Abstract
Surface modification techniques that create surfaces capable of killing adherent bacteria are promising solutions to infections associated with implantable medical devices. Antimicrobial peptoid oligomers (ampetoids) that were designed to mimic helical antimicrobial peptides were synthesized with a peptoid spacer chain to allow mobility and an adhesive peptide moiety for easy and robust immobilization onto substrates. TiO2 substrates were modified with the ampetoids and subsequently backfilled with an antifouling polypeptoid polymer in order to create polymer surface coatings composed of both antimicrobial (active) and antifouling (passive) peptoid functionalities. Confocal microscopy images show that the membranes of adherent E. coli were damaged after 2 h exposure to the modified substrates, suggesting that ampetoids retain antimicrobial properties even when immobilized onto substrates.
Keywords: ampetoids, antibacterial, antimicrobial, polypeptoid, antifouling
Introduction
Potential applications of surface-immobilized antimicrobial polymers include medical devices, water purification systems, food packaging, and hospital equipment (Kenawy et al., 2007). Bacterial infections on implanted medical devices such as catheters and pacemakers can lead to serious complications and often device removal because the adherent bacterial can form a biofilm, which provides a protective environment for bacteria against antibiotics and immune responses (Costerton et al., 1999; Habash & Reid, 1999).
Strategies to limit bacterial fouling of surfaces can incorporate either passive or active elements. Passive approaches to fouling prevention lack an active antibacterial component and typically involve the use of antifouling polymer surface coatings to provide steric resistance to physical attachment of bacteria. Examples of polymers that have been shown to reduce short-term bacterial attachment include poly(ethylene glycol) (PEG) (Kingshott et al., 2003; Harbers et al., 2007), self-assembled monolayers (SAMs) (Chapman et al., 2001), and peptide mimetic polymers (Statz et al., 2008).
Surface coatings may also contain active components capable of killing bacteria through direct contact or via a leachable compound. Examples of active antibacterial coatings include cationic polymers such as chitosan (Huh et al., 2001; Fu et al., 2005) and polymers containing quaternary ammonium (Lee et al., 2004; Cheng et al., 2005; Murata et al., 2007) and pyridinium (Tiller et al., 2001; Tiller et al., 2002; Cen et al., 2004; Krishnan et al., 2006) functional groups. The antibacterial effect of silver can be exploited by incorporation of silver salts or silver nanoparticles into coatings (Sambhy et al., 2006; Marini et al., 2007; Ramstedt et al., 2007a; Ramstedt et al., 2007b). Another reported approach for creating antibacterial surfaces involves the direct attachment of antibiotics such as vancomycin (Jose et al., 2005) and penicillin (Aumsuwan et al., 2007) to surfaces. Antimicrobial peptides (AMPs) offer activity against a wide range of organisms, while functioning with some selectivity for bacteria over mammalian cells (Hancock & Sahl, 2006), and there have been recent reports of AMP immobilization onto surfaces (Appendini & Hotchkiss, 2001; Gabriel et al., 2006).
Several non-natural mimics of antimicrobial peptides with high activity have recently been developed, providing advantages in terms of chemical diversity and significant resistance to protease degradation (Miller et al., 1995). For example, the linear cationic α-helical class of AMPs have been successfully mimicked as β-peptides and as poly-N-substituted glycines (peptoids) (Porter et al., 2002; Patch & Barron, 2003; Chongsiriwatana et al., 2008). Peptoids are non-natural mimics of polypeptides with the side chains appended to the amide nitrogen instead of the α-carbon (Zuckermann et al., 1992). Peptoids are well suited for antibacterial peptide use because they have been shown to be resistant to protease enzymes (Miller et al., 1995; Statz et al., 2008), the submonomer synthesis method allows for great versatility of the side-chain chemistry (Zuckermann et al., 1992), and helical secondary structures can be formed by incorporating bulky α-chiral side chains (Wu et al., 2001; Sanborn et al., 2002; Wu et al., 2003). Peptoid mimics of antimicrobial peptides (ampetoids) with helical structures that exhibit antibacterial activity in solution have been synthesized previously (Patch & Barron, 2003; Chongsiriwatana et al., 2008). Several ampetoids were recently tested for broad-spectrum activity and cytotoxicity, revealing peptoid sequences with antibacterial activity equivalent to cationic antimicrobial peptides and with strong selectivity for bacteria over mammalian cells (Chongsiriwatana et al., 2008).
Peptoids can also offer passive resistance to biofouling much like other antifouling polymers, as was first established with surfaces coated with a linear poly(N-methoxyethyl glycine) peptoid (Statz et al., 2005). Protein and cell adhesion onto TiO2 was dramatically reduced by coating with this peptoid, which has side-chains similar to the repeat unit of PEG. A follow-up study revealed a dramatic reduction in Staphylococcus epidermidis and Escherichia coli bacterial adhesion when compared to unmodified TiO2 substrates (Statz et al., 2008), presumably due to passive inhibition of bacterial cell attachment. In the current study, we combined the passive resistance of N-methoxyethyl glycine peptoids with an active antibacterial peptoid sequence identified in a previous ampetoid study (Chongsiriwatana et al., 2008). The results demonstrate a reduction in protein adsorption and fibroblast cell attachment. For surfaces incorporating the antibacterial peptoid sequence, a large fraction of attached bacteria had compromised membranes, confirming that the immobilized antibacterial peptoid remained active.
Materials and methods
Materials
The primary amines for peptoid synthesis, 2,2,2-trifluoroethylamine, (S)-(−)-1-phenylethylamine, (S)-(+)-sec-butylamine, methoxyethylamine and 1,4-diaminobutane were purchased from Aldrich (Milwaukee, WI). Dimethylformamide (DMF), diisopropylethylamine (DIPEA), acetonitrile, N-morpholinopropanesufonic acid (MOPS) buffer salt, 4-(2-hydroxyethyl)piperazine-1-ethanesulfonic acid (HEPES) buffer salt, 2-propanol, 1,1’-dioctadecyl-3,3,3’,3’tetramethylindocarbocyanine perchlorate (DiI) and fluorescein 5(6)-isothiocyanate (FITC) were also purchased from Aldrich (Milwaukee, WI). Rink amide MBHA resin was purchased from AnaSpec (San Jose, CA). Fmoc-Lys(Boc)-OH, Fmoc-Dopa(acetonide)-OH, and Fmoc-Pro-OH were purchased from Novabiochem (San Diego, CA). A protected submonomer (N-tert-butoxycarbonyl-1,4-butanediamine) was synthesized according to the published procedure (Krapcho & Kuell, 1990), while all other primary amines for peptoid synthesis were used as purchased. Acetic anhydride, 2-(1H-Benzotriazole-1-yl)-1,1,3,3-tetramethylaminium hexafluorophosphate (HBTU), and N-methylpyrrolidone (NMP) were purchased from Applied Biosystems (Foster City, CA). Trifluoroacetic acid (TFA) was obtained from Acros Organics (Belgium). Silicon wafers were purchased from University Wafer (South Boston, MA). Glass microscope slides and Lab-tek two-well slide chambers were purchased from Fisher Scientific (Pittsburgh, PA). 3T3-Swiss albino fibroblasts, Dulbecco's modified Eagle's medium, fetal bovine serum, penicillin/streptomycin, trypsin-EDTA, and Escherichia coli (ATCC 35218), Bacillus subtilis (ATCC 6633), Staphylococcus epidermidis RP62A (ATCC 12228) and Pseudomonas aeruginosa (ATCC 700829) were obtained from American Type Culture Collection (Manassas, VA). Mueller-Hinton broth (MHB) and agar were purchased from Becton, Dickinson and Co. (Sparks, MD). Lyophilized whole human serum (Control Serum N) was purchased from Roche Diagnostics (Indianapolis, IN). Ultrapure water (U.P. H2O) used for all experiments was purified (resistivity ≥ 18.2 MΩ-cm, total organic content ≤ 5 ppb) with a NANOpure Infinity System from Barnstead/Thermolyne Corp. (Dubuque, IA).
