Abstract
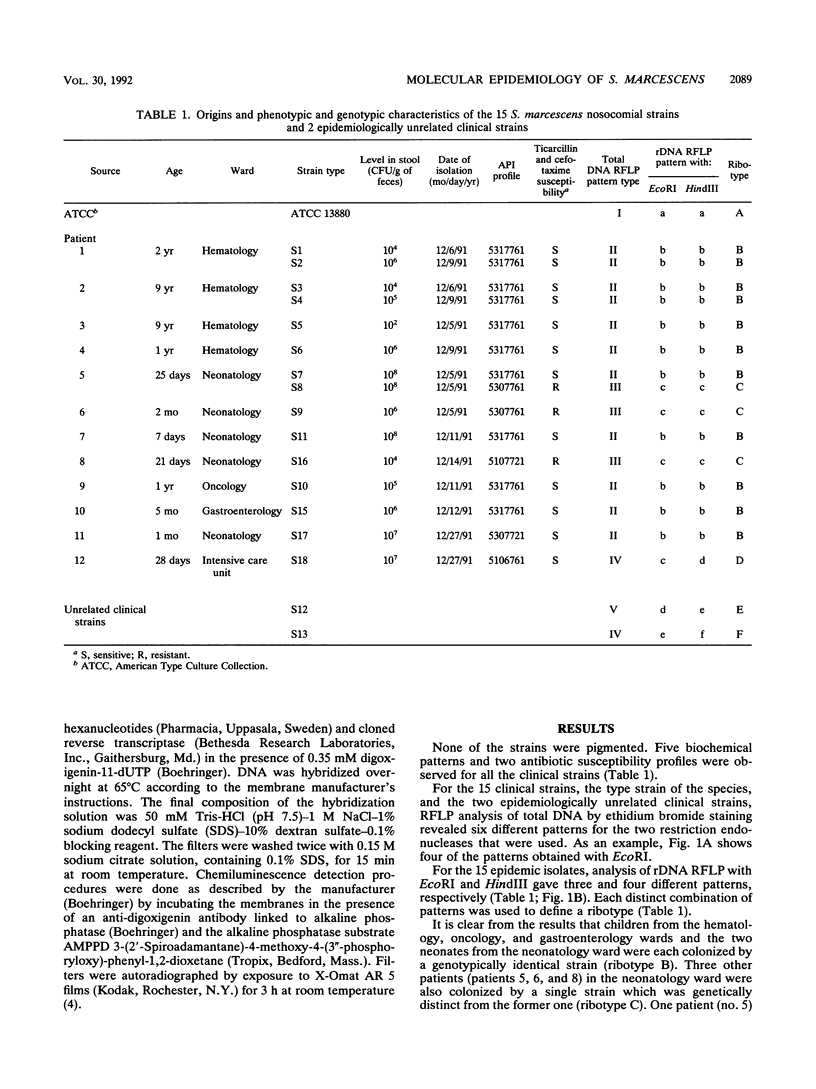
Ribotyping with a nonradioactive probing system was used for the epidemiological evaluation of 15 Serratia marcescens nosocomial strains isolated from the stools of 12 children with no apparent illness in five different hospital wards over a 20-day period. Our results indicate that the occurrence of S. marcescens colonization was the result of the spread of a single epidemiological strain in the hematology ward, the oncology ward, and the gastroenterology ward and in two neonates in the neonatology ward, suggesting cross-contamination between the patients in these four wards. This isolate was genotypically unrelated to the bacterial strain found in the three other patients in the neonatology ward. Interestingly, one patient in the neonatology ward harbored these two genotypically different strains. Finally, the patient in the intensive care unit was colonized with a different strain. We find ribotyping to be a more reliable technique than biochemical typing. The results of ribotyping are more easily interpreted than are those of total DNA analysis, with an equivalent degree of discrimination.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anagnostakis D., Fitsialos J., Koutsia C., Messaritakis J., Matsaniotis N. A nursery outbreak of Serratia marcescens infection. Evidence of a single source of contamination. Am J Dis Child. 1981 May;135(5):413–414. doi: 10.1001/archpedi.1981.02130290011005. [DOI] [PubMed] [Google Scholar]
- Baker C. J. Nosocomial septicemia and meningitis in neonates. Am J Med. 1981 Mar;70(3):698–701. doi: 10.1016/0002-9343(81)90599-4. [DOI] [PubMed] [Google Scholar]
- Bingen E. H., Denamur E., Lambert-Zechovsky N. Y., Bourdois A., Mariani-Kurkdjian P., Cezard J. P., Navarro J., Elion J. DNA restriction fragment length polymorphism differentiates crossed from independent infections in nosocomial Xanthomonas maltophilia bacteremia. J Clin Microbiol. 1991 Jul;29(7):1348–1350. doi: 10.1128/jcm.29.7.1348-1350.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bingen E., Denamur E., Lambert-Zechovsky N., Aujard Y., Brahimi N., Geslin P., Elion J. Analysis of DNA restriction fragment length polymorphism extends the evidence for breast milk transmission in Streptococcus agalactiae late-onset neonatal infection. J Infect Dis. 1992 Mar;165(3):569–573. doi: 10.1093/infdis/165.3.569. [DOI] [PubMed] [Google Scholar]
- Christensen G. D., Korones S. B., Reed L., Bulley R., McLaughlin B., Bisno A. L. Epidemic Serratia marcescens in a neonatal intensive care unit: importance of the gastrointestinal tract as a reservoir. Infect Control. 1982 Mar-Apr;3(2):127–133. doi: 10.1017/s0195941700055909. [DOI] [PubMed] [Google Scholar]
