Abstract
Coordination-driven self-assembly via the directional-bonding approach utilizes rigid transition metal acceptors and electron-rich donors to allow for complex, nanoscale 2D polygons and 3D polyhedra to be prepared under mild conditions and in high yields. To ensure proper rigidity and directionality, many acceptor and donor precursors contain largely carbon-rich aromatic and/or acetylenic moieties. This article introduces self-assembly as an alternative means of synthesizing carbon-rich materials and discusses the development, design, synthesis, and applications of carbon-rich supramolecular metallacycles and metallacages as well as the self-assembly of new diastereomeric carbon-rich supramolecular triangles.
1. Introduction
Carbon-rich materials1 have attracted the interest and attention of chemists ever since the extraction and identification of polycycles during petroleum analysis began in the late 1800’s. In the intervening years, seminal studies, led initially by Glaser, Scholl, and Clar, have transformed the science of carbon-rich materials from one primarily of analysis to one of planned preparation.2,3 Whereas it was once a distinct challenge to synthesize even a relatively simple polycyclic aromatic hydrocarbon (PAHs) such as naphthalene – a challenge originally overcome by Radziszewski4 in 1878 – it is now possible to carry out a chemical synthesis of such highly complex PAHs as C60, as was done by Scott5 in 2001. Even so, as the field of carbon-rich molecules expands further, continually branching out into new areas with new applications, there is a persistent need for new and innovative ways of preparing these important compounds. Recent advances in coordination-driven self-assembly have provided such a means and have enabled a wide variety of carbon-rich metal-organic supramolecules to be prepared.
Over the past 150 years a variety of methods have been devised for preparing carbon-rich compounds. Originally, most techniques relied upon fairly harsh conditions typically requiring high temperatures and/or strong oxidizing agents and often resulted in low yields. Milder, higher yielding methods have since been developed such as Clar’s Zn-dust reductions,2 oxidative couplings of terminal alkynes,6 Scholl-type intramolecular cyclodehydrogenation,7 hv-induced ring closures,8 cyclodehydration,9 and Diels-Alder cycloaddition,10 just to name a few. High temperature flash vacuum pyrolysis (FVP), however, remains a very popular means of preparing carbon-rich molecules despite its often-poor yields. Self-assembly, on the other hand, is a synthetic technique that is typified by mild reaction conditions and high yields.
1.1. Coordination-Driven Self-Assembly of Carbon-Rich Supramolecules
Molecular self-assembly11 is a process through which properly designed, complementary molecular subunits spontaneously assemble according to the specific noncovalent “information” encoded within their structures. The use of noncovalent interactions (e.g. hydrogen-bonding, π-π, donor-acceptor, charge-charge, etc.) to direct self-assembly enables product formation to be thermodynamically controlled. Individual building-blocks are able to continually and reversibly assemble in a variety of ways until the most thermodynamically stable product is obtained. More often than not, the result of such dynamic12 self-assembly processes is one highly symmetric, thermodynamically stable product in high and often quantitative yield. With appropriate planning, individual precursor building-blocks may be designed such that they lead specifically to a single desired supramolecule possessing unique architectural or topological13 characteristics. While many examples of self-assembled supramolecules involve flexible, significantly saturated carbon backbones11c,d,f and, thus, may not fit the typical definition of being carbon-rich compounds, there are also myriad ways of using the virtues of self-assembly (high yield, mild conditions, complex architectures) to prepare carbon-rich molecules.
As a design and synthesis methodology, coordination-driven14 self-assembly is especially useful for preparing carbon-rich molecules. Coordination-driven self-assembly relies upon the use of dative metal-organic interactions between electron-poor transition metal acceptors and electron-rich organic donors and is often used in conjunction with the directional-bonding15 approach to self-assembly. According to the directional-bonding protocol, discrete supramolecular assemblied will be formed in accordance to the specific geometric information – the “directionality” – stored within the pre-defined angles and symmetry of rigid precursors and the stoichiometric ratio in which those precursors are mixed. Rigid acceptor and donor precursors are necessary so that they retain their directionality, increasing the likelihood of a successful self-assembly, i.e. one that results in a high yield of a single discrete product as opposed to a mixture of polymeric, oligomeric, or other side products. With these design criteria in mind, the vast majority of examples that follow the directional-bonding approach to coordination-driven self-assembly utilize largely aromatic and alkynyl building-blocks. The resulting self-assembled metal-organic supramolecules are often quite carbon-rich.
1.2. Carbon-Rich Polygons and Polyhedra
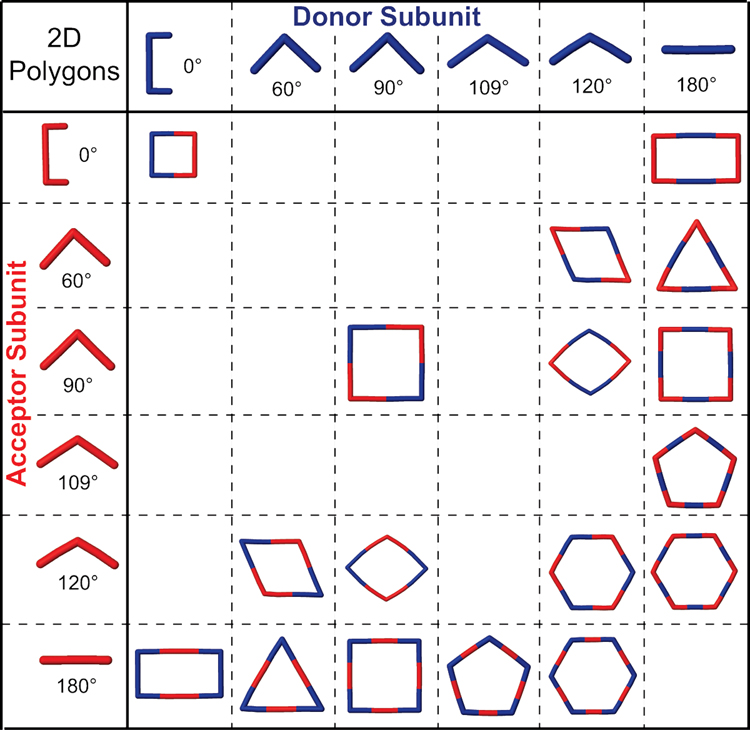
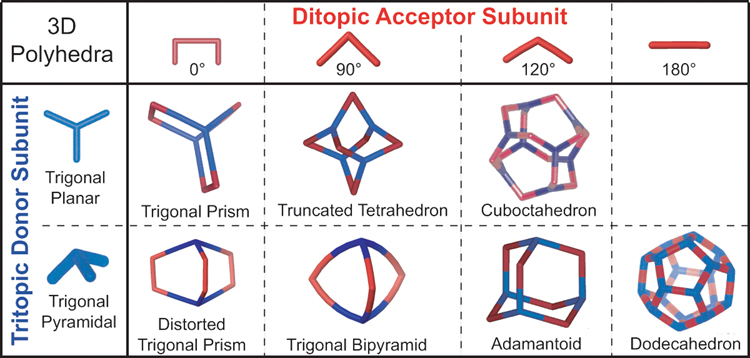
The use of coordination-driven self-assembly in the synthesis of carbon-rich compounds has many advantages. When precursors are carefully designed it is possible to self-assemble 2D metallacyclic polygons and 3D metallacage polyhedra while exerting precise control over the often highly-complex structures of the supramolecular products. Representative collections of 2D and 3D metallacycles that can be prepared by the directional-bonding approach are shown in Figure 1 and Figure 2, respectively. It would likely prove exceedingly difficult, if not altogether impossible, to prepare analogous structures in high yields by traditional synthetic methods. Dynamic self-assembly, on the other hand, as a thermodynamically controlled process, enables such complex structures to be prepared in significantly fewer synthetic steps and often in near quantitative yields. Preparation of these highly symmetric polygons and polyhedra is very modular, enabling both their architecture and properties to be easily manipulated simply by changing the size, shape, coordination number, and/or functionality of individual building units.
Figure 1.
The directional-bonding approach to the self-assembly of 2D metallacyclic polygons.
Figure 2.
The directional-bonding approach to the self-assembly of select 3D metallacages.