Synthesis
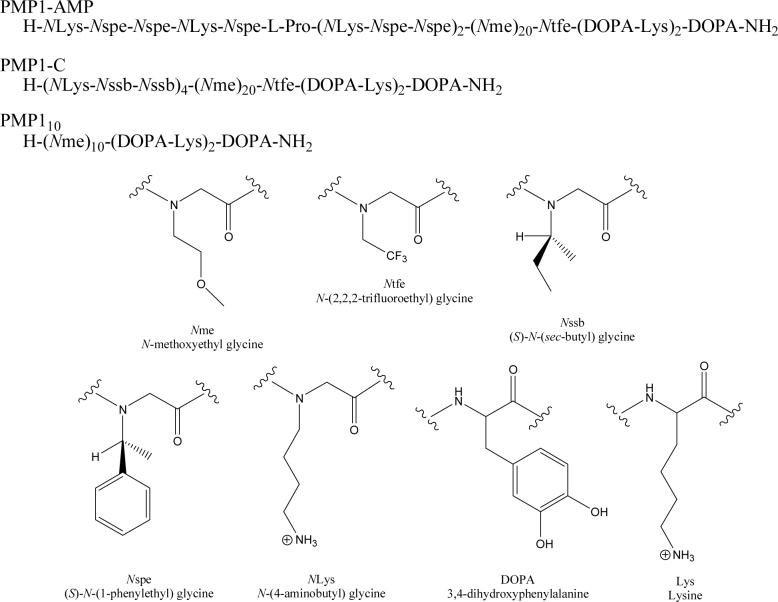
Polymer sequences and peptoid monomer side chains and amino acids are shown in Figure 1. Synthesis was performed on a C S Bio 036 (C S Bio Co., Menlo Park, CA) automated peptide synthesizer according to the previously described procedure (Statz et al., 2005). First, a C-terminal Dopa-Lys-Dopa-Lys-Dopa peptide was synthesized on rink amide MBHA resin using conventional Fmoc strategy of solid-phase peptide synthesis; this pentapeptide functions as an adhesive anchor for immobilization of the peptoid onto surfaces (Statz et al., 2005). A 20-mer N-methoxyethyl glycine (Nme) polypeptoid portion was then synthesized using a submonomer protocol (Zuckermann et al., 1992) and the resin was split. For PMP1-AMP and PMP1-C, submonomer synthesis was continued to add peptoid AMP (H-NLys-Nspe-Nspe-NLys-Nspe-Pro-[NLys-Nspe-Nspe]2-NH2) or peptoid C (H-[NLys-Nssb-Nssb]4-NH2) (Chongsiriwatana et al., 2008), respectively, onto the N-terminus of the Nme polypeptoid. During synthesis of PMP1-AMP and PMP1-C, a single residue of N-(2,2,2-trifluoroethyl) glycine (Ntfe) was added between the peptide and peptoid fragments, as a label for XPS compositional analysis. PMP110 consisted of a 10-mer sequence of Nme conjugated to the adhesive pentapeptide anchor, and was used as a passive ‘backfilling’ component in surfaces pre-adsorbed with PMP1-AMP.
Figure 1.
Polymer sequences, peptoid monomer structures and amino acid structures with abbreviations.
Polymers were cleaved from the resin and the amino acid side-chains were deprotected by treating the resin with 95% (v/v) TFA, 2.5% H2O and 2.5% TIS for 10 minutes, after which the cleaved polymer was removed by filtering and rinsing several times with TFA. Solvent was removed using a rotary evaporator; the product was dissolved in 50/50% water/acetonitrile, frozen and lyophilized. The crude products were purified by preparative reversed-phase high performance liquid chromatography (RP-HPLC) (Waters, Milford, MA) using a Vydac C18 column. The purity of each final product was confirmed by RP-HPLC and matrix-assisted laser desorption/ionization mass spectrometry (MALDI-MS) (Voyager DE-Pro, Perspective Biosystem, MA).
Antibacterial activity in solution
The minimum inhibitory concentration (MIC) for each polymer was determined according to Clinical Laboratory Standards Institute (CLSI) broth microdilution protocols (M7-A6). The procedure explained previously was followed (Chongsiriwatana et al., 2008); briefly, serial dilutions of the polymers were prepared in Mueller-Hinton broth (MHB) in 96-well microtiter plates and bacterial inoculum in MHB was added to each well (∼5.0 × 104 CFU/well). Optical density was monitored at 590 nm for 16 h at 35°C. The MIC was defined as the lowest concentration of peptoid/polymer necessary to completely inhibit bacterial growth for 16 h; experiments were repeated three times in duplicate for each bacterial strain including E. coli, B. subtilis, S. epidermidis and P. aeruginosa.
CD spectroscopy
CD measurements were conducted on a Jasco model 715 spectropolarimeter, using a quartz cylindrical cell (path length = 0.02 cm). Samples were dissolved at a concentration of 50 μM in 10 mM Tris-HCl (pH = 7.4). Scans were measured at 100 nm/min between 185 and 280 nm at 0.2 nm data pitch, 1 nm bandwidth, 2 s response, and 100 mdeg sensitivity. The plots contain the average data from 40 spectral accumulations.
