Abstract
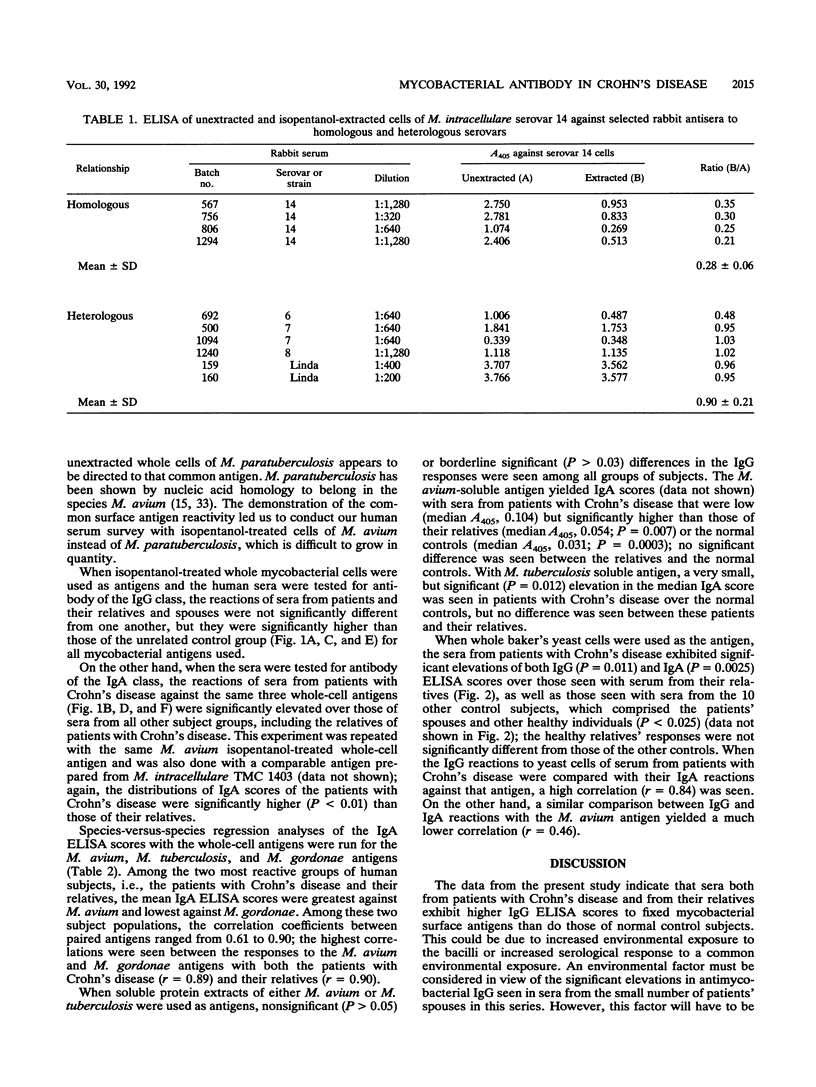
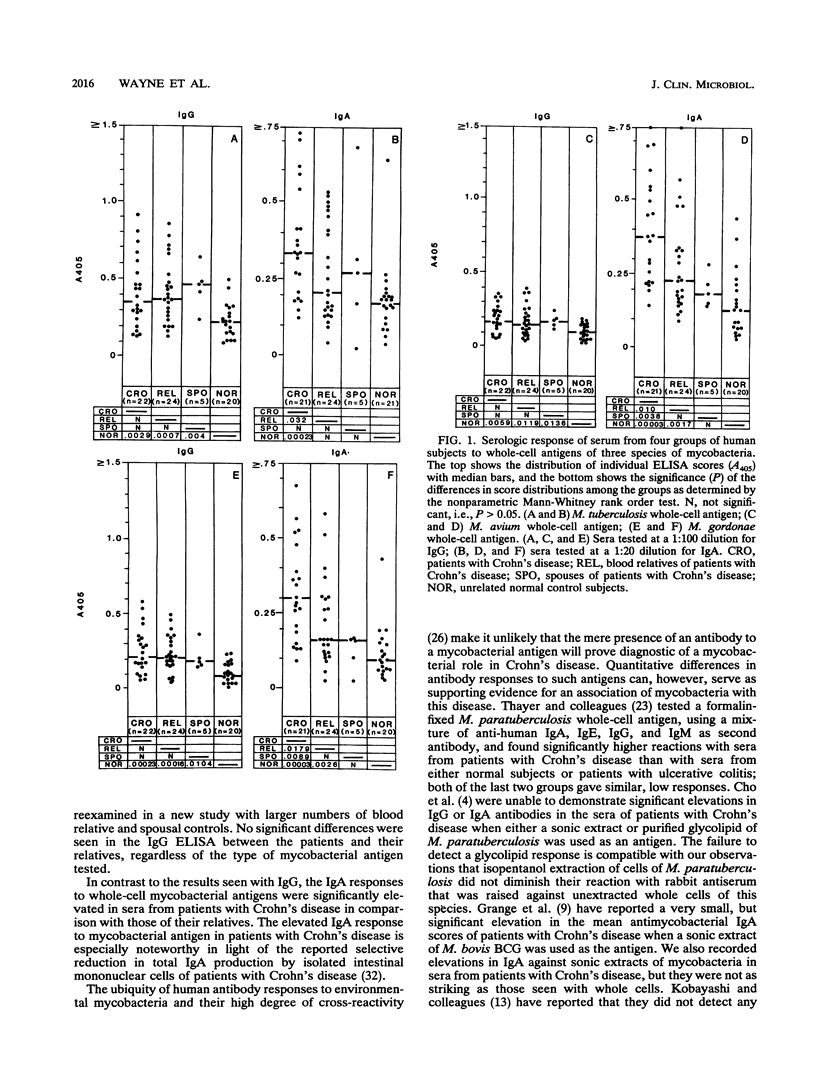
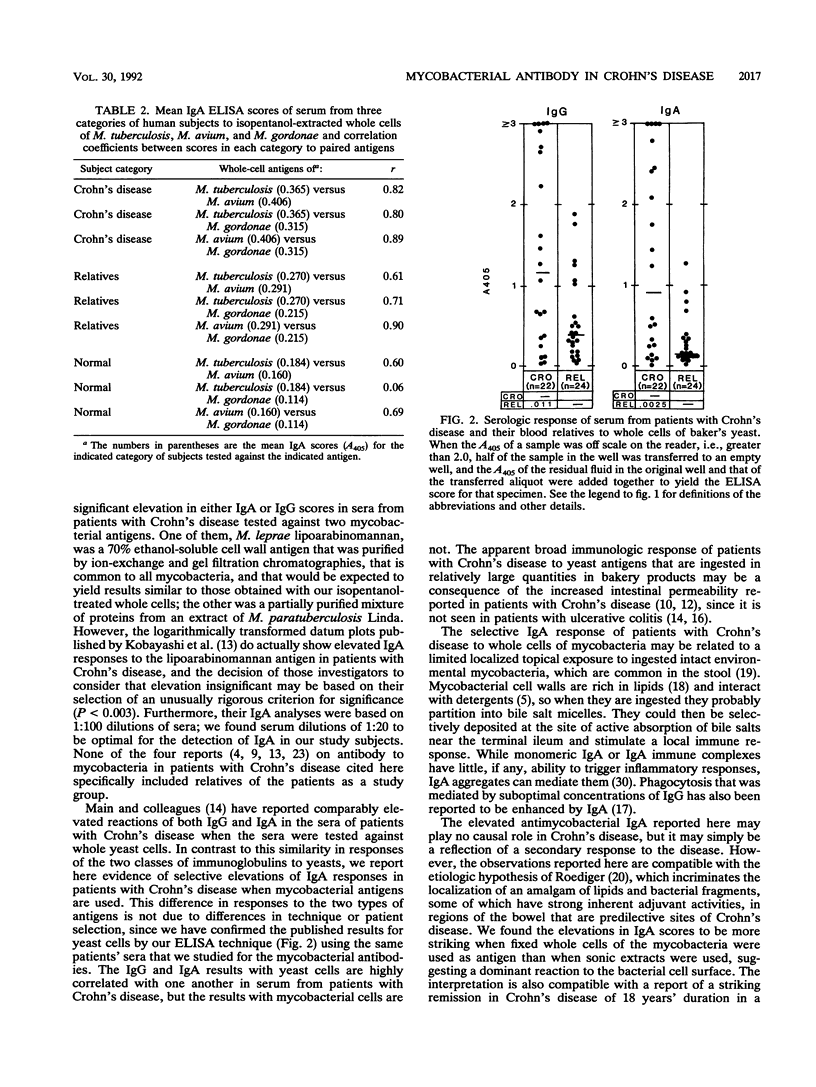
Sera from patients with Crohn's disease, their relatives, their spouses, and unrelated healthy controls were assayed by enzyme-linked immunosorbent assay for immunoglobulin G (IgG) and IgA antibodies to Mycobacterium tuberculosis, M. avium, and M. gordonae. The patients had significantly higher IgA responses to mycobacterial antigens than did either their relatives or the controls. On the other hand, both the patients and their relatives had significantly higher IgG responses against these antigens than did the controls. The elevated IgA response was more pronounced against isopentanol-extracted whole bacterial cells than it was against soluble protein extracts, and it appeared to be directed against fixed surface antigens that lie under the loosely bound peptidoglycolipid or glycolipid antigens of mycobacteria.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrow W. W., Ullom B. P., Brennan P. J. Peptidoglycolipid nature of the superficial cell wall sheath of smooth-colony-forming mycobacteria. J Bacteriol. 1980 Nov;144(2):814–822. doi: 10.1128/jb.144.2.814-822.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiodini R. J. Crohn's disease and the mycobacterioses: a review and comparison of two disease entities. Clin Microbiol Rev. 1989 Jan;2(1):90–117. doi: 10.1128/cmr.2.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiodini R. J., Van Kruiningen H. J., Thayer W. R., Merkal R. S., Coutu J. A. Possible role of mycobacteria in inflammatory bowel disease. I. An unclassified Mycobacterium species isolated from patients with Crohn's disease. Dig Dis Sci. 1984 Dec;29(12):1073–1079. doi: 10.1007/BF01317078. [DOI] [PubMed] [Google Scholar]
- Cho S. N., Brennan P. J., Yoshimura H. H., Korelitz B. I., Graham D. Y. Mycobacterial aetiology of Crohn's disease: serologic study using common mycobacterial antigens and a species-specific glycolipid antigen from Mycobacterium paratuberculosis. Gut. 1986 Nov;27(11):1353–1356. doi: 10.1136/gut.27.11.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitnick G., Collins J., Beaman B., Brooks D., Arthur M., Imaeda T., Palieschesky M. Preliminary report on isolation of mycobacteria from patients with Crohn's disease. Dig Dis Sci. 1989 Jun;34(6):925–932. doi: 10.1007/BF01540280. [DOI] [PubMed] [Google Scholar]
