Abstract
The factors necessary for normal pancreatic islet morphogenesis have not been well characterized. Here we report that connective tissue growth factor (CTGF) is involved in the establishment of normal islet endocrine cell ratio and architecture. CTGF is a secreted protein known to modulate several growth factor-signaling pathways including TGF-β, BMP, and Wnt. Although its role in pancreatic diseases such as pancreatitis and pancreatic cancer are well documented, a role for CTGF in normal pancreas development and function has heretofore not been examined. Using a lacZ-tagged CTGF allele, we describe for the first time the expression pattern of CTGF in the developing pancreas and the requirement of CTGF for normal islet morphogenesis and embryonic β-cell proliferation. CTGF is highly expressed in pancreatic ductal epithelium and vascular endothelium, as well as at lower levels in developing insulin+ cells, but becomes down-regulated in β-cells soon after birth. Pancreata from CTGF null embryos have an increase in glucagon+ cells with a concomitant decrease in insulin+ cells, and show defects in islet morphogenesis. Loss of CTGF also results in a dramatic decrease in β-cell proliferation at late gestation. Unlike CTGF null embryos, CTGF heterozygotes survive past birth and exhibit a range of islet phenotypes, including an intermingling of islet cell types, increased number of glucagon+ cells, and β-cell hypertrophy.
Global gene inactivation reveals that connective tissue growth factor (CTGF) is required for pancreatic endocrine lineage allocation, embryonic β-cell proliferation, and islet morphogenesis.
The mouse pancreas is formed from dorsal and ventral evaginations of the posterior foregut endoderm at embryonic d 9.5 (e9.5), which fuse later in development to become a single organ. As organ development proceeds, the pancreas becomes comprised of two functionally distinct cell populations: the exocrine pancreas, composed of acinar cell clusters and a ductal network, which produces and secretes digestive enzymes into the lumen of the small intestine; and the endocrine pancreas, which consists of microorgans known as islets of Langerhans, involved in the maintenance of glucose homeostasis. In both mice and humans, disruption of glucose homeostasis can lead to diabetes.
Pancreatic islets consist of α-, β-, δ-, ε-, and PP cells (which secrete glucagon, insulin, somatostatin, ghrelin, and pancreatic polypeptide, respectively). These endocrine cells are organized into a characteristic architecture in the mouse, with the insulin-producing β-cells located in the islet core, and the other cell types located at the islet periphery. Previous studies have suggested that maximal β-cell to β-cell contacts allow for optimal insulin secretion in response to glucose, and that the proper organization of the endocrine cell types may be critical for communication between endocrine cell types (1,2,3,4,5).
At e17.5 in the mouse, clusters of endocrine cells lie close to the pancreatic ducts from which these cells originate (6,7,8). As endocrine cells are specified and differentiate, they must delaminate from the ductal epithelium. Beginning around e18.5 and continuing after birth, cells destined to populate the mature islet organize in the acinar parenchyma to form typical islet clusters. Cell biological processes involved in islet morphogenesis include cell adhesion, cell migration, cell sorting, and extracellular matrix (ECM) degradation and remodeling (6,9,10,11). Although all endocrine cells arise from a common neurogenin 3 (ngn3)-expresssing progenitor cell population, approximately 80% of these cells differentiate as β-cells (12). Relatively little is known about the specific factors that regulate allocation to the different endocrine lineages or their subsequent organization into islets.
Connective tissue growth factor (CTGF) is a member of the CCN family of secreted proteins named for its founding members, cysteine rich 61 (CCN1), CTGF (CCN2), and nephroblastoma overexpressed (Nov/CCN3). CTGF is a modular protein with four domains encoded by separate exons: an N-terminal region with homology to IGF-binding proteins and Twisted Gastrulation, which modulates bone morphogenetic protein (BMP) signaling (13,14); a von Willebrand factor repeat, with similarities to the cysteine repeats (CR) in the BMP antagonist Chordin (15,16,17); a thrombospondin type 1 repeat (18); and a C-terminal domain with similarity to the C terminus of the protein Slit, which is involved in axon guidance and cell migration (19,20). This last domain contains a cysteine knot structure, which is also present in other growth factors such as TGF-β, platelet-derived growth factor, and nerve growth factor.
CTGF is expressed in a variety of cell and tissue types, including fibroblasts, vascular smooth muscle, neurons, endothelial cells, and various epithelial cell types (21). Although a specific CTGF receptor has not yet been identified, it functionally interacts with integrins, including αvβ3 (22), and elicits biological effects specific to the signaling properties of the integrin combination expressed in a particular tissue. CTGF interacts with several growth factor signaling pathways, including BMP, Wnt, and TGF-β. The most studied of these interactions is that between CTGF and the TGF-β signaling pathway. CTGF is produced at high levels in human foreskin fibroblasts in response to TGF-β treatment (23), and a TGF-β/Smad response element has been identified in the CTGF promoter, suggesting that CTGF expression is directly regulated by TGF-β signaling (24). CTGF interacts directly with TGF-β extracellularly through its von Willebrand factor repeat, resulting in enhancement of TGF-β signaling and a positive feedback loop (17), whereas it inhibits BMP and Wnt signaling through interactions with its CR and C-terminal domains, respectively. CTGF inhibits Wnt signaling by binding to the Wnt coreceptor LRP6 (25), whereas its antagonizing effect on BMP signaling is caused by prevention of BMP binding to its cognate receptors (17).
