.3) analysed separately, although because of reduced sample size the result was significant only in women. There was no interaction between sex and duration of breast feeding, and we therefore conducted further analyses on the combined cohort. Participants who had been exclusively breast fed for less than four months had similar distensibility to those who had never been breast fed (distensibility in bottle fed=0.1388 mm Hg-1 and breast fed less than four months=0.1457 mm Hg-1, mean difference=0.007 mm Hg-1, 95% confidence interval −0.026 to 0.012, P=0.46). Those who had been breast fed for four months or more had significantly lower distensibility than those who had been breast fed for less than four months (distensibility for those breast fed for four months or more=0.1200 mm Hg-1, mean difference from those breast fed for less than four months=−0.026 mm Hg-1, −0.038 to −0.048, P=0.02).
Relation of duration of breast feeding to cardiovascular risk factors and other variables
Duration of breast feeding was unrelated to lipid profile, body size, body mass index, smoking patterns, blood pressure, social class, birthweight, or prevalence of premature cardiovascular disease in relatives (regression coefficient between duration of breast feeding and cholesterol concentration=−0.16 months/mmol/l, 95% confidence interval −0.49 to 0.17, P=0.4). The strength of the association between duration of breast feeding and distensibility was unaffected by inclusion of current lipid profile, body mass index, height, weight, or social class in the regression model (table 3).
Table 3.
Linear regression coefficients for relation between length of breast feeding and distensibility with adjustments for pulse pressure, age, sex, and resting vessel plus cholesterol concentration, body mass index, and socioeconomic factors
| Variable | Regression coefficient (95% CI) (μm/month) | P value |
|---|---|---|
| Length of breast feeding | −3.9 (−7.3 to −0.6) | 0.02 |
| +Total cholesterol | −3.8 (−7.2 to −0.4) | 0.03 |
| +Body mass index | −3.9 (−7.2 to −0.5) | 0.02 |
| +Social class | −3.9 (−7.3 to −0.6) | 0.02 |
Discussion
We found that distensibility of the brachial artery in young adults was related to the duration of breast feeding in infancy. Longer duration of breast feeding was associated with stiffer arteries. As we studied young adults, our results are less likely to be confounded by the effects of other cardiovascular risk factors that are present in older cohorts. The results suggest that the effect of breast feeding on cardiovascular risk occurs early in life.
Possible explanations for the effect
Breast feeding evolved in mammals over millions of years and is generally considered optimal for humans. The commonly cited advantages over formula feeding include reduced infection and atopy and increased cognitive development.12 The optimal duration of breast feeding remains inadequately researched. We found an apparent adverse outcome in people who had had prolonged breast feeding. This finding is consistent with the results of an observational study in which men who had been breast fed at 1 year had an increased risk of ischaemic heart disease 60 years later.2 This finding was suggested to be due to programming of cholesterol metabolism in response to the unique lipid or hormone contents of breast milk. These factors might alter expression of hepatic enzymes or low density lipoprotein receptors, changing the response to a high fat diet later in life.13–16 This hypothesis is consistent with the experimental studies in primates, where breastfed baboons given a Western style high saturated fat diet had more early atherosclerotic changes in adulthood than formula fed animals.
In our study, however, longer duration of breast feeding was not associated with significantly higher total cholesterol or lipoprotein concentration in adulthood. Furthermore, models relating breast feeding to distensibility were not affected by inclusion of current lipid profile. Thus our results do not support a hypothesis of deranged blood lipid profiles in adulthood.
An alternative hypothesis would be that breast feeding influences lipid metabolism much earlier, during the infant feeding period, resulting in early formation of arterial fatty streaks. Animal studies show arterial elasticity is reduced during fatty streak formation in infancy before other pathophysiological changes.17 Cholesterol is laid down in the vessel wall from the first months, with a peak prevalence of fatty streaks during the first year18 when cholesterol concentrations are higher in breastfed infants.19,20 If breast feeding is extended throughout infancy, the vessel wall is exposed to raised circulating cholesterol concentrations for longer. Most arterial lesions seem to regress initially, but in the primate studies above, animals breast fed as infants and then placed on a high saturated fat diet had more fatty streaks in adulthood.1 In humans, such early arterial changes might persist into later life if subsequent diet interferes with their natural regression. A small, inconclusive postmortem study of adolescents showed more arterial lesions in those who had been breast fed, further supporting an early effect of breast feeding.21 More work is needed to investigate dietary differences in vascular lipid deposition in early life.
Relevance of arterial distensibility
Arterial distensibility is known to diminish with age in relation to cardiovascular risk factors and is reduced in subjects with frank coronary disease.22,23 Indeed, the potential impact of such risk factors is apparent in this study, which showed reduced arterial distensibility in young adults with higher blood pressure, cholesterol, or body mass index. Although further investigation is needed, it seems likely that arterial distensibility is related to risk of vascular disease.