2. Metallacyclic Polygons
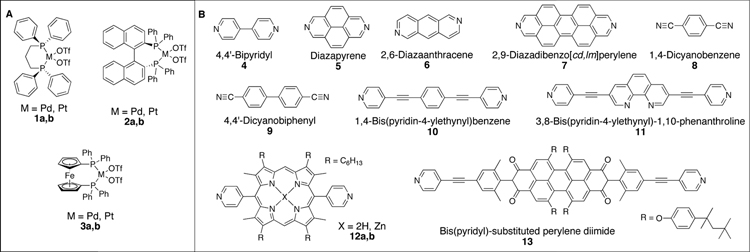
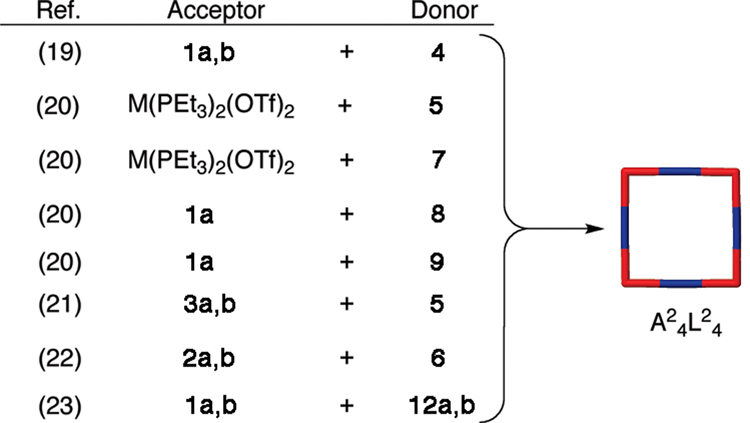
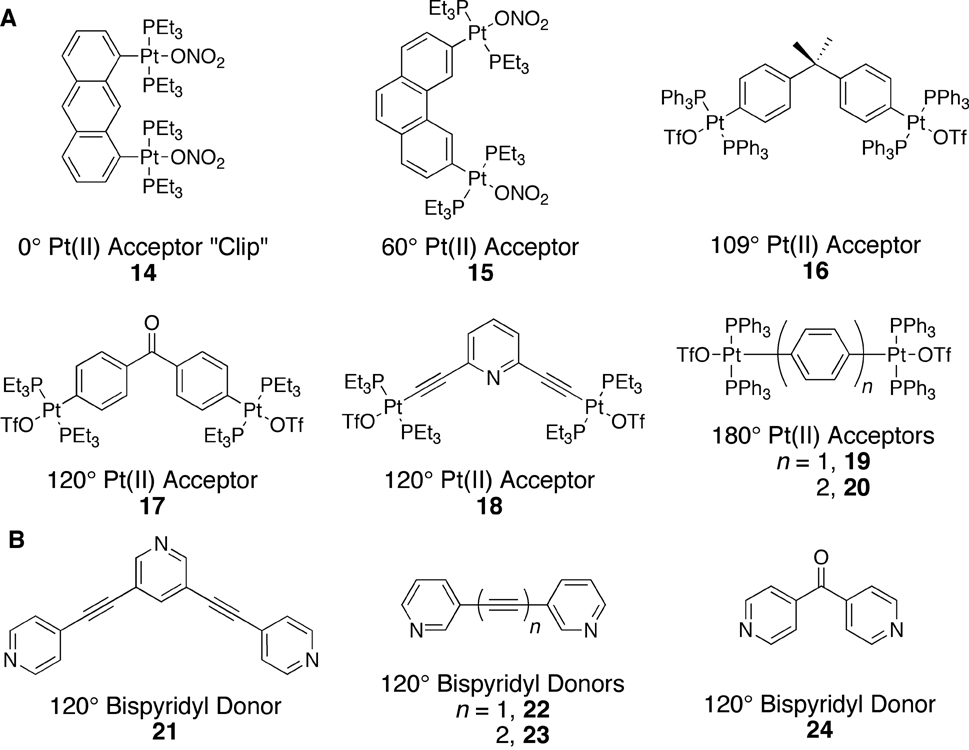
While earlier examples of metal-organic self-assembled metallacages exist, most notably from Maverick and coworkers16 in 1984, it wasn’t until about 1990 that systematic approaches to metallacycles began to appear.17 Most initial examples of self-assembled metallacycles took the form of supramolecular squares. One of the most commonly utilized protocols for designing a supramolecular square calls for a combination of four linear ditopic units (L2) and four complementary angular ditopic units (A2), generating a square of type A24L24. Square planar Pt(II) and Pd(II) metals have provided the basis for 90° angular corner units of myriad carbon-rich supramolecular squares in conjunction with rigid pyridyl or nitryl substituted linear donors.18–22 Example carbon-rich angular and linear components are shown in Figure 3, while Scheme 1 summarizes the self-assembly of a number of A24L24 supramolecular squares.
Figure 3.
Carbon-rich ditopic 90° metal acceptors (A) and ditopic 180° donors (B).
Scheme 1.
The self-assembly of carbon-rich metallacyclic squares. In all cases the acceptor and donor are mixed in a 1:1 molar ratio. M = Pt, Pd.
It is also possible to form related squares of type A22A22 wherein two sets of ditopic 90° angular units, one donor and one acceptor, are combined in a 1:1 ratio.23
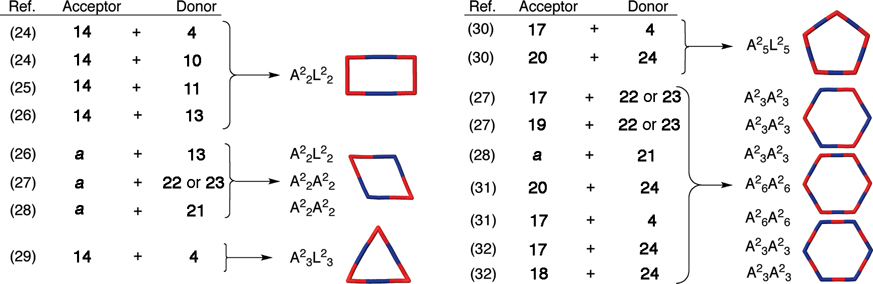
By employing ditopic donor and/or acceptor building-blocks with turning angles that deviate from 90°, a variety of other carbon-rich 2D metallacycles can be prepared (Figure 1). Representative examples of ditopic acceptors and donors used in the construction of carbon-rich supramolecular rectangles,24–26 rhomboids,26–28 triangles,29 pentagons,30 and hexagons27–28,31–32 are shown in Figure 4. As can be seen in Scheme 2, the variety of complementary angular and linear ditopic building units provides access to a series of metallacyclic polygons.
Figure 4.
Carbon-rich Pt(II) acceptors with turning angles of 0°, 60°, 109°, 120°, and 180° (A) as well as non-linear ditopic donors (B).
Scheme 2.
Summary of protocols for self-assembling a variety of carbon-rich 2D polyhedra. In each case the donor and acceptor precursors are mixed in a 1:1 molar ratio. a = Pt(PMe3)2(OTf)2.
Rigid supramolecular rectangles can be prepared upon mixing the 0° molecular “clip” with linear donor ligands such as 4, 10, 11, and 13. Given the close proximity of the two Pt(II) acceptors in 14, care must be taken when designing a linear donor ligand because of steric crowding. Given that a rhomboid can be defined as any four sided parallelogram that is neither a square nor a rectangle, a variety of donor and acceptor combinations may be used to prepare metallacyclic rhomboids. Carbon-rich supramolecular rhomboids have thus been prepared using 180° donor bis(pyridyl)-substituted perylene diimide 13 as well as 120° donors 21–23. It is possible to form a rhomboid structure using the linear donor 13 because its length, ~3.5 nm, confers enough overall flexibility to self-assemble when combined with a 90° metal acceptor.
Combining three ditopic 60° phenanthrene-based Pt(II) acceptors (15) with three ditopic 180° 4,4'-bipyridyl linear donors (4) results in a metallacyclic triangle.29 Changing the acceptor unit from a 60° phenanthrene-based 15 to a 120° benzophenone-based Pt(II) acceptor (17) results in a carbon-rich supramolecular hexagon when mixed with the same linear donor (4) in a 1:1 molar ratio.31 The modularity of the coordination-driven directional-bonding approach is easily seen when comparing some of the many carbon-rich metallacyclic hexagons that have been self-assembled. Hexagons of type A23A23, devoid of linear building blocks, have been prepared27 as have large hexagons of type A26L26 wherein complementary pairs of 120° acceptors and 180° donors and analogous 120° donors and 180° acceptors have both been used. Pentagonal metallacycles may be formed by using a slightly more flexible 109° acceptor, however, they are often in competition with related hexagonal metallacycles.