In other cell types, CTGF mediates several processes known to occur during normal islet morphogenesis, including cell adhesion and migration (26) and ECM production and degradation (27,28). CTGF localization and function in the developing mouse pancreas have never been described, although CTGF has been linked to cell survival and proliferation in pancreatic cancer, and is up-regulated in chronic pancreatitis (29,30). Despite the strong evidence that CTGF plays a role in disease states and in cell lines, very little is known about its role in developmental processes. CTGF is critical for normal chondrogenesis and angiogenesis during skeletal development but its role in other tissues has not yet been examined (31). CTGFneo null mutant mice die immediately after birth due to respiratory failure. To date, nothing is known about the normal role of CTGF in the developing pancreas.
We recently found that CTGF expression was down-regulated in a mouse model of islet dysmorphogenesis (32). Here we examined the expression of CTGF during pancreas development and studied the effects of CTGF inactivation on endocrine cell development, islet morphogenesis, and function. This study presents the generation and characterization of a CTGFlacZ allele and is the first to use this mouse to characterize CTGF as a factor critical for proper endocrine lineage allocation, islet morphogenesis, and embryonic β-cell proliferation.
Results
CTGF is expressed in the developing mouse pancreas
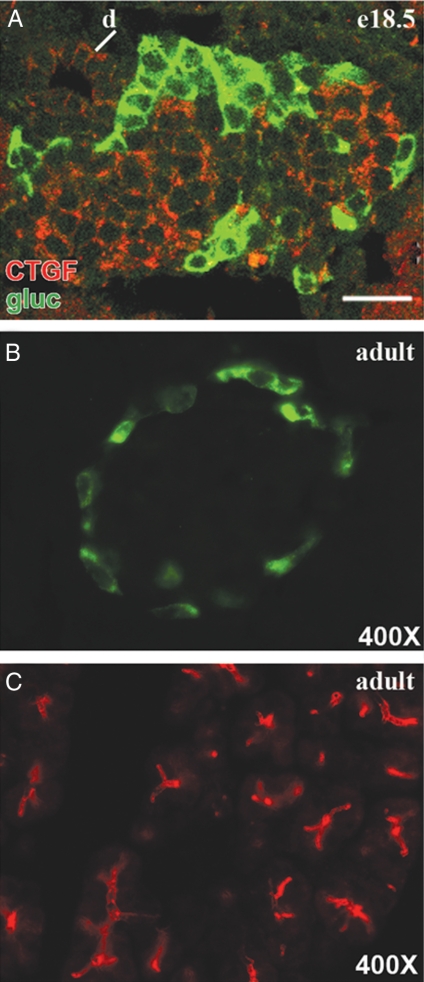
Previous studies in our laboratory detected a 2-fold decrease in CTGF expression in a mouse model of islet dysmorphogenesis using microarray analysis (32). This prompted us to examine the expression of CTGF in the normal developing pancreas. Antibody labeling of embryonic pancreas at e18.5, a time when islet morphogenesis is occurring, showed CTGF expression in the core of the islets where β-cells are located. CTGF was not expressed in α-cells at this or any stage of endocrine development examined (Fig. 1A and data not shown). CTGF was still present in islets at postnatal d 1 (P1), but was not detectable in the endocrine pancreas at P3 (data not shown), and remained undetectable in adult islets (Fig. 1B). In addition to expression in β-cells, CTGF was localized to structures consistent with ducts and blood vessels in the developing pancreas. The ductal/vascular expression remained detectable in adult pancreas (Fig. 1C).
Figure 1.
CTGF is expressed in the mouse pancreas. A, CTGF (red) was expressed in the islets, ducts (d), and blood vessels (data not shown) at e18.5. CTGF expression did not overlap with glucagon (gluc; green). Scale bar, 25 μm. B, Endocrine-specific CTGF expression was extinguished in adult islets. C, CTGF expression in the ductal network was maintained in the adult pancreas.
Generation and characterization of CTGFlacZ allele
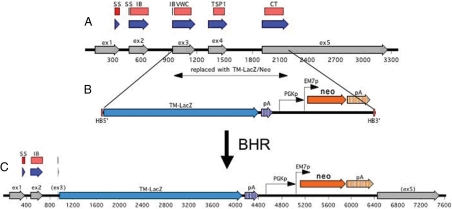
To generate a lacZ-tagged CTGF null allele, the coding sequence of CTGF from the start of its von Willebrand factor type C domain in exon 3 to the end of the coding sequence in exon 5 was replaced with a transmembrane domain-lacZ cassette (Fig. 2; see Materials and Methods for detail). This results in the production of a CTGF N terminus/β-galactosidase (β-gal) fusion protein, the presence of which can be detected at the plasma membrane with an antibody against β-gal (data not shown).
Figure 2.