Surface modification
Silicon wafers and glass slides were coated with a 20 nm-thick layer of TiO2 by electron beam evaporation (Edwards Auto306; <10−5 Torr); the coated wafers were cut into 1-cm2 pieces. The substrates were cleaned ultrasonically for ten minutes in 2-propanol, dried under N2, and then exposed to O2 plasma (Harrick Scientific, Ossinging, USA) at ≤150 Torr and 100 W for three minutes. Optical waveguide lightmode spectroscopy (OWLS) waveguides were purchased from MicroVacuum Ltd. (Budapest, Hungary) (Voros et al., 2002) and coated with a 10 nm-thick layer of TiO2 by electron beam evaporation as described above. Sensors were cleaned following the same procedure as TiO2 substrates. After use, OWLS waveguides were regenerated for subsequent use by 10-minute sonication cycles in 0.1 M HCl, U.P. H2O and 2-propanol followed by exposure to O2 plasma to remove adsorbates.
Clean substrates and sensors were immersed in a 0.5 mg/ml solution of the appropriate ampetoid in U.P. H2O at 25°C. After 2 hours, substrates were removed and rinsed with U.P. H2O to remove any unbound polymer, and then dried in a stream of filtered N2. Next the substrates were immersed in a 1.0 mg/ml solution of PMP110 in 3 M NaCl buffered with 0.1M MOPS, pH = 6 (C.P. buffer) at 50°C for 6 hours. After modification, substrates were extensively rinsed with U.P. H2O to remove any unbound polymer, and then dried in a stream of filtered N2.
X-ray photoelectron spectroscopy (XPS)
Survey and high resolution XPS spectra were collected on an Omicron ESCALAB (Omicron, Taunusstein, Germany) configured with a monochromated Al Kα (1486.8 eV) 300-W X-ray source, 1.5 mm circular spot size, a flood gun to counter charging effects, and an ultrahigh vacuum (<10−8 Torr). Substrates were prepared and analyzed as previously described (Statz et al., 2008).
Optical waveguide lightmode spectroscopy (OWLS)
OWLS was used to determine the optimum adsorption conditions for the polymers on TiO2 surfaces. Clean TiO2 sensors were inserted into the measurement head of an OWLS110 (MicroVacuum Ltd.) and exposed to the appropriate buffer solution through the flow-through cell (16 μl volume) for several hours to allow for equilibration. Ampetoid solutions were injected into the flow-through cell in stop-flow mode and allowed to adsorb for various times. The waveguide sensors were subsequently rinsed with buffer, and allowed to equilibrate for another 30 minutes. Between polymer adsorption steps, buffers were exchanged and the system was allowed to equilibrate for at least 10 hours. The measured incoupling angles, αTM and αTE were converted to refractive indices NTM and NTE by the MicroVacuum software, and changes in refractive index at the sensor surface were converted to adsorbed mass using de Feijter's formula (de Feijter et al., 1978). The refractive indices of solutions were measured using a refractometer (J157 Automatic Refractometer, Rudolph Research) under identical experimental conditions. A refractive index value of 1.35616 was used for the C.P. buffer, and 0.159 cm3/g and 0.129 cm3/g were used for dn/dc values for the ampetoids and PMP110, respectively.
OWLS was also used for in situ protein adsorption experiments; TiO2 coated waveguide sensors were modified with the polymers as explained previously. After adequate equilibration of the sensors in HEPES buffer, human serum solution was injected and allowed to adsorb for 20 minutes at 37°C before rinsing with HEPES buffer. A refractive index value of 1.33127 was used for the HEPES buffer, and a standard value of 0.182 cm3/g was used for dn/dc in the protein-adsorption calculations (Pasche et al., 2003). Averages and standard deviations from 3 replicates are reported. Statistical significance was assessed using a one-way ANOVA and Tukey's post-hoc test with 95% confidence intervals (SPSS, Chicago, IL).
Antibacterial activity on surfaces
E. coli were streaked from frozen stocks onto Mueller-Hinton agar and incubated overnight at 37°C. A few colonies were then used to inoculate 25 ml of sterile Mueller-Hinton broth (MHB) and grown overnight at 37°C. TiO2-coated glass slides were modified with the polymers using the previously described procedure. Two-well chambers were clamped to the slides and sealed by injecting silastic resin, which was allowed to cure overnight. Slides were sterilized by exposure to UV light for 10 minutes. The bacterial suspension was concentrated by centrifugation and resuspended in PBS at a concentration of 5 × 107 CFU/ml; 2 ml of the E. coli suspension was added to each slide chamber. Slide chambers were covered and placed in a humidified incubator at 37°C; after 2 h, nonadherent bacteria were removed by inverted centrifugation for 2 min at 30 rcf in sealed bags filled with PBS (Jensen et al., 2004). Adherent bacteria were stained with FITC (6 μg/ml) in PBS for 15 min at 37°C; FITC has been observed to only penetrate into cells with compromised membranes (Makovitzki et al., 2006). Slides were rinsed with PBS and imaged by confocal microscopy equipped with an inverted microscope (Leica TCS SP2). Phase contrast and fluorescent (488-nm band-pass filter for excitation of FITC) images were taken at identical locations to determine bacterial cell count and percent stained with FITC. The microscopy images were quantified using thresholding in ImageJ, and averages with standard deviations for at least nine images from one slide are reported. While slides were prepared in duplicate or triplicate and consistency was qualitatively confirmed between experimental samples, the reported counts and images were obtained from a single slide for each condition.
Fibroblast adhesion assay
3T3-Swiss albino fibroblasts were maintained at 37°C and 5% CO2 in Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal bovine serum (FBS) and 100 U/ml of penicillin/streptomycin. Immediately before use, fibroblasts of passage 12−16 were harvested using 0.25% trypsin-EDTA, resuspended in DMEM with 10% FBS and counted using a hemacytometer. Modified and unmodified TiO2 substrates were sterilized by exposure to UV light for 10 minutes; cells were seeded on each substrate at a density of 2.9 × 103 cells/cm2 and maintained in DMEM with FBS at 37°C and 5% CO2 for 4 hours, after which adherent cells were fixed in 3.7% paraformaldehyde for 5 minutes and stained with 5 μM 1,1’-dioctadecyl-3,3,3’,3’tetramethylindocarbocyanine perchlorate (DiI) for epifluorescent microscope counting.
Quantitative cell attachment data were obtained by acquiring nine images (10× magnification) from random locations on each substrate using a Leica epifluorescent microscope (W. Nuhsbaum Inc., McHenry, IL) equipped with a SPOT RT digital camera (Diagnostics Instruments, Sterling Heights, MI). Three identical substrates for each experiment were analyzed, and total projected cellular area was quantified using thresholding in Metamorph (Molecular Devices, Downingtown, PA); the mean and standard deviation are reported. Statistical significance was assessed using a one-way ANOVA and Tukey's post-hoc test with 95% confidence intervals (SPSS, Chicago, IL).