3. Metallacage Polyhedra
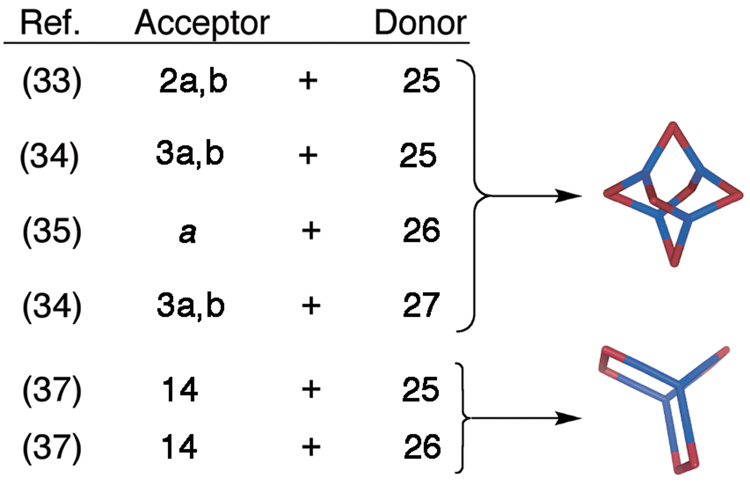
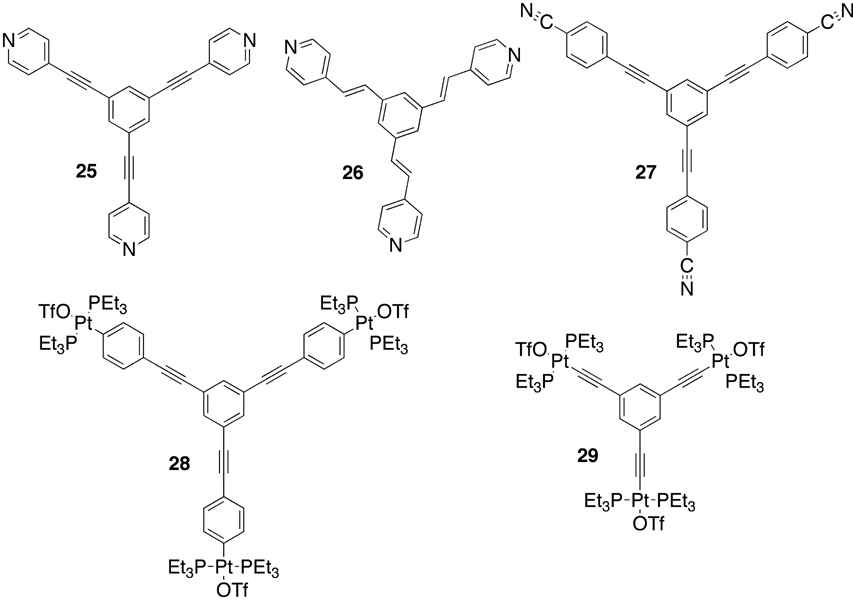
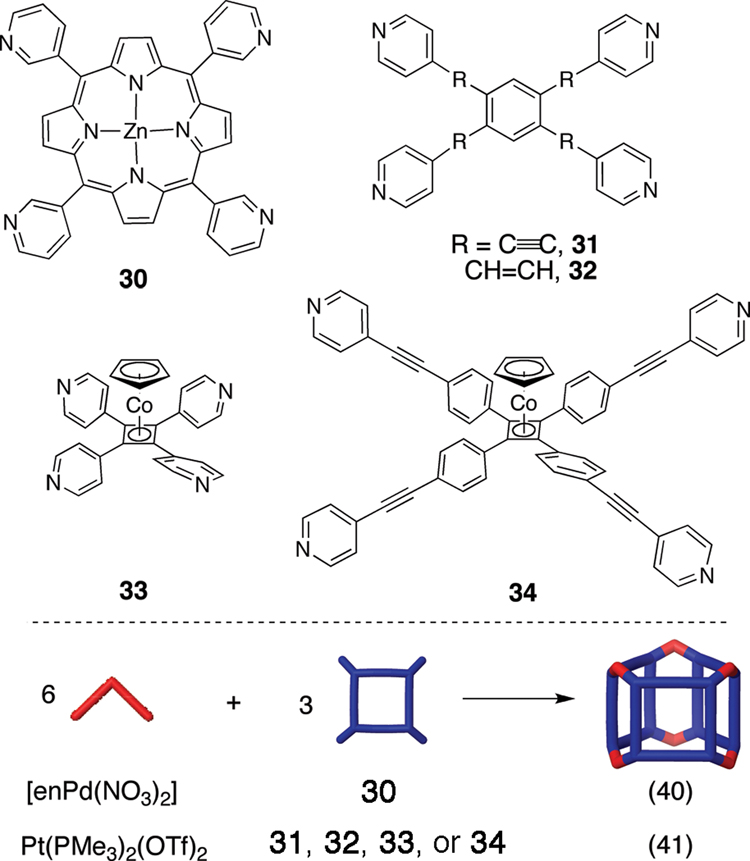
More recently, considerable attention has been paid to expanding these coordination-driven self-assembly techniques to the 3rd dimension. The protocol for preparing 3D metallacages only requires the preparation of donor and/or acceptor precursors with greater than two binding sites. As shown in Figure 2, combining carbon-rich tritopic and ditopic building units allows for the construction of metallacages with truncated tetrahedral, distorted and non-distorted trigonal prism, cuboctahedral, adamantoid, and dodecahedral geometries (Scheme 3).14b,c,e Combining carbon-rich, 3-fold symmetric planar tritopic donors or acceptors (Figure 5) with complementary 90° units in a 3:2 ratio allows the self-assembly of truncated tetrahedral assemblies.33–35 In addition to these carbon-rich examples, Fujita and coworkers have also prepared related nitrogen-rich analogues based on tritopic trispyridyl donors with a triazabenzene core.36 If the self-assembly involving donors 25 or 26 is performed with a 0° molecular “clip” rather than a 90° acceptor, also in a 3:2 ratio, the result becomes a trigonal prism.37,38 An alternative method for preparing a carbon-rich trigonal prism has been achieved by Fujita using a combination of two tritopic trigonal planar donors, three ditopic linear donors, and six 90° metal acceptors.39
Scheme 3.
Use of trigonal planar tritopic donors to self-assemble carbon-rich truncated tetrahedra and trigonal prisms. a = Pt(PMe3)2(OTf)2.
Figure 5.
Trigonal planar tritopic donors and acceptors.
Other trigonal prism geometries may be formed using three carbon-rich tetratopic donors that function as planar “walls” that are linked using six 90° metal acceptors as “corners” as shown in Scheme 4. Tetrapyridyl-substituted porphyrins40 as well as tetrapyridyl-substituted benzene and cyclobutadiene-cyclopentadieneyl cobalts41 have been used as the carbon-rich walls of such trigonal prism geometries. In the case of tetratopic donor 34, restricted rotation of pyridyl ligands in a trigonal prism renders the assembly helically chiral with MMM and PPP enantiomers.
Scheme 4.
The self-assembly of “walled” trigonal prism geometries using carbon-rich tetratopic donors and 90° metal acceptors in a 3:6 molar ratio.
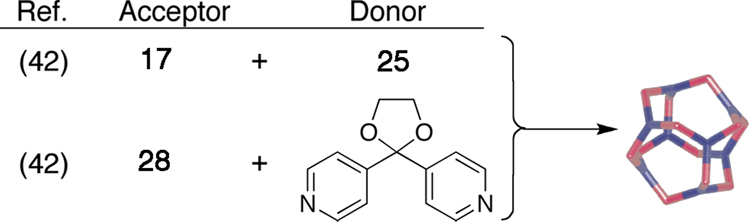
An entirely different geometry, that of a cuboctahedron, is obtained when planar tritopic units such as carbon-rich donor 25 or acceptor 28 are combined in an 8:12 ratio with complementary 120° ditopic units (Scheme 5).42 Robson and coworkers43 have self-assembled a metallacage with cuboctahedral geometry by combining a 2,4,6-triazophenyl-1,3,5-trioxybenzene trianion and Cu(NO3)2 in a 2:3 molar ratio. By using “naked” Pd metals in conjunction with 120° dipyridyl donors, Fujita and coworkers44 have prepared a variety of cuboctahedral complexes of the form M12L24. The large variety of carbon-rich 3D architectures available simply using planar triropic of tetratopic units with 0°, 90°, or 120° units – resulting in assembly geometries that range from truncated tetrahedral, to trigonal prisms, to cuboctahedra, respectively – demonstrates the power and modularity of the directional-bonding, coordination-driven approach.
Scheme 5.
The self-assembly of cuboctahedra from a 12 ditopic 120° precursors and 8 complementary tritopic trigonal planar precursors.
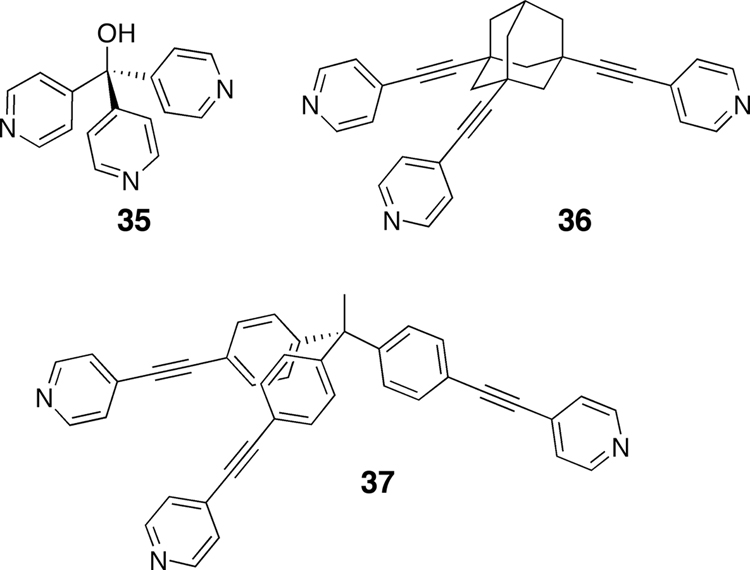
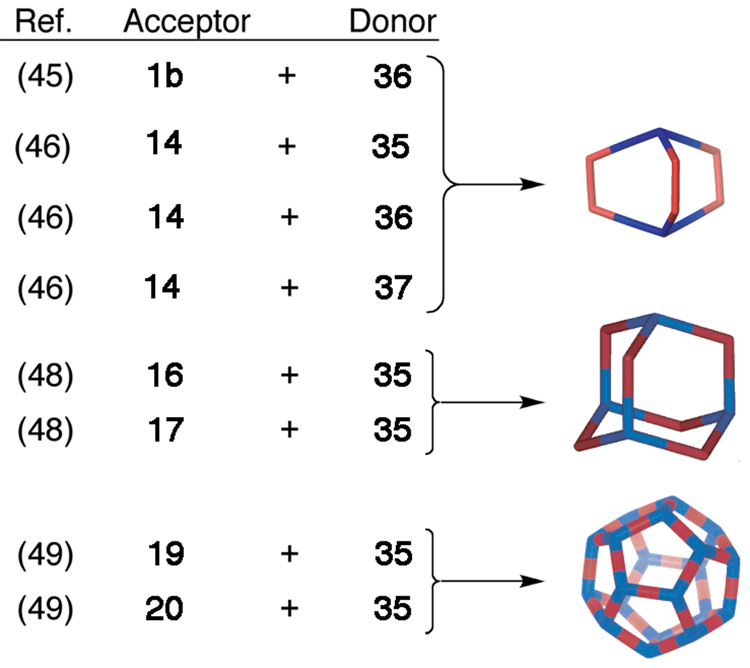
Using non-planar tritopic donors (Figure 6) opens the door to an even greater variety of 3D metallacages. Combining trigonal pyramidal tritopic donors with 0° or 90° acceptors in a 2:3 molar ratio results in the formation of distorted trigonal prisms45–46 or, more precisely, trigonal bipyramids (Scheme 6). The extent of distortion from a “planar” trigonal prism geometry is controlled by the size and geometry of the tritopic donor and the angle of the acceptor, with a greater acceptor angle resulting in greater distortion from a trigonal prism geometry.