There was no direct record of feeding method or duration. We used maternal recall, which has been independently validated for collecting such data9 and provides reproducible data 30 or more years after birth.24 Further early dietary information was limited as the study was retrospective. Prospective investigations could take account of age of weaning and subsequent diet as well as the more recent secular changes in infant feeding, notably duration of use and design of infant formula.
Comparison of the relative influence of risk factors on arterial distensibility suggests that breast feeding has an important effect. Extending breast feeding by two months has an effect on arterial distensibility that is broadly equivalent to that produced by a 1 mmol/l rise in cholesterol or 4 mm Hg increase in blood pressure. Although the effect is apparently larger in women, there was no significant difference between sexes. This may be because the underlying arterial changes were established before development of the hormonal protection that reduces the incidence of coronary disease in women during reproductive life.
Implications
We emphasise that our observational data do not establish a causal relation between length of breast feeding and cardiovascular disease. Nevertheless, our findings are supported by previous human and experimental animal data.1,2 We tried to minimise confounding by studying a young cohort and adjusted for potential influences on distensibility that might also relate to duration of breast feeding. Further confounding should be explored in future studies.
Since breast feeding is widely accepted and promoted as the best nutrition for infants, any suggestion of an association between duration of breast feeding and vascular health requires careful and critical examination. We took full account of the feeding period and showed a graded relation between length of breast feeding and the decline in distensibility. However, arterial distensibility was similar in those breast fed for up to four months to that in participants who were not breast fed.
At this stage our findings should not influence current advice on the importance of breast feeding. Even if prolonged breast feeding were confirmed to have disadvantages, these would need to be carefully weighed against the advantages. For example, in developing countries the benefit of prolonged breast feeding in infants and toddlers would be an overriding consideration. Nevertheless, there is an urgent public health need to study further the possible influence of a longer period of breast feeding on the evolution of arterial disease.
What is already known on this topic
No data exist on the optimal duration of breast feeding
One study found that men who had been breast fed at 1 year had higher rates of ischaemic heart disease
What this study adds
Arterial distensibility (a marker of early cardiovascular disease) in adults aged 20-28 decreased with increasing duration of breast feeding
No difference in distensibility was found between participants who had been bottle fed and those breast fed for less than four months
Duration of breast feeding was not related to cholesterol concentration in adulthood
Any effect of breast feeding on cardiovascular risk probably occurs in infancy
Figure.
Mean (SE) distensibility coefficient for brachial artery in relation to duration of breast feeding
Acknowledgments
We thank Ian Merryweather, Sarah Jones, and Tristan Cope for help with data collection and laboratory analysis and Dr David Muller for coordinating the biochemical work.
Editorial by Booth
Footnotes
Funding: The work was funded by the Medical Research Council. All work in the Institute of Child Health and Great Ormond Street Hospital also benefits from NHS research and development funding.
Competing interests: The centre has collaborated with the infant food industry for its outcome studies on nutrition.
References
- 1.Mott GE, Lewis DS, McGill HC. Ciba Foundation symposium. The childhood environment and adult disease: Chichester: Wiley; 1991. Programming of cholesterol metabolism by breast and formula feeding; pp. 128–174. [DOI] [PubMed] [Google Scholar]
- 2.Fall CH, Barker DJP, Osmond C, Winter PD, Clark PM, Hales CN. Relation of infant feeding to adult serum cholesterol concentration and death from ischaemic heart disease. BMJ. 1992;304:801–805. doi: 10.1136/bmj.304.6830.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lucas A. Ciba Foundation symposium. The childhood environment and adult disease. Chichester: Wiley; 1991. Programming by early nutrition in man; pp. 38–55. [PubMed] [Google Scholar]