Results and discussion
Antimicrobial peptoid design
The antimicrobial peptoids incorporated previously identified sequences that mimic helical antimicrobial peptides (Patch & Barron, 2003; Chongsiriwatana et al., 2008). PMP1-AMP contained an N-terminal 12-mer peptoid sequence composed of α-chiral aromatic (S)-N-(1-phenylethyl)glycine (Nspe), achiral cationic N-(4-aminobutyl)glycine (NLys) and 1 proline residue, selected based on its reported low MIC and high selectivity ratio for bacterial cells over mammalian cells (Chongsiriwatana et al., 2008). PMP1-C contained a terminal 12-mer peptoid sequence composed of bulky α-chiral side-chains (S)-N-(sec-butyl)glycine (Nssb) and cationic N-(4-aminobutyl)glycine (NLys) residues, selected as a control because this sequence exhibited very low antibacterial and hemolytic activity (Patch & Barron, 2003). Both polymers were tethered to the substrate surface via a peptide/peptoid construct composed of adhesive peptides (DOPA, Lys) and antifouling peptoids (Nme). The (Nme)20-(DOPA-Lys)2-DOPA construct (PMP1) was previously shown to adsorb strongly to TiO2 substrates and provide excellent protein and cell fouling-resistant properties (Statz et al., 2005; Statz et al., 2008). A shorter version of this construct, PMP110, was synthesized in order to ‘backfill’ the surfaces with a passive antifouling peptoid after modification with PMP1-AMP. The polymers were purified using RP-HPLC, and the purity and molecular weight of the resulting fractions were determined using RP-HPLC and MALDI-MS (Figures S1 and S2). Chemical structures for PMP1-AMP, PMP1-C, and PMP110 are shown in Figure 1.
Antimicrobial activity - solution assay
The polymers were initially tested to determine their antibacterial properties in the solution state. Antibacterial activities of the compounds were tested using the methods described above for both Gram-negative and Gram-positive bacterial strains. The minimum inhibitory concentrations (MICs) are reported in Table 1. While PMP1-AMP was active against all bacterial strains, the MIC was 2−4 times greater than that for AMP alone; this increase can likely be explained by the presence of the additional 26 residues contained in the Nme tether and adhesive anchor portion of the polymer. As expected, peptoid C, PMP1-C and PMP110 were not active at the tested concentrations. While MIC values are useful predictors for antimicrobials in solution, these measurements do not directly correlate to the amount required for surface-immobilized applications because interactions between the bacteria and the immobilized polypeptoids are expected to differ from the interactions when the ampetoids are free in solution.
Table 1.
Antibacterial activities (MICs) of the ampetoids and polymer components with E. coli, B. subtilis, S. epidermidis, and P. aeruginosa.
| MIC (uM) | ||||
|---|---|---|---|---|
| E. coli |
B. subtilis |
S. epidermidis |
P. aeruginosa |
|
| PMP1-AMP | 50 | 3.1 | 3.1 | 200 |
| AMP | 12.5 | 1.6 | 1.6 | 25 |
| PMP1-C | > 100 | > 100 | > 100 | > 100 |
| Peptoid C | > 100 | > 100 | > 100 | > 100 |
| PMP1 | > 100 | > 100 | > 100 | > 100 |
The effects of the additional peptide and peptoid residues on the secondary structure of AMP were investigated by measuring the overall helix structure of the polymers using circular dichroism (CD) spectroscopy (Figure S3). PMP1-AMP and AMP exhibited similar spectral features, indicating the presence of a defined helical structure as was shown previously for AMP (Chongsiriwatana et al., 2008). The helical structures of PMP1-AMP and AMP are due to the incorporation of bulky α-chiral side chains which cause steric constraints (Armand et al., 1998; Wu et al., 2001; Sanborn et al., 2002; Wu et al., 2003). Peptoid C, PMP1-C and PMP110 did not have helical structures.
Surface characterization
Next we investigated adsorption of the polymers onto TiO2 substrates using OWLS and XPS. In order to create surfaces with both active antibacterial and passive antifouling properties, we modified surfaces with PMP1-AMP or PMP1-C first and then backfilled with the shorter PMP110. Modifying surfaces with a two-step approach involving grafting of a longer polymer followed by backfilling with a shorter one, has been shown to be an effective strategy for enhancing antifouling performance of polymer brushes (Fang et al., 2005; Satomi et al., 2007; Uchida et al., 2007). In the present case, backfilling with PMP110 should facilitate extension of the active AMP moiety away from the surface for interaction with bacteria that encounter the modified surface. Like antimicrobial peptides, ampetoids must interact with the bacterial membranes (Pag et al., 2004; Hancock & Sahl, 2006; Jenssen et al., 2006); therefore we expect that mobility of the longer ampetoid chains is important for antibacterial activity. Gabriel et al. demonstrated that poly(ethylene glycol) spacers were necessary when immobilizing cathelin LL37 on titanium surfaces because lateral mobility of surface-bound AMPs and parallel orientation of the peptide helices are required for interaction of the peptides with bacterial membranes (Gabriel et al., 2006).
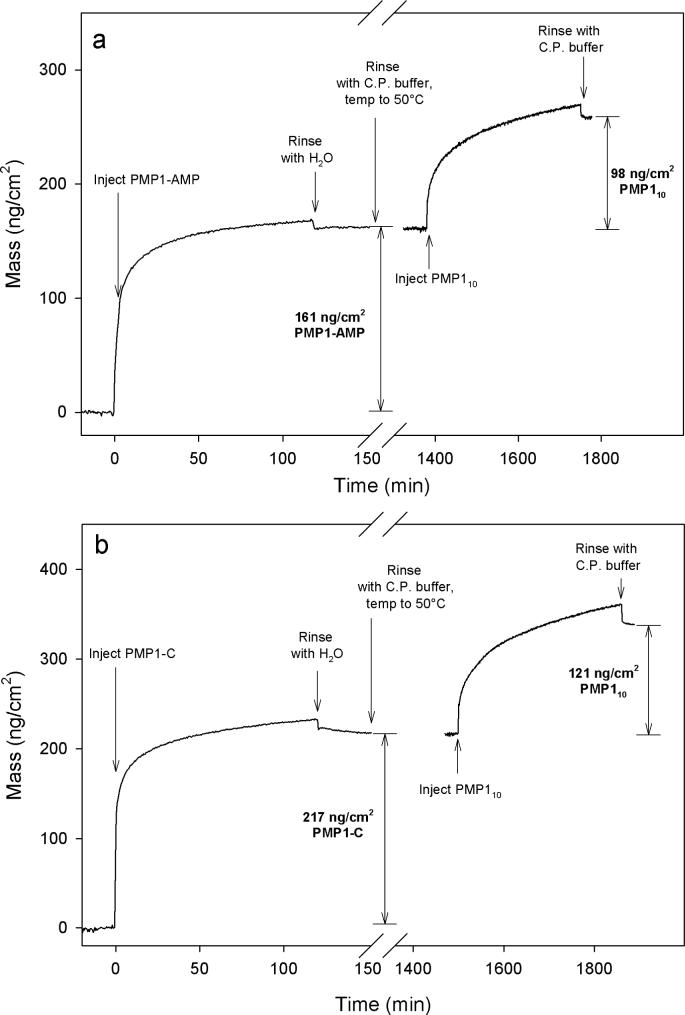
Optimum modification conditions were determined by conducting OWLS experiments; PMP1-AMP was adsorbed onto TiO2 waveguides using various concentrations and modification conditions. The goal was to adsorb a sub-monolayer coating of the polymer onto the surface. Subsequently, PMP110 was adsorbed on the waveguide containing the PMP1-AMP layer in order to backfill between the ampetoid chains with this antifouling polymer. While the PMP110 may be able to adsorb on top of the PMP1-AMP layer, most likely the PMP110 will adsorb to any remaining exposed areas of the TiO2 waveguide due to the strong interactive forces between DOPA and metal oxides (Statz et al., 2005; Lee et al., 2006). The OWLS mass plot, shown in Figure 2a, represents the optimum conditions for two-stage adsorption: 2 h adsorption of a 0.5 mg/ml solution of PMP1-AMP in H2O, followed by 6 h adsorption of a 1.0 mg/ml solution of PMP110 in C.P. buffer. The resulting adsorbed masses correlate to a polymer coating composed of approximately 40% PMP1-AMP antibacterial polymer and 60% of PMP110 antifouling polymer. The experiments were repeated using identical adsorption conditions for PMP1-C, shown in Figure 2b, yielding a surface composed of approximately 43% antibacterial polymer and 57% antifouling polymer. These conditions were selected for preparation of mixed surfaces in all subsequent experiments.