Figure 6.
Examples of three carbon-rich trigonal pyramidal tritopic donors.
Scheme 6.
Self-assembly of trigonal bipyrimidal, adamantoid, and dodecahedral polygons. Component acceptor and donor precursors are mixed in 3:2, 6:4, and 30:20 molar ratios, respectively.
Fujita has self-assembled a “double-square” metallacage47 by combining four equivalents of a tritopic trispyridyl methyl donor derivative with six equivalents of a 90° Pd(II) acceptor. Whereas a 90° Pd(II) acceptor in the previous case is used to prepare a double-square, a 109–120° Pt(II) can be combined with a related tritopic trispyridyl methyl derivative (35) to self-assemble an adamantoid structure (Scheme 6).48 We have also prepared a variety of carbon-rich dodecahedral assemblies using a trispyridylmethanol tritopic donor and different sized 1,4-substituted benzene and 4,4'-biphenyl substituted Pt(II) linear (180°) ditopic acceptors.49 Combining the tritopic donors and ditopic acceptors in a 30:20 ratio results in carbon-rich supramolecular metallacages with dodecahedral geometries. These dodecahedra, with diameters ranging from 5–7 nm, represent some of the largest discrete supramolecular assemblies prepared to date.
4. Materials Applications
As with many other carbon-rich molecules, which have found applications in materials such as organic field-effect transistors (OFETs), artificial photosynthetic systems, and luminescent devices, the potential materials applications of carbon-rich supramolecular assemblies have also been explored. Our phenanthroline-containing supramolecular rectangle,25 for example, has been shown to function as an optical sensor for Ni(II), Cd(II), and Cr(III). Fujita and coworkers have demonstrated the ability of a carbon-rich supramolecular tetrahedron cage to selectively recognize and bind sequence-specific peptides50 and, in the case of a supramolecular trigonal prism, capable of folding an Ala-Ala-Ala tripeptide in to a β-turn on account of hydrophobic encapsulation.51 The use of largely aromatic moieties in various precursors enables many supramolecular cages to act as hosts for other aromatic guests through a combination of π-π and hydrophobic interactions.39,52 Both Hupp53 and Mirkin54 have recently used porphyrin-based supramolecular rectangles as catalysts for epoxidation and acyl transfer reactions, respectively.
4.1. Microporous Molecular Materials
In addition to aforementioned host-guest applications, rigid supramolecular 2D polygons offer unique opportunities to be used as highly-selective nanofilters. The size and shape of nanoscale cavities present in metallacyclic polygons can be easily modified in order to allow for size-specific discrimination of various molecular permeants. Hupp and coworkers55 have extensively explored the use of metal-organic supramolecules as selective nanofilters. Small and large supramolecular squares, for example, composed of Re(I)Cl(CO)3 corners and either pyrazine or dipyridyl porphyrin edges, respectively, were shown capable of filtering planar and spherical permeants based upon their size.56 Further studies utilizing thin-films of Mn(I) and Re(I) carbon-rich rectangles demonstrated that only those molecular permeants that were narrow in at least one dimension, similar to the metallacyclic rectangles, were able to pass through the intramolecular rectangular cavities whereas permeants lacking a narrow dimension were blocked.57 In addition to applications as highly-modular nanofilters, the gas adsorption properties of carbon-rich supramolecular metal-organic assemblies are currently being explored.58
4.2. Higher-Order Self-Assembly on Surfaces
Recently, in collaboration with Wan and coworkers, we have explored the self-assembly of supramolecular assemblies on Au(111) and HOPG surfaces. Both polygonal (rectangular59 and square60) and polyhedral (trigonal bipyramid60 and chiral trigonal prism61) structures have been assembled onto surfaces and their structures characterized. Results of these studies have shed considerable light on the factors that influence the orientation of these supramolecules on various surfaces. For example, one carbon-rich supramolecular rectangle investigated adopts an edge-down orientation on HOPG while the same rectangle adopts a face-down orientation on Au(111).59 In the case of chiral trigonal prisms, deposition of a racemic mixture of supramolecular assemblies resulted in spontaneous symmetry-breaking and formation of enantiomerically-segregated chiral domains.61 Furthermore, we have demonstrated the ability of using a molecular template62 to promote the formation of a well-ordered, monodisperse monolayer of molecular rectangles on Au(111) whereas without the template only a disordered adlayer is observed. Having the ability to prepare well-ordered arrays of these carbon-rich supramolecules and control their orientation on surfaces may lead to a variety of applications as sensors, electronic, photonic, or magnetic materials, or catalysis, especially in the case of chiral 2D assemblies which may have a range of heterogeneous stereoselective synthetic applications.
4.3. Functionalized Metal-Organic Assemblies
Another development in the use of carbon-rich supramolecular metallacycles and metallacages has been in their functionalization. The rigid structures and precise, unique geometries of many supramolecular assemblies makes them very effective “scaffolds” for a variety of functionalities simply by preparing derivatized donors and/or acceptors. Given the rigid structural characteristics of precursor building-blocks and the fact that donors and acceptors are mixed in specific ratios, the location and stoichiometry of covalently linked functional moieties attached to carbon-rich aupramolecules can be precisely controlled. We have recently prepared 120° donors functionalized with Frechét-type dendritic,63 crown-ether,64 and ferrocenyl65 moieties attached. Combining these donors with complementary 90°, 120°, or 180° Pt(II) acceptors allows for the formation of bisfunctionalized rhomboids as well as tris- and hexakisfunctionalized hexagons, respectively. We have further demonstrated the ability of crown-ether derivatized assemblies to act as hosts for multiple dibenzylammonium cations64 and have explored the electrochemistry of ferrocenyl metallacycles, which may have applications as multiple electron redox catalysts or as electron sponges.65 Fujita and coworkers66 have also successfully prepared carbon-rich 3D cuboctahedra that have been functionalized endohedrally with azobenzene,66a,c oligo(ethyleneglycol),66a polyfluoro,66b and polymerizable methyl methacrylate units66d and exohedrally with saccharide moieties.66e These 3D assemblies have found uses in a variety of host-guest applications.
5. Novel Stereoisomeric Supramolecular Triangles
Chirality is a ubiquitous and important phenomenon throughout nature in general and chemistry in particular. The general lack of tetrahedral carbon atoms throughout most carbon-rich compounds usually rules out the possibility of synthesizing carbon-rich molecules with point chirality. There are, however, ways to introduce chirality into carbon-rich molecules by means of helicity. For example, beginning in 1967, Martin and coworkers67 prepared helically chiral hepta-, octa-, and nonahelicenes. Building upon these purely aromatic helically chiral structures, in 1972 Staab and coworkers68 prepared a carbon-rich helically chiral benzannulene structure composed solely of benzene and acetylene moieties. Since that time a rich chemistry of chiral benzannulene compounds has been developed.69 Similarly, chirality can be introduced into carbon-rich systems through the incorporation of chiral moieties, such as binaphthyl units.
Coordination-driven self-assembly techniques provide a means of synthesizing stereoisomers of carbon-rich supramolecules in high yield under thermodynamic control. In the mid-1990’s, Stang and coworkers used a chiral binaphthyl-substituted 90° Pt(II) acceptor to prepare optically active carbon-rich 2D metallacyclic squares21 and 3D metallacage truncated tetrahedra.33 It is also possible to use self-assembly to synthesize carbon-rich supramolecules from completely achiral building blocks. To demonstrate the utility of coordination-driven self-assembly we report herein the design and synthesis of stereoisomeric carbon-rich metallacyclic triangles.