- Graham D. Y., Markesich D. C., Yoshimura H. H. Mycobacteria and inflammatory bowel disease. Results of culture. Gastroenterology. 1987 Feb;92(2):436–442. doi: 10.1016/0016-5085(87)90139-9. [DOI] [PubMed] [Google Scholar]
- Grange J. M., Gibson J., Nassau E., Kardjito T. Enzyme-linked immunosorbent assay (ELISA): a study of antibodies to Mycobacterium tuberculosis in the IgG, IgA and IgM classes in tuberculosis, sarcoidosis and Crohn's disease. Tubercle. 1980 Sep;61(3):145–152. doi: 10.1016/0041-3879(80)90003-3. [DOI] [PubMed] [Google Scholar]
- Hollander D., Vadheim C. M., Brettholz E., Petersen G. M., Delahunty T., Rotter J. I. Increased intestinal permeability in patients with Crohn's disease and their relatives. A possible etiologic factor. Ann Intern Med. 1986 Dec;105(6):883–885. doi: 10.7326/0003-4819-105-6-883. [DOI] [PubMed] [Google Scholar]
- James S. P. Remission of Crohn's disease after human immunodeficiency virus infection. Gastroenterology. 1988 Dec;95(6):1667–1669. doi: 10.1016/s0016-5085(88)80094-5. [DOI] [PubMed] [Google Scholar]
- Katz K. D., Hollander D., Vadheim C. M., McElree C., Delahunty T., Dadufalza V. D., Krugliak P., Rotter J. I. Intestinal permeability in patients with Crohn's disease and their healthy relatives. Gastroenterology. 1989 Oct;97(4):927–931. doi: 10.1016/0016-5085(89)91499-6. [DOI] [PubMed] [Google Scholar]
- Kobayashi K., Brown W. R., Brennan P. J., Blaser M. J. Serum antibodies to mycobacterial antigens in active Crohn's disease. Gastroenterology. 1988 Jun;94(6):1404–1411. doi: 10.1016/0016-5085(88)90679-8. [DOI] [PubMed] [Google Scholar]
- Main J., McKenzie H., Yeaman G. R., Kerr M. A., Robson D., Pennington C. R., Parratt D. Antibody to Saccharomyces cerevisiae (bakers' yeast) in Crohn's disease. BMJ. 1988 Oct 29;297(6656):1105–1106. doi: 10.1136/bmj.297.6656.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadden J. J., Butcher P. D., Thompson J., Chiodini R., Hermon-Taylor J. The use of DNA probes identifying restriction-fragment-length polymorphisms to examine the Mycobacterium avium complex. Mol Microbiol. 1987 Nov;1(3):283–291. doi: 10.1111/j.1365-2958.1987.tb01934.x. [DOI] [PubMed] [Google Scholar]