Figure 2.
OWLS plot of mass adsorption for PMP1-AMP and PMP110 (a) and PMP1-C and PMP110 (b).
The elemental compositions of the polymer layers are reported in Table 2, derived from the XPS survey scan spectra shown in Figure S4. C, N and O signals are representative of the peptide/peptoid backbone and side-chains of the polymers. Ti signal was detected for all samples from the underlying TiO2 substrates. The decrease in Ti signal for PMP110-modified substrates indicates successful modification, and the further decrease in Ti % for the PMP1-AMP and PMP1-C surfaces backfilled with PMP110 suggests that the polymer coating becomes thicker during the backfilling step. The detected F signal is from the single trifluoroethyl glycine residue included in the PMP1-AMP and PMP1-C polymers; the presence of the F signal after backfilling indicates that PMP1-AMP and PMP1-C remained on the surface after backfilling with PMP110.
Table 2.
Atomic compositions of bare and modified TiO2 substrates as determined from high-resolution XPS spectra.
| Experimental Composition (atom %) |
|||||
|---|---|---|---|---|---|
| Substrates | C | N | O | F | Ti |
| TiO2 | 16.8 | 0.2 | 54.8 | 0.0 | 28.2 |
| PMP110 only | 48.5 | 6.9 | 34.8 | 0.0 | 9.8 |
| PMP1-AMP* | 54.1 | 8.3 | 29.5 | 0.2 | 7.9 |
| PMP1-C* | 52.8 | 7.3 | 32.0 | 0.2 | 7.7 |
back-filled with PMP110
Antibacterial activity - substrates
Bacterial experiments on modified TiO2 slides were performed by exposing the surfaces to an E. coli suspension for two hours and then centrifuging to remove unattached and weakly attached bacteria. The bacteria remaining on the surfaces were imaged in phase contrast for determination of cell numbers, and in fluorescence after staining with FITC to detect cells with compromised membranes. Images of representative areas of the substrates are shown in Figure 3. From these images, total number of adherent bacteria (from phase contrast) and percent of attached cells with compromised membranes (from FITC staining) were determined and are shown in Table 3.
Figure 3.
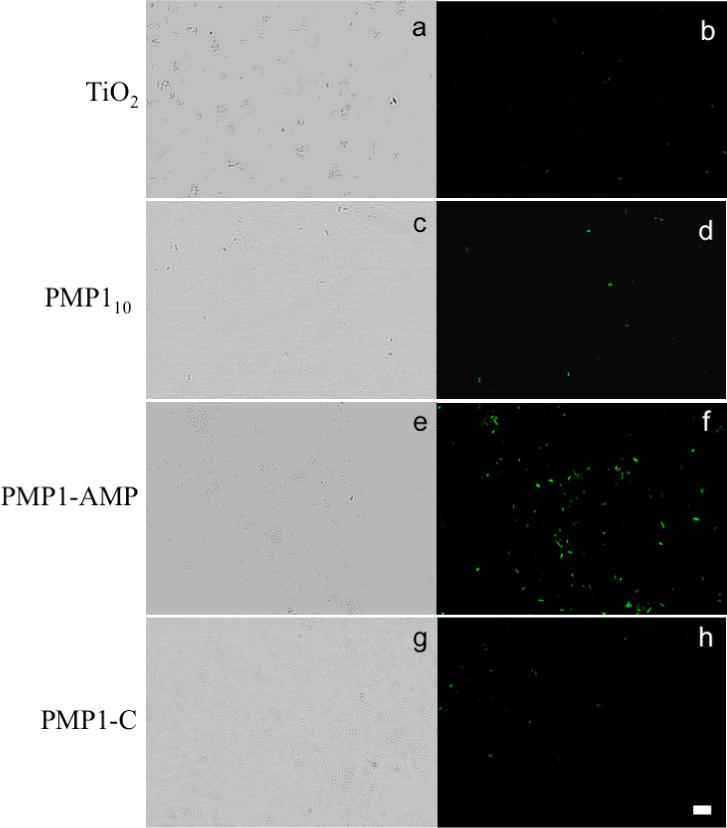
Confocal microscopy images (phase contrast images on left and fluorescent images on right) for E. coli after 2 h incubation on: bare TiO2 (a and b), PMP110 (c and d), PMP1-AMP (e and f), and PMP1-C (g and h) modified substrates. Scale bar = 10 μm.
Table 3.