- Grimont F., Grimont P. A. Ribosomal ribonucleic acid gene restriction patterns as potential taxonomic tools. Ann Inst Pasteur Microbiol. 1986 Sep-Oct;137B(2):165–175. doi: 10.1016/s0769-2609(86)80105-3. [DOI] [PubMed] [Google Scholar]
- Grimont P. A., Grimont F. Biotyping of Serratia marcescens and its use in epidemiological studies. J Clin Microbiol. 1978 Jul;8(1):73–83. doi: 10.1128/jcm.8.1.73-83.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan P. U., Pereira B., Macaden R. Epidemiological study of an outbreak of Serratia marcescens in a haemodialysis unit. J Hosp Infect. 1991 May;18(1):57–61. doi: 10.1016/0195-6701(91)90093-n. [DOI] [PubMed] [Google Scholar]
- McGeer A., Low D. E., Penner J., Ng J., Goldman C., Simor A. E. Use of molecular typing to study the epidemiology of Serratia marcescens. J Clin Microbiol. 1990 Jan;28(1):55–58. doi: 10.1128/jcm.28.1.55-58.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newport M. T., John J. F., Michel Y. M., Levkoff A. H. Endemic Serratia marcescens infection in a neonatal intensive care nursery associated with gastrointestinal colonization. Pediatr Infect Dis. 1985 Mar-Apr;4(2):160–167. doi: 10.1097/00006454-198503000-00010. [DOI] [PubMed] [Google Scholar]
- Sifuentes-Osornio J., Ruiz-Palacios G. M., Gröschel D. H. Analysis of epidemiologic markers of nosocomial Serratia marcescens isolates with special reference to the Grimont biotyping system. J Clin Microbiol. 1986 Feb;23(2):230–234. doi: 10.1128/jcm.23.2.230-234.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simor A. E., Ramage L., Wilcox L., Bull S. B., Bialkowska-Hobrzanska H. Molecular and epidemiologic study of multiresistant Serratia marcescens infections in a spinal cord injury rehabilitation unit. Infect Control. 1988 Jan;9(1):20–27. [PubMed] [Google Scholar]
- Smith P. J., Brookfield D. S., Shaw D. A., Gray J. An outbreak of Serratia marcescens infections in a neonatal unit. Lancet. 1984 Jan 21;1(8369):151–153. doi: 10.1016/s0140-6736(84)90074-6. [DOI] [PubMed] [Google Scholar]
- Stull T. L., LiPuma J. J., Edlind T. D. A broad-spectrum probe for molecular epidemiology of bacteria: ribosomal RNA. J Infect Dis. 1988 Feb;157(2):280–286. doi: 10.1093/infdis/157.2.280. [DOI] [PubMed] [Google Scholar]
- Tancrède C. H., Andremont A. O. Bacterial translocation and gram-negative bacteremia in patients with hematological malignancies. J Infect Dis. 1985 Jul;152(1):99–103. doi: 10.1093/infdis/152.1.99. [DOI] [PubMed] [Google Scholar]