- McKenzie H., Main J., Pennington C. R., Parratt D. Antibody to selected strains of Saccharomyces cerevisiae (baker's and brewer's yeast) and Candida albicans in Crohn's disease. Gut. 1990 May;31(5):536–538. doi: 10.1136/gut.31.5.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mestecky J., McGhee J. R. Immunoglobulin A (IgA): molecular and cellular interactions involved in IgA biosynthesis and immune response. Adv Immunol. 1987;40:153–245. doi: 10.1016/s0065-2776(08)60240-0. [DOI] [PubMed] [Google Scholar]
- Portaels F., Larsson L., Smeets P. Isolation of mycobacteria from healthy persons' stools. Int J Lepr Other Mycobact Dis. 1988 Sep;56(3):468–471. [PubMed] [Google Scholar]
- Roediger W. E. A new hypothesis for the aetiology of Crohn's disease--evidence from lipid metabolism and intestinal tuberculosis. Postgrad Med J. 1991 Jul;67(789):666–671. doi: 10.1136/pgmj.67.789.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salcedo J. R., Hernández R. S., Célis H. Extraction of detergents from hydrophobic proteins with isopentanol: application to electrophoretic analysis of photosynthetic bacterial hydrophobic proteins. Anal Biochem. 1983 Jul 15;132(2):324–327. doi: 10.1016/0003-2697(83)90014-3. [DOI] [PubMed] [Google Scholar]
- Thayer W. R., Jr, Coutu J. A., Chiodini R. J., Van Kruiningen H. J., Merkal R. S. Possible role of mycobacteria in inflammatory bowel disease. II. Mycobacterial antibodies in Crohn's disease. Dig Dis Sci. 1984 Dec;29(12):1080–1085. doi: 10.1007/BF01317079. [DOI] [PubMed] [Google Scholar]
- Tsang V. C., Peralta J. M., Simons A. R. Enzyme-linked immunoelectrotransfer blot techniques (EITB) for studying the specificities of antigens and antibodies separated by gel electrophoresis. Methods Enzymol. 1983;92:377–391. doi: 10.1016/0076-6879(83)92032-3. [DOI] [PubMed] [Google Scholar]