Quantification of bacterial adhesion and membrane permeation on bare and modified TiO2 substrates. Standard deviations from the mean for nine images are reported.
| Substrates | Total Cell Count (cells/cm2) |
% FITC-stained |
|---|---|---|
| TiO2 | 74 ± 31 | 5 ± 3 |
| PMP110 only | 20 ± 7 | 27 ± 15 |
| PMP1-AMP* | 87 ± 13 | 69 ± 25 |
| PMP1-C* | 189 ± 82 | 4 ± 3 |
back-filled with PMP110
E. coli attachment to TiO2 was reduced upon modification with PMP110, which is in general agreement with a previous study on attachment of S. epidermidis and E. coli to TiO2 modified with a 20-mer Nme peptoid (Statz et al., 2008). The number of E. coli attached to PMP1-AMP was comparable to bare TiO2 surfaces, whereas attachment to PMP1-C was much higher than PMP1-AMP and TiO2 surfaces. Compared to PMP110, increased bacterial attachment to PMP1-AMP and PMP1-C surfaces could be explained either by direct interaction of the N-terminal peptoid sequences with the bacterial cell membrane, or through attachment of the bacteria to an adsorbed protein layer. In the case of PMP1-C the terminal peptoid is unlikely to be membrane active since this construct has no antibacterial properties in solution, suggesting a role for protein adsorption. With this in mind, we determined protein adsorption to PMP110, and PMP1-AMP and PMP1-C backfilled with PMP110. The results shown in Table 4 indicate that while serum protein adsorption was increased on PMP1-AMP and PMP1-C substrates compared to PMP110 only substrates, the adsorbed masses are significantly lower than on unmodified TiO2 sensors (p < 0.05). That the peptoid side-chains of the N-terminal segments of PMP1-AMP and PMP1-C increase fouling by macromolecules is not surprising given the hydrophobic nature of the Nspe and Nssb residues in these sequences (Ostuni et al., 2001).
Table 4.
Quantification of short-term (20 min) human serum protein adsorption on bare and modified TiO2 sensors using OWLS. Standard deviations from the mean for 3 replicates are reported.
| Substrate | Adsorbed Serum Mass (ng/cm2) |
|---|---|
| Bare TiO2 | 342 ± 21 |
| PMP110 | 51 ± 7 |
| PMP1-AMP* | 119 ± 40 |
| PMP1-C* | 88 ± 30 |
back-filled with PMP110
Staining of adhered E. coli with FITC revealed the highest percentage of bacterial cells with compromised membranes on the active PMP1-AMP polymer surface when compared to PMP1-C, PMP110 only and TiO2 surfaces (Table 3). While the percent death on the PMP110 substrates was surprisingly high, overall cell adhesion is much lower than on the PMP1-AMP substrates; therefore the total number of FITC-stained bacteria on the active surface is more than ten times greater than on the PMP110 surfaces. Percent FITC-stained bacteria on the PMP1-C substrates was low and comparable to the control TiO2 substrates, indicating no antimicrobial effects from this peptoid. While antibacterial activity was only demonstrated for E. coli, similar results are expected for B. subtilis, S. epidermidis and P. aeruginosa based on the MIC values determined for the ampetoids in solution (Table 1).
Antifouling properties of substrates-mammalian cells
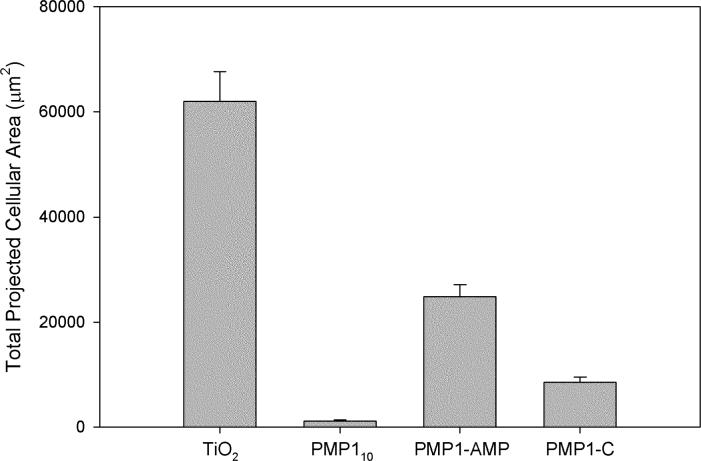
To further assess the antifouling properties of these substrates a short-term fibroblast adhesion assay was conducted, the results of which are shown in Figure 4. While the total projected area of adherent cells was greater on the polymer-modified surfaces containing the ampetoids than on the PMP110 substrates, all modified substrates had a significant reduction (p < 0.05) in cell adhesion compared to bare TiO2 substrates. The adherent cells appeared to be less spread on the ampetoid surfaces than the bare TiO2 substrates, suggesting that while the fibroblasts may be able to weakly attach, they are not capable of spreading on the surface. These fibroblast adhesion results are consistent with the relative levels of serum protein adsorption (Table 4), suggesting that the modestly higher cell adhesion on the ampetoid-containing surfaces can be explained by the higher adsorbed protein on these surfaces.
Figure 4.
Total projected cellular area for 4 h 3T3 fibroblast adhesion assay on bare TiO2 and modified TiO2 substrates.
Conclusions
In summary, peptoids are promising alternatives to conventional antimicrobials because of their stability, ease of synthesis and low cytotoxicity. Our results suggest that peptoid mimics of antimicrobial peptides can be immobilized onto surfaces, rendering these surfaces capable of compromising the membranes of attached bacteria. The antibacterial activity of the ampetoids was initially demonstrated in solution-based assays, and subsequently shown for E coli when immobilized onto surfaces. Substrates modified with PMP1-AMP exhibited increased bacterial adhesion, and a significant percentage of the adherent bacteria had compromised membranes, indicating that the surface-immobilized ampetoids were capable of interacting with the bacteria. By modifying the surfaces with both the ampetoids and PMP110, the passive resistance to protein and mammalian cell fouling was improved compared to bare TiO2, while maintaining sufficient ampetoid concentration for antibacterial activity. It remains to be seen whether the benefits of having an active antibacterial component on the surface may outweigh the disadvantages of slightly increased bacterial adhesion. More detailed future studies involving fine-tuning the ratio of adsorbed passive and active components, or the composition of the active component, may reveal a surface composition that combines even greater resistance to protein and bacterial adhesion with an antibacterial effect. For some medical device applications, more sophisticated designs incorporating hydrolytic or enzymatic cleavage sites within the PMP1-AMP backbone might allow for cleavage of the antibacterial domain from the coating once the risk of postoperative infection had passed, leaving behind a protein and cell resistant PMP1 coating.
Supplementary Material
Acknowledgements
This research was supported by NIH grants R37 DE014193 (PBM), R01 AI072666 and R01 EB003806 (AEB). XPS surface analysis was performed at KeckII/NUANCE and confocal microscopy was performed at the Biological Imaging Facility at Northwestern University.