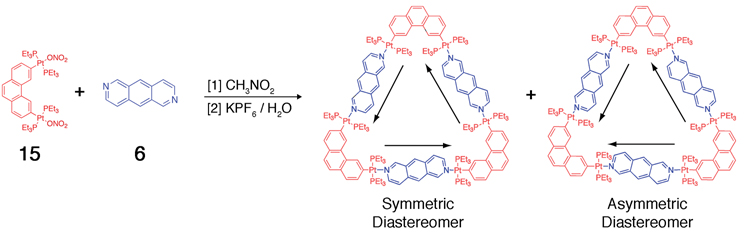
The combination of 2,6-diazaanthracene (6) and the 60° Pt(II) acceptor 15 in a 1:1 ratio results, theoretically, in the formation of two possible A23L23 metallacyclic triangle diastereomers (Scheme 7), which are both meso compounds. The diastereomers differ in the relative orientations of their donor components as indicated by arrows in the Scheme. In the symmetric diastereomer, all three donor moieties are oriented in the same direction while in the asymmetric diastereomer, one donor moiety is oriented opposite the other two. Rotation around the Pt-N bonds in each assembly is restricted,70 preventing direct interconversion between stereoisomers through dynamic bond rotations.
Scheme 7.
Self-assembly of “symmetric” and “asymmetric” diastereomers of a carbon-rich supramolecular triangle from achiral building blocks.
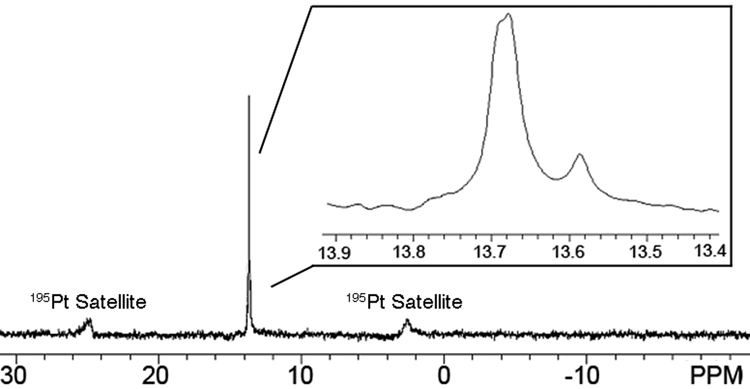
Stirring 6 and 15 in dry CH3NO2 for 2 h results in a homogeneous yellow solution. Following the exchange of the NO3− counterions for weakly coordinating PF6− counterions the formation of the new metallacyclic triangles was initially investigated by 31P and 1H NMR spectroscopies. Analysis of the carbon-rich triangles with 31P{1H} NMR revealed the presence of a sharp signal around 13.7 ppm, indicating the formation of discrete supramolecules as opposed to oligomeric species. Close examination of the sharp signal, however, revealed that it is in fact composed of two peaks: one high intensity signal at 13.69 ppm and another lower intensity signal at 13.59 ppm (Figure 7). The observation of two peaks indicates that both carbon-rich diastereomers are present in solution, though more of one stereoisomer is formed than the other. Both peaks are upfield shifted by about 5.3 ppm as compared to the starting 60° acceptor 15 due to back donation from the platinum atoms. Further evidence of back donation and Pt-N coordination was also observed by the decrease in coupling of the 195Pt satellite peaks (ΔJ = 109 Hz). Examination of the 1H NMR spectrum also revealed the presence of highly symmetric species, though proton signals were broadened slightly as a result of the presence of two different stereoisomers. It was further observed that hindered Pt-N rotation gives rise to in equivalent signals for donor 6, the four protons that point inward with respect to the core of the supramolecular triangle display are shifted slightly relative to the four that point outward (see Supporting Information). Further confirmation of the formation of discrete supramolecular triangles was provided by Electro-Spray Ionization mass spectrometry (ESI-MS). A peak was found at m/z = 986.8, corresponding to the [M – 4PF6−]4+ peak of the carbon-rich A23L23 triangle, and the distribution of peaks matched their theoretical values (see Supporting Information) establishing the molecularity of the assembly.
Figure 7.
31P NMR spectrum (121.5 MHz, CD3COCD3, 298 K) of the self-assembled carbon-rich supramolecular diastereomers. The inset shows that what appears to be a single sharp peak is actually composed of two peaks of differing intensity, indicating one diastereomer is formed preferentially.
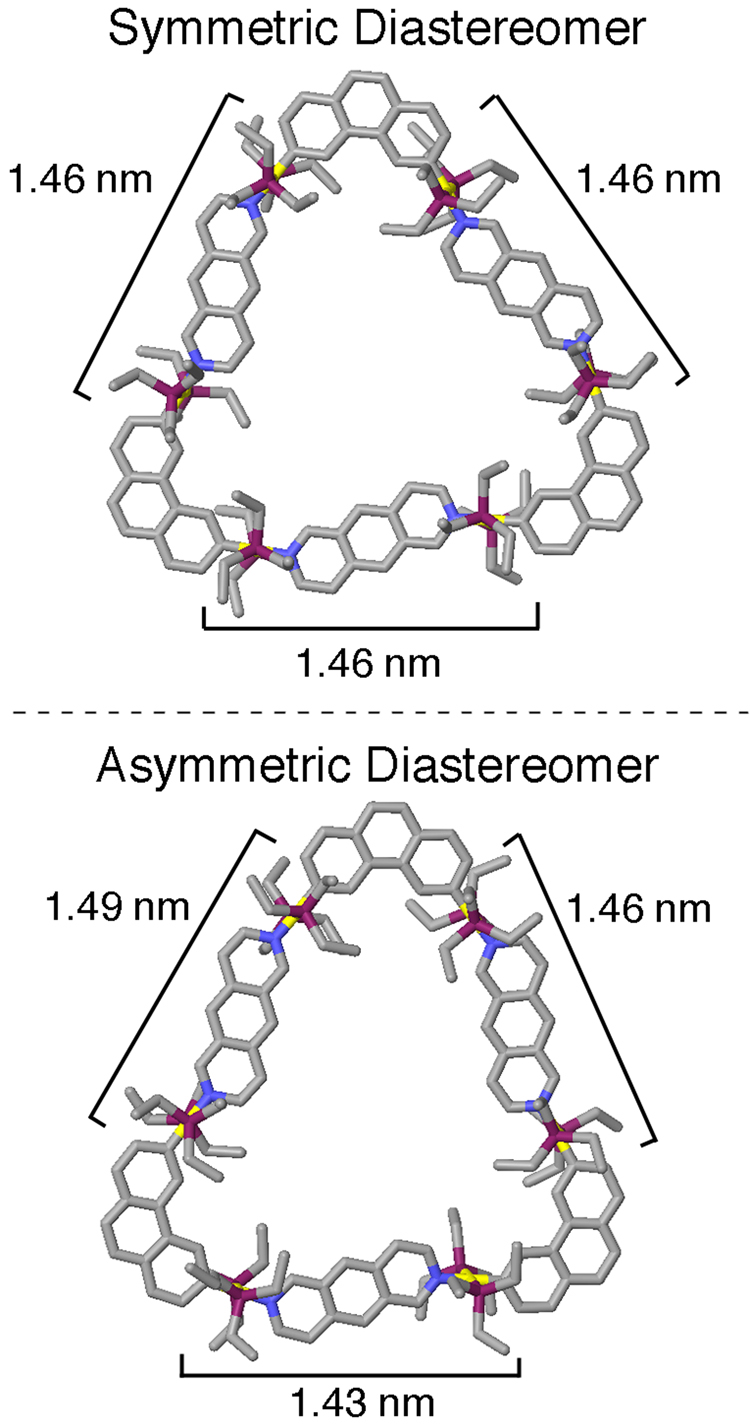
The observation of two peaks of different intensity in the 31P NMR spectrum of the carbon-rich supramolecular triangle can be rationalized through molecular modeling studies. The supramolecular structures of both diastereomers were built within the input mode of the program Maestro v8.01.10 and were subjected to a 1.0 ns molecular dynamics simulation (MMFF force field, 300 K, gas phase) in order to equilibrate their structures, followed by energy minimization to full convergence (Figure 8). The fully equilibrated and minimized structure of the “symmetric” supramolecular triangle, having all three donor moieties oriented in the same direction, was found to be 6.9 kcal/mol more stable than the asymmetric supramolecular triangle. This difference in stability likely arises from differences in strain energy induced by the relative orientations of the donor moieties in the two diastereomers. In the asymmetric diastereomer two of the three donors are oriented opposite to each other, increasing the structural strain in the supramolecule and causing the asymmetric triangle to deviate from a perfectly triangular structure as shown in Figure 8b. The aligned arrangement of the three donor moieties in the symmetric triangle distributes and equalizes any strain, resulting in a more stable, and more triangular, structure (Figure 8a). The differences in structural strain can be seen, in part, in comparing the distances between neighboring phenanthrene units as measured from molecular modeling: all three distances are 1.46 nm in the symmetric diastereomer while in the asymmetric diastereomer they range from 1.43–1.49 nm. Due to the energetic difference between the two diastereomeric carbon-rich triangles it is likely that the symmetric triangles are formed preferentially, giving rise to the 31P NMR signal of higher intensity, while the less stable asymmetric triangles can be accounted for by the 31P NMR signal of lower intensity.