- 4.Celermajer DS, Sorenson KE, Gooch VM, Spiegelhalter DJ, Miller OT, Sullivan I, Deanfield JE. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet. 1992;340:1111–1115. doi: 10.1016/0140-6736(92)93147-f. [DOI] [PubMed] [Google Scholar]
- 5.Hoeks APG, Brands PJ, Smeets FAM, Reneman RS. Assessment of the distensibility of superficial arteries. Ultrasound Med Biol. 1990;16:121–128. doi: 10.1016/0301-5629(90)90139-4. [DOI] [PubMed] [Google Scholar]
- 6.Leeson CPM, Whincup PH, Cook DG, Donald AE, Papacosta O, Lucas A, et al. Flow mediated dilation in 9-11 year old children: the influence of childhood and intrauterine factors. Circulation. 1997;96:2233–2238. doi: 10.1161/01.cir.96.7.2233. [DOI] [PubMed] [Google Scholar]
- 7.Leeson CPM, Whincup PH, Cook DG, Mullen MJ, Donald AE, Seymour CA, et al. Cholesterol and arterial distensibility in the first decade of life: a population-based study. Circulation. 2000;101:1533–1538. doi: 10.1161/01.cir.101.13.1533. [DOI] [PubMed] [Google Scholar]
- 8.Office of Population Censuses and Surveys. Occupational mortality decennial supplement 1979-80; 1982-83. London: HMSO; 1986. [Google Scholar]
- 9.Kark JD, Troya G, Friedlander Y, Slater PE, Stein Y. Validity of maternal reporting of breast feeding history and the association with blood lipids in 17 year olds in Jerusalem. J Epidemiol Community Health. 1984;38:218–225. doi: 10.1136/jech.38.3.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leeson CPM, Thorne S, Donald AE, Mullen MJ, Clarkson P, Deanfield JE. Non-invasive measurement of endothelial function: effect on brachial artery dilation of graded endothelial dependent and independent stimuli. Heart. 1997;78:22–27. doi: 10.1136/hrt.78.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Office of Population Censuses and Surveys. Health survey of England 1995. London: HMSO; 1997. [Google Scholar]
- 12.American Academy of Pediatrics. Breast feeding and the use of human milk. Pediatrics. 1997;100:1035–1039. doi: 10.1542/peds.100.6.1035. [DOI] [PubMed] [Google Scholar]
- 13.Phillips DI, Barker DJP, Osmond C. Infant feeding, fetal growth and adult thyroid function. Acta Endocrinol Copenh. 1993;129:134–138. doi: 10.1530/acta.0.1290134. [DOI] [PubMed] [Google Scholar]
- 14.Mott GE, Jackson EM, DeLallo L, Lewis DS, McMahan CA. Differences in cholesterol metabolism in juvenile baboons are programmed by breast versus formula feeding. J Lipid Res. 1995;36:299–307. [PubMed] [Google Scholar]
- 15.Wong WW, Hachey DL, Insull W, Opekun AR, Klein PD. Effect of dietary cholesterol on cholesterol synthesis in breast fed and formula fed infants. J Lipid Res. 1993;34:1403–1411. [PubMed] [Google Scholar]
- 16.Mott GE, DeLallo L, Driscoll DM, McMahan CA, Lewis DS. Influence of breast and formula feeding on hepatic concentrations of apolipoprotein and low density lipoprotein receptor mRNAs. Biochem Biophys Acta. 1993;1169:59–65. doi: 10.1016/0005-2760(93)90082-k. [DOI] [PubMed] [Google Scholar]
- 17.Hironaka K, Yano M, Kohno M, Tanigawa T, Obayashi M, Konishi M, et al. In vivo aortic wall characteristics at the early stage of atherosclerosis in rabbits. Am J Physiol. 1997;273:H1142–H1147. doi: 10.1152/ajpheart.1997.273.3.H1142. [DOI] [PubMed] [Google Scholar]
- 18.Stary HC. Evolution and progression of atherosclerotic lesions in coronary arteries in children and young adults. Arteriosclerosis. 1989;99(suppl I):I 19–32. [PubMed] [Google Scholar]
- 19.Friedman G, Goldberg SJ. Concurrent and subsequent serum cholesterol of breast and formula fed infants. Am J Clin Nutr. 1975;281:42–45. doi: 10.1093/ajcn/28.1.42. [DOI] [PubMed] [Google Scholar]
- 20.Akeson PM, Axelsson IE, Raiha NC. Plasma lipids and apolipoproteins in breast-fed and formula-fed Swedish infants. Acta Paediatr. 1999;881:1–6. doi: 10.1111/j.1651-2227.1999.tb01258.x. [DOI] [PubMed] [Google Scholar]
- 21.Burr ML, Beasley WH, Fisher CB. Breast feeding, maternal smoking and early atheroma. Eur Heart J. 1984;5:588–591. doi: 10.1093/oxfordjournals.eurheartj.a061709. [DOI] [PubMed] [Google Scholar]
- 22.Benetos A, Laurent S, Hoeks APG, Bontonyrie PH, Safar ME. Arterial alterations with ageing and high blood pressure. Arterioscler Thromb. 1993;13:90–97. doi: 10.1161/01.atv.13.1.90. [DOI] [PubMed] [Google Scholar]
- 23.Dart AM, Lacombe F, Yeoh JK, Cameron JD, Jennings GL, Laufer E, et al. Aortic distensibility in patients with isolated hypercholesterolaemia, coronary artery disease or cardiac transplant. Lancet. 1991;338:696–697. doi: 10.1016/0140-6736(91)90415-l. [DOI] [PubMed] [Google Scholar]
- 24.Tomeo CA, Rich-Edwards JW, Michels KB, Berkey CS, Hunter DJ, Frazier AL, et al. Reproducibility and validity of maternal recall of pregnancy-related events. Epidemiology. 1999;10:774–777. [PubMed] [Google Scholar]