References
- Appendini P, Hotchkiss JH. Surface modification of poly(styrene) by the attachment of an antimicrobial peptide. Journal of Applied Polymer Science. 2001;81:609–616. [Google Scholar]
- Armand P, Kirshenbaum K, Goldsmith RA, Farr-Jones S, Barron AE, Truong KTV, Dill KA, Mierke DF, Cohen FE, Zuckermann RN, Bradley EK. NMR determination of the major solution conformation of a peptoid pentamer with chiral side chains. Proceedings of the National Academy of Science USA. 1998;95:4309–4314. doi: 10.1073/pnas.95.8.4309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aumsuwan N, Heinhorst S, Urban MW. Antibacterial Surfaces on Expanded Polytetrafluoroethylene; Penicillin Attachment. Biomacromolecules. 2007;8:713–718. doi: 10.1021/bm061050k. [DOI] [PubMed] [Google Scholar]
- Cen L, Neoh KG, Ying L, Kang ET. Surface modification of polymeric films and membranes to achieve antibacterial properties. Surface and Interface Analysis. 2004;36:716–719. [Google Scholar]
- Chapman RG, Ostuni E, Liang MN, Meluleni G, Kim E, Yan L, Pier G, Warren HS, Whitesides GM. Polymeric Thin Films That Resist the Adsorption of Proteins and the Adhesion of Bacteria. Langmuir. 2001;17:1225–1233. [Google Scholar]
- Cheng Z, Zhu X, Shi ZL, Neoh KG, Kang ET. Polymer Microspheres with Permanent Antibacterial Surface from Surface-Initiated Atom Transfer Radical Polymerization. Ind Eng Chem Res. 2005;44:7098–7104. [Google Scholar]
- Chongsiriwatana NP, Patch JA, Czyzewski AM, Dohm MT, Ivankin A, Gidalevitz D, Zuckermann RN, Barron AE. Peptoids that mimic the structure, function, and mechanism of helical antimicrobial peptides. Proceedings of the National Academy of Science USA. 2008;105:2794–2799. doi: 10.1073/pnas.0708254105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costerton JW, Stewart PS, Greenberg EP. Bacterial Biofilms: A Common Cause of Persistent Infections. Science. 1999;284:1318–1322. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- de Feijter JA, Benjamins J, Veer FA. Ellipsometry as a Tool to Study Adsorption Behavior of Synthetic and Biopolymers at Air-Water-Interface. Biopolymers. 1978;17:1759–1772. [Google Scholar]
- Fang F, Satulovsky J, Szleifer I. Kinetics of Protein Adsorption and Desorption on Surfaces with Grafted Polymers. Biophys J. 2005;89:1516–1533. doi: 10.1529/biophysj.104.055079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu J, Ji J, Yuan W, Shen J. Construction of anti-adhesive and antibacterial multilayer films via layer-by-layer assembly of heparin and chitosan. Biomaterials. 2005;26:6684–6692. doi: 10.1016/j.biomaterials.2005.04.034. [DOI] [PubMed] [Google Scholar]
- Gabriel M, Nazmi K, Veerman EC, NieuwAmerongen AV, Zentner A. Preparation of LL-37-Grafted Titanium Surfaces with Bactericidal Activity. Bioconjugate Chem. 2006;17:548–550. doi: 10.1021/bc050091v. [DOI] [PubMed] [Google Scholar]
- Habash M, Reid G. Microbial biofilms: their development and significance for medical device-related infections. J Clin Pharmacol. 1999;39:887–898. doi: 10.1177/00912709922008506. [DOI] [PubMed] [Google Scholar]
- Hancock REW, Sahl H-G. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat Biotech. 2006;24:1551–1557. doi: 10.1038/nbt1267. [DOI] [PubMed] [Google Scholar]
- Harbers GM, Emoto K, Greef C, Metzger SW, Woodward HN, Mascali JJ, Grainger DW, Lochhead MJ. Functionalized Poly(ethylene glycol)-Based Bioassay Surface Chemistry That Facilitates Bio-Immobilization and Inhibits Nonspecific Protein, Bacterial, and Mammalian Cell Adhesion. Chem Mater. 2007;19:4405–4414. doi: 10.1021/cm070509u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh MW, Kang I-K, Lee DH, Kim WS, Lee DH, Park LS, Min KE, Seo KH. Surface characterization and antibacterial activity of chitosan-grafted poly(ethylene terephthalate) prepared by plasma glow discharge. Journal of Applied Polymer Science. 2001;81:2769–2778. [Google Scholar]
- Jensen TW, Hu BH, Delatore SM, Garcia AS, Messersmith PB, Miller WM. Lipopeptides incorporated into supported phospholipid monolayers have high specific activity at low incorporation levels. Journal of the American Chemical Society. 2004;126:15223–15230. doi: 10.1021/ja048684o. [DOI] [PubMed] [Google Scholar]
- Jenssen H, Hamill P, Hancock REW. Peptide Antimicrobial Agents. Clin Microbiol Rev. 2006;19:491–511. doi: 10.1128/CMR.00056-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jose B, Antoci V, Jr., Zeiger AR, Wickstrom E, Hickok NJ. Vancomycin covalently bonded to titanium beads kills Staphylococcus aureus. Chemistry & Biology. 2005;12:1041–1048. doi: 10.1016/j.chembiol.2005.06.013. [DOI] [PubMed] [Google Scholar]
- Kenawy E-R, Worley SD, Broughton R. The chemistry and applications of antimicrobial polymers: a state-of-the-art review. Biomacromolecules. 2007;8:1359–1384. doi: 10.1021/bm061150q. [DOI] [PubMed] [Google Scholar]
- Kingshott P, Wei J, Bagge-Ravn D, Gadegaard N, Gram L. Covalent Attachment of Poly(ethylene glycol) to Surfaces, Critical for Reducing Bacterial Adhesion. Langmuir. 2003;19:6912–6921. [Google Scholar]
- Krapcho AP, Kuell CS. Mono-protected diamines. N-tert-butoxycarbonyl-αω-alkanediamines from αω-alkanediamines. Synthetic Communications. 1990;20:2559–2564. [Google Scholar]
- Krishnan S, Ward RJ, Hexemer A, Sohn KE, Lee KL, Angert ER, Fischer DA, Kramer EJ, Ober CK. Surfaces of fluorinated pyridinium block copolymers with enhanced antibacterial activity. Langmuir. 2006;22:11255–11266. doi: 10.1021/la061384v. [DOI] [PubMed] [Google Scholar]
- Lee H, Scherer NF, Messersmith PB. Single-molecule mechanics of mussel adhesion. Proceedings of the National Academy of Sciences. 2006;103:12999–13003. doi: 10.1073/pnas.0605552103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SB, Koepsel RR, Morley SW, Matyjaszewski K, Sun Y, Russell AJ. Permanent, Nonleaching Antibacterial Surfaces. 1. Synthesis by Atom Transfer Radical Polymerization. Biomacromolecules. 2004;5:877–882. doi: 10.1021/bm034352k. [DOI] [PubMed] [Google Scholar]