Figure 8.
Structures of symmetric and asymmetric diastereomers obtained from molecular modeling. Measurements of the distance between neighboring phenanthrene units partially demonstrates differences in structural strain between the diastereomers. Color scheme: C = grey, N = blue, P = purple, Pt = yellow.
These results demonstrate how self-assembly may be used to prepare different stereoisomers of carbon-rich supramolecular assemblies from achiral precursors. Though not the first examples of chiral carbon-rich molecules or supramolecules, the preferential formation of one stereoisomer at the expense of another indicates that it may be possible to prepare enantiomerically pure carbon-rich supramolecular assemblies by coordination-driven self-assembly. In addition to structural strain it may be possible to use other driving forces such as electrostatics to bias the formation of one desired stereoisomer. Having the ability to prepare novel, enantiomerically pure carbon-rich compounds in high yield under the mild reaction conditions of self-assembly is very desirable. Experiments aimed at such chiral discrimination during self-organizing self-assembly are currently underway.
6. Conclusions
In conclusion, these examples demonstrate many of the ways in which coordination-driven self-assembly can be used to prepare carbon-rich supramolecular 2D and 3D assemblies. The key characteristics of metal-ligand self-assembly – mild reaction conditions, high yields, thermodynamic control, and a high degree of modulatiry – combine to make the directional-bonding approach to self-assembly a very valuable addition to the many other synthetic protocols available for the synthesis of carbon-rich materials. While carbon-rich molecular polygons and polyhedra have also been prepared via traditional covalent means – from triangles71 and rectangles72 composed purely of aromatic and acetylenic moieties, to molecules such as Kohnkene,73 to Diederich’s synthesis of expanded cubane74 – the overall number of synthetic steps and yields of such synthesis often limit their practicality. Self-assembly allows access to related supramolecular systems at significantly lower synthetic cost. It has also been demonstrated that coordination-driven self-assembly can be used to prepare diastereomeric supramolecular triangles and that the structural characteristics of these metallacyclic systems are able to bias the formation of one stereoisomer over another. Such observations may lead to the development of methods that utilize self-assembly to form enantiomerically pure carbon-rich supramolecules. Furthermore, the unique geometries and physical, electronic, and magnetic properties of these metal-organic structures opens a wide range of potential applications. It is likely that as their solution, solid-state, and surface-confined properties continue to be studied, and as new functionalized examples of this already important class of compounds continue to be investigated, researchers will find an ever widening number of applications in materials and nanosciences. They are certainly a welcome recent addition to the diverse world of carbon-rich materials.
7. Experimental
Self assembly of diastereomeric supramolecular triangles
The 2,6-diazaanthracene donor 6 and 60° Pt(II) acceptor 15 were prepared according to literature procedures.21,29 In a glass vial equipped with a magnetic stir bar was placed 15 (32.2 mg, 0.0277 mmol). In another glass vial was placed solid 6 (5.0 mg, 0.0277 mmol). Donor 6 was then dissolved in 0.5 mL dry CH3NO2, transferred to the vial with acceptor 15, and then washed with additional CH3NO2 (3 × 0.5 mL) to ensure quantitative transfer. The suspension was stirred for 5 h at room temperature, resulting in a homogeneous clear yellow solution. The solvent was removed under reduced pressure, the NO3− counterions were exchanged for PF6− using a concentrated aqueous solution of KPF6, and the resulting precipitate was dried to completeness. Yield 39 mg (yellow solid), 93%. 1H NMR (CD3COCD3, 300 MHz): δ 10.43 (s, 3H, He), 10.42 (s, 3H, He’), 9.58 (s, 3H, Hf), 9.54 (s, 3H, Hf’), 9.20-9.13 (m, 6H, HH), 9.03-8.93 (m, 6H, Hg), 8.61 (br, 6H, Ha), 7.86 (d, 6H, Hb, J = 7.7 Hz), 7.75 (d, 6H, Hc, J = 7.7 Hz), 7.71 (s, 6H, Hd), 1.42 (m, 72H, PCH2CH3), 1.19 (m, 108H, PCH2CH3). 31P{1H} NMR (CD3COCD3, 121.4 MHz):δ 13.7 (major), 13.6 (minor) (1JPt-P = 2662.7 Hz). MS (ESI) calcd for [M – 4 PF6]4+ m/z 986.8, found 986.8.
The majority of synthetic procedures outlined in Scheme 1–Schemes 6 involve the self-assembly of pyridyl or nitryl donors and Pt(II) metal acceptors as either their OTf− or NO3− salts. The following two synthetic self-assembly protocols, one for a Pt(II) triflate salt and one for a Pt(II) nitrate salt, serve as representative examples of the procedures used to prepare 2D and 3D supramolecules.
Self assembly of supramolecular rectangles.24
In a glass vial equipped with a magnetic stir bar were placed solid 1,8-bis(trans-Pt(PEt3)2(NO3))anthracene (14) (20.0 mg, 0.0172 mmol) and an equimolar amount of the appropriate linear bridging ligand (e.g. 4, 10, 11, 13). Next, 0.5 mL of CD3COCD3 and 0.5 mL of D2O were added to the vial, which was then sealed with Teflon tape and heated in an oil bath at 50–55° C, with stirring. After 10–15 h, the initial pale yellow suspension gradually turned bright orange and became homogeneous. The orange solution was then transferred to an NMR tube for analysis. The product was precipitated with KPF6, collected on a frit, washed with excess water, and dried in vacuo.
Self assembly of an A26L26 supramolecular hexagon.30
In a glass vial equipped with a magnetic stir bar were placed solid bis(4-trans-Pt(PEt3)2(OTf)2)methanone (17) (20.0 mg, 0.0149 mmol) and 4,4'-bipyridyl (4) (2.3 mg, 0.0149 mmol). Next, 1.0 mL of CD2Cl2 was added to the vial, which was then stirred at 298 K for 30 min, resulting in quantitative formation (as observed by 1H and 31P NMR spectroscopy) of the desired supramolecular hexagon.
For details of the synthetic procedures used in the preparation of 1–37 please see the original texts.
Supplementary Material
Acknowledgments
The authors thank our co-workers and collaborators who have made this work possible. We are also grateful to Dr. Rik Tykwinski for guidance and materials. P.J.S. thanks the NIH (Grant GM-057052) and the NSF (Grant CHE-0306720) for financial support. B.H.N. thanks the NIH (Grant GM-080820) for financial support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Haley MM, Tykwinski RR, editors. Carbon-Rich Compounds. Weinheim: Wiley-VCH; 2006. [Google Scholar]
- 2.Clar E. Polycyclic Hydrocarbons. Vol. I–II. London: Academic Press; 1964. [Google Scholar]
- 3.(a) Harvey RG. Polycyclic Aromatic Hydrocarbons. New York: Wiley-VCH; 1997. [Google Scholar]; (b) Modern Arene Chemistry. Weinheim: Wiley-VCH; 2002. [Google Scholar]
- 4.Radaziszewski B. Ber. Dtsh. Chem. Ges. 1876;9:260–262. [Google Scholar]
- 5.(a) Boorum MM, Vasil’ev YV, Drewello T, Scott LT. Science. 2001;294:828–831. doi: 10.1126/science.1064250. [DOI] [PubMed] [Google Scholar]; (b) Scott LT, Boorum MM, McMahon B, Hagen S, Mack J, Blank J, Wegner H, de Meijere A. Science. 2002;295:1500–1503. doi: 10.1126/science.1068427. [DOI] [PubMed] [Google Scholar]
- 6.(a) Glaser C. Ber. Dtsh. Chem. Ges. 1869;2:422–424. [Google Scholar]; (b) Baeyer A, Landsberg L. Ber. Dtsh. Chem. Ges. 1882;15:50–56. 57–61. [Google Scholar]; (c) Sondheimer F. Acc. Chem. Res. 1972;5:81–91. [Google Scholar]; (d) Siemsen P, Livingston RC, Dietrich F. Angew. Chem. Int. Ed. 2000;39:2632–2657. [PubMed] [Google Scholar]
- 7.Kovacic P, Jones MB. Chem. Rev. 1987;87:357–379. [Google Scholar]
- 8.Muszkat KA. Top. Curr. Chem. 1981;88:89–143. [Google Scholar]
- 9.See, for example, Harvey RG, Pataki J, Cortez C, DiRaddo P, Yang C. J. Org. Chem. 1991;56:1210–1217.
- 10.See, for example, Zander M. Chem. Ber. 1959;92:2740–2743. LeHoullier CS, Gribble GW. J. Org. Chem. 1983;48:1682–1685.