- Wayne L. G., Anderson B., Chetty K., Light R. W. Antibodies to mycobacterial peptidoglycolipid and to crude protein antigens in sera from different categories of human subjects. J Clin Microbiol. 1988 Nov;26(11):2300–2306. doi: 10.1128/jcm.26.11.2300-2306.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayne L. G., Sramek H. A. Agents of newly recognized or infrequently encountered mycobacterial diseases. Clin Microbiol Rev. 1992 Jan;5(1):1–25. doi: 10.1128/cmr.5.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayne L. G., Sramek H. A. Antigenic differences between extracts of actively replicating and synchronized resting cells of Mycobacterium tuberculosis. Infect Immun. 1979 May;24(2):363–370. doi: 10.1128/iai.24.2.363-370.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayne L. G. The "atypical" mycobacteria: recognition and disease association. Crit Rev Microbiol. 1985;12(3):185–222. doi: 10.3109/10408418509104429. [DOI] [PubMed] [Google Scholar]
- Wayne L. G., Young L. S., Bertram M. Absence of mycobacterial antibody in patients with acquired immune deficiency syndrome. Eur J Clin Microbiol. 1986 Jun;5(3):363–365. doi: 10.1007/BF02017802. [DOI] [PubMed] [Google Scholar]
- Winter S. M., Bernard E. M., Gold J. W., Armstrong D. Humoral response to disseminated infection by Mycobacterium avium-Mycobacterium intracellulare in acquired immunodeficiency syndrome and hairy cell leukemia. J Infect Dis. 1985 Mar;151(3):523–527. doi: 10.1093/infdis/151.3.523. [DOI] [PubMed] [Google Scholar]
- Wu K. C., Mahida Y. R., Priddle J. D., Jewell D. P. Immunoglobulin production by isolated intestinal mononuclear cells from patients with ulcerative colitis and Crohn's disease. Clin Exp Immunol. 1989 Oct;78(1):37–41. [PMC free article] [PubMed] [Google Scholar]
- Yoshimura H. H., Graham D. Y., Estes M. K., Merkal R. S. Investigation of association of mycobacteria with inflammatory bowel disease by nucleic acid hybridization. J Clin Microbiol. 1987 Jan;25(1):45–51. doi: 10.1128/jcm.25.1.45-51.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura H. H., Graham D. Y. Nucleic acid hybridization studies of mycobactin-dependent mycobacteria. J Clin Microbiol. 1988 Jul;26(7):1309–1312. doi: 10.1128/jcm.26.7.1309-1312.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]