- Makovitzki A, Avrahami D, Shai Y. Ultrashort antibacterial and antifungal lipopeptides. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:15997–16002. doi: 10.1073/pnas.0606129103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marini M, De Niederhausern S, Iseppi R, Bondi M, Sabia C, Toselli M, Pilati F. Antibacterial activity of plastics coated with silver-doped organic-inorganic hybrid coatings prepared by sol-gel processes. Biomacromolecules. 2007;8:1246–1254. doi: 10.1021/bm060721b. [DOI] [PubMed] [Google Scholar]
- Miller SM, Simon RJ, Ng S, Zuckermann RN, Kerr JM, Moos WH. Comparison of the proteolytic susceptibilities of homologous L-amino acid, D-amino acid, and N-substituted glycine peptide and peptoid oligomers. Drug Development Research. 1995;35:20–32. [Google Scholar]
- Murata H, Koepsel RR, Matyjaszewski K, Russell AJ. Permanent, non-leaching antibacterial surfaces--2: How high density cationic surfaces kill bacterial cells. Biomaterials. 2007;28:4870–4879. doi: 10.1016/j.biomaterials.2007.06.012. [DOI] [PubMed] [Google Scholar]
- Ostuni E, Chapman RG, Holmlin RE, Takayama S, Whitesides GM. A Survey of Structure-Property Relationships of Surfaces that Resist the Adsorption of Protein. Langmuir. 2001;17:5605–5620. doi: 10.1021/la0015258. [DOI] [PubMed] [Google Scholar]
- Pag U, Oedenkoven M, Papo N, Oren Z, Shai Y, Sahl HG. In vitro activity and mode of action of diastereomeric antimicrobial peptides against bacterial clinical isolates. Journal of Antimicrobial Chemotherapy. 2004;53:230–239. doi: 10.1093/jac/dkh083. [DOI] [PubMed] [Google Scholar]
- Pasche S, De Paul SM, Voros J, Spencer ND, Textor M. Poly(L-lysine)-graft-poly(ethylene glycol) assembled monolayers on Niobium Oxide surfaces: a quantitative study of the influence of polymer interfacial architecture on resistance to protein adsorption by ToF-SIMS and in situ OWLS. Langmuir. 2003;19:9216–9225. [Google Scholar]
- Patch JA, Barron AE. Helical Peptoid Mimics of Magainin-2 Amide. Journal of the American Chemical Society. 2003;125:12092–12093. doi: 10.1021/ja037320d. [DOI] [PubMed] [Google Scholar]
- Porter EA, Weisblum B, Gellman SH. Mimicry of Host-Defense Peptides by Unnatural Oligomers: Antimicrobial beta-Peptides. Journal of the American Chemical Society. 2002;124:7324–7330. doi: 10.1021/ja0260871. [DOI] [PubMed] [Google Scholar]
- Ramstedt M, Cheng N, Azzaroni O, Mossialos D, Mathieu HJ, Huck WT. Synthesis and characterization of poly(3-sulfopropylmethacrylate) brushes for potential antibacterial applications. Langmuir. 2007a;23:3314–3321. doi: 10.1021/la062670+. [DOI] [PubMed] [Google Scholar]
- Ramstedt M, Houriet R, Mossialos D, Dieter H, Mathieu HJ. Wet chemical silver treatment of endotracheal tubes to produce antibacterial surfaces. Journal of Biomedical Materials Research Part B: Applied Biomaterials. 2007b;83B:169–180. doi: 10.1002/jbm.b.30781. [DOI] [PubMed] [Google Scholar]
- Sambhy V, MacBride MM, Peterson BR, Sen A. Silver bromide nanoparticle/polymer composites: dual action tunable antimicrobial materials. Journal of the American Chemical Society. 2006;128:9798–9808. doi: 10.1021/ja061442z. [DOI] [PubMed] [Google Scholar]
- Sanborn TJ, Wu CW, Zuckerman RN, Barron AE. Extreme stability of helices formed by water-soluble poly-N- substituted glycines (polypeptoids) with alpha-chiral side chains. Biopolymers. 2002;63:12–20. doi: 10.1002/bip.1058. [DOI] [PubMed] [Google Scholar]
- Satomi T, Nagasaki Y, Kobayashi H, Otsuka H, Kataoka K. Density Control of Poly(ethylene glycol) Layer To Regulate Cellular Attachment. Langmuir. 2007;23:6698–6703. doi: 10.1021/la0624384. [DOI] [PubMed] [Google Scholar]
- Statz AR, Meagher RJ, Barron AE, Messersmith PB. New Peptidomimetic Polymers for Antifouling Surfaces. Journal of the American Chemical Society. 2005;127:7972–7973. doi: 10.1021/ja0522534. [DOI] [PubMed] [Google Scholar]
- Statz AR, Barron AE, Messersmith PB. Protein, cell and bacterial fouling resistance of polypeptoid-modified surfaces: effect of side-chain chemistry. Soft Matter. 2008;4:131–139. doi: 10.1039/B711944E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiller JC, Liao CJ, Lewis K, Klibanov AM. Designing surfaces that kill bacteria on contact. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:5981–5985. doi: 10.1073/pnas.111143098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiller JC, Lee SB, Lewis K, Klibanov AM. Polymer surfaces derivatized with poly(vinyl-N-hexylpyridinium) kill airborne and waterborne bacteria. Biotechnology & Bioengineering. 2002;79:465–471. doi: 10.1002/bit.10299. [DOI] [PubMed] [Google Scholar]
- Uchida K, Hoshino Y, Tamura A, Yoshimoto K, Kojima S, Yamashita K, Yamanaka I, Otsuka H, Kataoka K, Nagasaki Y. Creation of a mixed poly(ethylene glycol) tethered-chain surface for preventing the nonspecific adsorption of proteins and peptides. Biointerphases. 2007;2:126–130. doi: 10.1116/1.2800754. [DOI] [PubMed] [Google Scholar]
- Voros J, Ramsden JJ, Csucs G, Szendro I, De Paul SM, Textor M, Spencer ND. Optical grating coupler biosensors. Biomaterials. 2002;23:3699–3710. doi: 10.1016/s0142-9612(02)00103-5. [DOI] [PubMed] [Google Scholar]
- Wu CW, Sanborn TJ, Huang K, Zuckermann RN, Barron A. Peptoid oligomers with achiral, aromatic side chains: Sequence requirements for the formation of stable peptoid helices. Journal of the American Chemical Society. 2001;123:6778–6784. doi: 10.1021/ja003154n. [DOI] [PubMed] [Google Scholar]
- Wu CW, Kirshenbaum K, Sanborn TJ, Patch JA, Huang K, Dill KA, Zuckermann RN, Barron AE. Structural and spectroscopic studies of peptoid oligomers with alpha-chiral aliphatic side chains. Journal of the American Chemical Society. 2003;125:13525–13530. doi: 10.1021/ja037540r. [DOI] [PubMed] [Google Scholar]
- Zuckermann RN, Kerr JM, Kent SBH, Moos WH. Efficient Method For the Preparation of Peptoids [Oligo(N- Substituted Glycines)] By Submonomer Solid-Phase Synthesis. Journal of the American Chemical Society. 1992;114:10646–10647. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.