- 11.(a) Lindesy JS. New J. Chem. 1991;15:153–180. [Google Scholar]; (b) Lehn J-M. Supramolecular Chemistry. Weinheim: Wiley-VCH; 1995. [Google Scholar]; (c) Rebek J., Jr Acc. Chem. Rec. 1999;32:278–286. [Google Scholar]; (d) Philip D, Stoddart JF. Angew. Chem. Int. Ed. 2003;42:1210–1250. [Google Scholar]; (e) Brancato G, Coutrot F, Leigh DA, Murphy A, Wong JKY, Zerbetto F. Proc. Natl. Acad. Sci. USA. 2002;99:4967–4971. doi: 10.1073/pnas.072695799. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Yaghi OM, O’Keeffe M, Ockwig NW, Chae HK, Eddaoudi M, Kim J. Nature. 2003;423:705–714. doi: 10.1038/nature01650. [DOI] [PubMed] [Google Scholar]
- 12.Huc I, Lehn J-M. Proc. Natl. Acad. Sci. USA. 1997;94:2106–2110. doi: 10.1073/pnas.94.6.2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lukin O, Godt A, Vötgle F. Chem. Eur. J. 2004;10:1878–1883. doi: 10.1002/chem.200305203. [DOI] [PubMed] [Google Scholar]
- 14.(a) Stang PJ, Olenyuk B. Acc. Chem. Res. 1997;30:502–518. [Google Scholar]; (b) Leininger S, Olenyuk B, Stang PJ. Chem. Rev. 2000;100:853–907. doi: 10.1021/cr9601324. [DOI] [PubMed] [Google Scholar]; (c) Seidel SR, Stang PJ. Acc. Chem. Res. 2002;35:972–983. doi: 10.1021/ar010142d. [DOI] [PubMed] [Google Scholar]; (d) Schwab PFH, Levin MD, Michl J. Chem. Rev. 1999;99:1863–1933. doi: 10.1021/cr970070x. [DOI] [PubMed] [Google Scholar]; (e) Gianneschi NC, Masar MS, III, Mirkin CA. Acc. Chem. Res. 2005;38:825–837. doi: 10.1021/ar980101q. [DOI] [PubMed] [Google Scholar]; (f) Cotton FA, Lin C, Murillo CA. Acc. Chem. Res. 2001;34:759–771. doi: 10.1021/ar010062+. [DOI] [PubMed] [Google Scholar]; (g) Fujita M, Tominaga M, Hori A, Therrien B. Acc. Chem. Res. 2005;38:369–378. doi: 10.1021/ar040153h. [DOI] [PubMed] [Google Scholar]; (h) Fiedler D, Leung DH, Bergman RG, Raymond KN. Acc. Chem. Res. 2005;38:349–358. doi: 10.1021/ar040152p. [DOI] [PubMed] [Google Scholar]; (i) Steel PJ. Acc. Chem. Res. 2005;38:243–250. doi: 10.1021/ar040166v. [DOI] [PubMed] [Google Scholar]
- 15.(a) Lehn J-M. Supramolecular Chemistry Concepts and Perspectives. Weinheim: VCH; 1995. pp. 139–160. [Google Scholar]; (b) Chambron J-C, Dietrich-Buchner C, Sauvage J-P. In: Comprehensive Supramolecular Chemistry. Lehn J-M, Atwood JL, Davies JED, MacNicol DD, Vötgle F, editors. Vo. 9. Oxford: Pergamon; 1996. pp. 43–83. [Google Scholar]; (c) Holliday BJ, Mirkin CA. Angew. Chem. Int. Ed. 2001;40:2022–2043. [PubMed] [Google Scholar]; (d) Cotton FA, Lin C, Murillo CA. Acc. Chem. Res. 2001;34:759–771. doi: 10.1021/ar010062+. [DOI] [PubMed] [Google Scholar]
- 16.Maverick AW, Klavetter FE. Inorg. Chem. 1984;23:4129–4130. [Google Scholar]
- 17.Fujita M, Yazaki J, Ogura K. J. Am. Chem. Soc. 1990;112:5645–5647. [Google Scholar]
- 18.Stang PJ, Cao DH. J. Am. Chem. Soc. 1994;116:4981–4982. [Google Scholar]
- 19.Stang PJ, Cao DH, Saito S, Arif AM. J. Am. Chem. Soc. 1995;117:6273–6283. [Google Scholar]
- 20.Stang PJ, Olenyuk B, Fan J, Arif AM. Organometallics. 1996;15:904–908. [Google Scholar]
- 21.Stang PJ, Olenyuk B. Angew. Chem. Int. Ed. Engl. 1996;35:732–736. [Google Scholar]
- 22.Stang PJ, Fan J, Olenyuk B. Chem. Commun. 1997:1453–1454. [Google Scholar]
- 23.For examples of A22A22 squares utilizing mixed Pt and Pd metals see: Stang PJ, Whiteford JA. Organometallics. 1994;13:3776–3777. Whiteford JA, Lu CV, Stang PJ. J. Am. Chem. Soc. 1997;119:2524–2533.. For examples of A22A22 squares utilizing hypervalent iodonium corners see: Stang PJ, Chen K. J. Am. Chem. Soc. 1995;117:1667–1668. Stang PJ, Chen K, Arif AM. J. Am. Chem. Soc. 1995;117:8793–8797.. For examples of A22A22 squares utilizing 5,10-dipyridyl substituted porphyrins as 90° units see: Drain CM, Lehn JM. J. Chem. Soc., Chem. Commun. 1994:2313–2315. Fan J, Whiteford JA, Olenyuk B, Levin MD, Stang PJ, Fleischer EB. J. Am. Chem. Soc. 1999;121:2741–2752.
- 24.Kuehl CJ, Huang SD, Stang PJ. J. Am. Chem. Soc. 2001;123:9634–9641. doi: 10.1021/ja0114355. [DOI] [PubMed] [Google Scholar]
- 25.Resendiz MJE, Noveron JC, Disteldorf H, Fischer S, Stang PJ. Org. Lett. 2004;6:651–653. doi: 10.1021/ol035587b. [DOI] [PubMed] [Google Scholar]
- 26.Addicott C, Oesterling I, Yamamoto T, Mullen K, Stang PJ. J. Org. Chem. 2005;70:797–802. doi: 10.1021/jo048239b. [DOI] [PubMed] [Google Scholar]
- 27.Chi K-W, Addicott C, Arif AM, Das N, Stang PJ. J. Org. Chem. 2003;68:9798–9803. doi: 10.1021/jo0353785. [DOI] [PubMed] [Google Scholar]
- 28.Yamamoto T, Arif AM, Stang PJ. J. Am. Chem. Soc. 2003;125:12309–12317. doi: 10.1021/ja0302984. [DOI] [PubMed] [Google Scholar]
- 29.Kryschenko YK, Seidel SR, Arif AM, Stang PJ. J. Am. Chem. Soc. 2003;125:5193–5198. doi: 10.1021/ja030018k. [DOI] [PubMed] [Google Scholar]
- 30.For a discussion of the equilibrium between metallacyclic pentagons and hexagons that exists when complementary 109° and 180° precursors are mixed in a 1:1 ratio, see 14b 882.
- 31.Stang PJ, Perskyc NE, Manna J. J. Am. Chem. Soc. 1997;119:4777–4778. [Google Scholar]
- 32.Leininger S, Schmitz M, Stang PJ. Org. Lett. 1999;1:1921–1923. doi: 10.1021/ol9911425. [DOI] [PubMed] [Google Scholar]
- 33.Stang PJ, Olenyuk B, Muddiman DC, Smith RD. Organometallics. 1997;16:3094–3096. [Google Scholar]
- 34.Leininger S, Fan J, Schmitz M, Stang PJ. Proc. Natl. Acad. Sci. USA. 2000;97:1380–1384. doi: 10.1073/pnas.030264697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schweiger M, Yamamoto T, Stang PJ, Bläser D, Boese R. J. Org. Chem. 2005;70:4861–4864. doi: 10.1021/jo050469i. [DOI] [PubMed] [Google Scholar]
- 36.Fujita M, Yazaki J, Oka H, Yamaguchi K, Ogura K. Nature. 1995;378:469–471. [Google Scholar]
- 37.Kuehl CJ, Yamamoto T, Seidel SR, Stang PJ. Org. Lett. 2002;4:913–915. doi: 10.1021/ol017296d. [DOI] [PubMed] [Google Scholar]
- 38.For a more recent example of carbon-rich, flexible trigonal prisms see: Yang H-B, Ghosh K, Das N, Stang PJ. Org. Lett. 2006;8:3991–3994. doi: 10.1021/ol0614626.
- 39.Kumazawa K, Biradha K, Kusukawa T, Okano T, Fujita M. Angew. Chem. Int. Ed. 2003;42:3909–3913. doi: 10.1002/anie.200351797. [DOI] [PubMed] [Google Scholar]
- 40.Kumazawa K, Biradha K, Fujita M, Sakamoto S, Yamaguchi KA. Angew. Chem. Int. Ed. 2001;40:1718–1721. [PubMed] [Google Scholar]
- 41.Caskey DC, Yamamoto T, Addicott C, Shoemaker RK, Vacek J, Hawkridge AM, Muddimann DC, Kottas GS, Michl J, Stang PJ. J. Am. Chem. Soc. 2008;130:7620–7628. doi: 10.1021/ja710715e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Olenyuk B, Whiteford JA, Fechtenkötter A, Stang PJ. Nature. 1999;398:796–799. doi: 10.1038/19740. [DOI] [PubMed] [Google Scholar]
- 43.Abrahams BF, Egan SJ, Robson R. J. Am. Chem. Soc. 1999;121:3535–3536. [Google Scholar]
- 44.Tominaga M, Suzuki K, Kawano M, Kusukawa T, Ozeki T, Sakamoto S, Yamaguchi K, Fujita M. Angew. Chem. Int. Ed. 2004;43:5621–5625. doi: 10.1002/anie.200461422. [DOI] [PubMed] [Google Scholar]
- 45.Radhakrishan U, Schweiger M, Stang PJ. Org. Lett. 2001;3:3141–3143. doi: 10.1021/ol0164684. [DOI] [PubMed] [Google Scholar]
- 46.Kuehl C, Kryschenko YK, Radhakrishnan U, Seidel SR, Huang SD, Stang PJ. Proc. Natl. Acad. Sci. USA. 2002;99:4932–4936. doi: 10.1073/pnas.012540799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fujita M, Yu S-Y, Kusukawa T, Funaki H, Ogura K, Yamaguchi K. Angew. Chem. Int. Ed. 1998;37:2082–2085. doi: 10.1002/(SICI)1521-3773(19980817)37:15<2082::AID-ANIE2082>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 48.Schweiger M, Seidel SR, Schmitz M, Stang PJ. Org. Lett. 2000;2:1255–1257. doi: 10.1021/ol005781n. [DOI] [PubMed] [Google Scholar]
- 49.(a) Olenyuk B, Levin MD, Whiteford JA, Shield JE, Stang PJ. J. Am. Chem. Soc. 1999;121:10434–10435. [Google Scholar]; (b) Levinc MD, Stang PJ. J. Am. Chem. Soc. 2000;122:7428–7429. [Google Scholar]
- 50.Tashiro S, Tominaga M, Kawano M, Therrien B, Ozeki T, Fujita M. J. Am. Chem. Soc. 2005;127:4546–4547. doi: 10.1021/ja044782y. [DOI] [PubMed] [Google Scholar]
- 51.Tashiro S, Kobayashi M, Fujita M. J. Am. Chem. Soc. 2006;128:9280–9281. doi: 10.1021/ja061722e. [DOI] [PubMed] [Google Scholar]
- 52.(a) Kumazawa K, Yamanoi Y, Yoshizawa M, Kusukawa T, Fujita M. Angew. Chem. Int. Ed. 2004;43:5936–5940. doi: 10.1002/anie.200460868. [DOI] [PubMed] [Google Scholar]; (b) Yoshizawa M, Nakagawa J, Kumazawa K, Nagao M, Kawano M, Ozeki T, Fujita M. Angew. Chem. Int. Ed. 2005;44:1810–1813. doi: 10.1002/anie.200462171. [DOI] [PubMed] [Google Scholar]
- 53.Merlau ML, del Pilar Mejia M, Nguyen ST, Hupp JT. Angew. Chem. Int. Ed. 2001;40:4239–4242. doi: 10.1002/1521-3773(20011119)40:22<4239::AID-ANIE4239>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 54.Oliveri CG, Gianneschi NC, Nguyen ST, Mirkin CA, Stern CL, Wawrzak Z, Pink M. J. Am. Chem. Soc. 2006;128:16286–16296. doi: 10.1021/ja0661010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dinolfo PH, Hupp JT. Chem. Mater. 2001;13:3113–3125. and references therein. [Google Scholar]
- 56.Czaplewski KF, Hupp JT, Snurr RQ. Adv. Mater. 2001;13:1895–1897. [Google Scholar]
- 57.Williams ME, Benkstein KD, Abel C, Dinolfo PH, Hupp JT. Proc. Natl. Acad. Sci. USA. 2002;99:5171–5177. doi: 10.1073/pnas.082643199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mulfort KL, Hupp JT. J. Am. Chem. Soc. 2007;129:9604–9605. doi: 10.1021/ja0740364. [DOI] [PubMed] [Google Scholar]
- 59.Gong J-R, Wan L-J, Yuan Q-H, Bai C-L, Jude H, Stang PJ. Proc. Natl. Acad. Sci. USA. 2005;102:971–974. doi: 10.1073/pnas.0409145102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yuan Q-H, Wan L-J, Jude H, Stang PJ. J. Am. Chem. Soc. 2005;127:16279–16286. doi: 10.1021/ja0549300. [DOI] [PubMed] [Google Scholar]
- 61.Yuan Q-H, Yan C-J, Yan H-J, Wan L-J, Northrop BH, Jude H, Stang PJ. J. Am. Chem. Soc. 2008;130:8878–8879. doi: 10.1021/ja801934w. [DOI] [PubMed] [Google Scholar]
- 62.Li S-S, Yan H-J, Wan L-J, Yang H-B, Northrop BH, Stang PJ. J. Am. Chem. Soc. 2007;129:9268–9269. doi: 10.1021/ja0733282. [DOI] [PubMed] [Google Scholar]
- 63.(a) Yang H-B, Das N, Huang F, Hawkridge AM, Muddiman DC, Stang PJ. J. Am. Chem. Soc. 2006;128:10014–10015. doi: 10.1021/ja063377z. [DOI] [PubMed] [Google Scholar]; (b) Yang H-B, Hawkridge AM, Huang SD, Das N, Bunge SD, Muddiman DC, Stang PJ. J. Am. Chem. Soc. 2007;129:2120–2129. doi: 10.1021/ja066804h. [DOI] [PubMed] [Google Scholar]
- 64.(a) Yang H-B, Ghosh K, Northrop BH, Zheng Y-R, Lyndon MM, Muddiman DC, Stang PJ. J. Am. Chem. Soc. 2007;129:14187–14189. doi: 10.1021/ja073744m. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Ghosh K, Yang H-B, Northrop BH, Lyndon MM, Zheng Y-R, Muddiman DC, Stang PJ. J. Am. Chem. Soc. 2008;130:5320–5334. doi: 10.1021/ja711502t. [DOI] [PubMed] [Google Scholar]
- 65.Yang H-B, Ghosh K, Zhao Y, Northrop BH, Lyndon MM, Muddiman DC, White HS, Stang PJ. J. Am. Chem. Soc. 2008;130:839–841. doi: 10.1021/ja710349j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.(a) Tominaga M, Suzuki K, Murase T, Fujita M. J. Am. Chem. Soc. 2005;127:11950–11951. doi: 10.1021/ja054069o. [DOI] [PubMed] [Google Scholar]; (b) Sato S, Iida J, Suzuki K, Kawano M, Ozeki T, Fujita M. Science. 2006;313:1273–1276. doi: 10.1126/science.1129830. [DOI] [PubMed] [Google Scholar]; (c) Murase T, Sato S, Fujita M. Angew. Chem. Int. Ed. 2007;46:5133–5136. doi: 10.1002/anie.200700793. [DOI] [PubMed] [Google Scholar]; (d) Murase T, Sato S, Fujita M. Angew. Chem. Int. Ed. 2007;46:1083–1085. doi: 10.1002/anie.200603561. [DOI] [PubMed] [Google Scholar]; (e) Kamiya N, Tominaga M, Sato S, Fujita M. J. Am. Chem. Soc. 2007;129:3816–3817. doi: 10.1021/ja0693082. [DOI] [PubMed] [Google Scholar]
- 67.(a) Flammang-Barbieux M, Nasielski J, Martin RH. Tetrahedron Lett. 1967:743–744. [Google Scholar]; (b) Martin RH, Flammang-Barbieux M, Cosyn JP, Gelbcke M. Tetrahedron Lett. 1968:3507–3510. [Google Scholar]
- 68.Staab HA, Wehinger E, Thorwart W. Chem. Ber. 1972;105:2290–2309. [Google Scholar]
- 69.Campbell K, Tykwinski RR. Chiral Carbon-Rich Macrocycles and Cyclophanes. In: Haley MM, Tykwinski RR, editors. Carbon-Rich Compounds. Weinheim: Wiley-VCH; 2006. [Google Scholar]
- 70.(a) Fuss M, Siehl H-U, Olenyuk B, Stang PJ. Organometallics. 1999;18:758–769. [Google Scholar]; (b) Vacek J, Caskey DC, Horinek D, Shoemaker RK, Stang PJ, Michl J. J. Am. Chem. Soc. 2008;130:7629–7638. doi: 10.1021/ja801341m. [DOI] [PubMed] [Google Scholar]
- 71.For an example of a purely covalent, carbon rich triagular dehydrobenzo[12]annulene see: Campbell ID, Eglinton G, Henderson W, Raphael RA. Chem. Commun. 1966:87–89. Staab HA, Graf F. Tetrahedron Lett. 1966:751–757.
- 72.For an example of a purely covalent, carbon-rich 1,8-diethynylanthracene rectangle see: Akiyama S, Nakagawa M. Chem. Ind. 1960:346–347.
- 73.(a) Kohnke FH, Slawin AMZ, Stoddart JF, Williams DJ. Angew. Chem. Int. Ed. Engl. 1987;26:892–894. [Google Scholar]; (b) Kohnke FH , Mathias JP, Stoddart JF. Angew. Chem. Int. Ed. Engl. 1989;28:1103–1110. [Google Scholar]
- 74.Manini P, Amrein W, Gramlich V, Diederich F. Angew. Chem. Int. Ed. 2002;41:4339–4343. doi: 10.1002/1521-3773(20021115)41:22<4339::AID-ANIE4339>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.