Generation of the CTGFlacZ allele. Map of the CTGF endogenous locus (A), targeting vector (B), and mutant allele (C) produced by homologous recombination. Only part of the CTGF gene is shown: exons 1–5 (gray arrows) with 100 bp flanking each end. Blue arrows indicate coding sequence. Red boxes indicate the different protein domains as predicted by Pfam. The sequence being replaced by TM-lacZ is marked and encompasses the coding sequence of the VWC domain (constituting the greater part of exon 3) to the Stop codon (in exon 5). The donor fragment used for BHR is shown below the map of the CTGF gene. HB5′ and HB3′ denote the homology boxes (50 bp each) used for BHR. Expression of NeoR is driven by two promoters: PGK (for expression in mammalian cells) and EM7 (for expression in E. coli) and is flanked by loxP sites (blue boxes) on either side. TM-lacZ is followed by an simian virus 40 polyadenylation signal and simian virus 40-derived associated sequence (65), whereas the NeoR open reading frame is followed by the mouse PGK polyadenylation signal and associated sequence (66). All of these elements are the standard elements used by Velocigene (56). The replacement afforded into the CTGF BAC by BHR is also translated in an identical manner into the mouse genome during targeting. Therefore, all the features shown above are those present in the modified CTGF locus in the targeted ES cells. SS, Signal sequence; IB, IGF binding; TSP, thrombospondin; CT, C terminal; pA, poly A; Neo, Rneomycin resistance. PGK, Phosphoglycerate kinase.
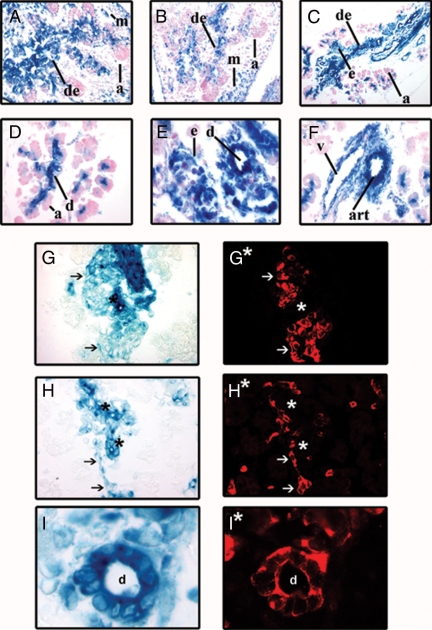
X-gal staining of CTGFlacZ/+ embryos was used to determine when and where CTGF was expressed in the developing pancreas. These data were compared with the CTGF expression pattern detected by immunofluorescence described above. X-gal staining revealed CTGF gene activity in the pancreas as early as e12.5 (Fig. 3A) and further confirmed the CTGF immunofluorescence results (Fig. 1): CTGFlacZ was strongly expressed within the developing ductal epithelium and blood vessels and within the endocrine cords (Fig. 3, A–C). X-gal staining also revealed that within the pancreatic mesenchyme, CTGFlacZ was broadly expressed at e12.5 but declined as embryogenesis progressed (Fig. 3, A–C). Although β-gal activity was detected in the small intralobular ducts of the acinar clusters, it was not present in the exocrine cells themselves, even from the earliest stages (Fig. 3, A–F). Colocalization studies using pancreatic cell type-specific markers on X-gal-stained tissue confirmed high levels of CTGF expression within the ducts (cytokeratin) and blood vessels [platelet-endothelial cell adhesion molecule (PECAM)], and lower levels of expression in the β-cells (insulin) of the developing pancreas at e18.5 (Fig. 3, G–I). By P3, CTGF gene expression, as assessed by β-gal activity, was no longer detected in the endocrine cells of the islets, but was still detectable in the microvessels within the islet, again consistent with our antibody analyses (data not shown). Endocrine-specific β-gal activity remained undetectable in adult animals; however, CTGFlacZ was reexpressed in the islets of pregnant females at every stage of pregnancy examined [e7.5, e10.5, e12.5, e13.5, e15.5, e17.5, e18.5 (supplemental Fig. 1 published as supplemental data on The Endocrine Society’s Journals Online web site at http://mend.endojournals.org and data not shown)]. This reexpression during pregnancy was also confirmed with the CTGF antibody (supplemental Fig. 1).
Figure 3.
Characterization of CTGFlacZ expression. A–F, X-gal staining of CTGFlacZ pancreata at e12.5 (panel A), e14.5 (panel B), e16.5 (panel C), and e18.5 (panels D–I). CTGFlacZ was expressed in the developing ductal epithelium (de), and mesenchyme (m), but not in acinar tissue (a) at e12.5–e16.5. D, At e18.5 CTGFlacZ was expressed in intralobular ducts (d) of the exocrine pancreas but not in the majority of acinar cells (a) themselves. E, CTGFlacZ expression in pancreatic ducts (d) and adjacent endocrine clusters (e). F, CTGFlacZ was expressed in the vasculature [artery (art) and vein (v)] of the developing pancreas. G–I, Colocalization of CTGFlacZ with cell type-specific markers. CTGF expression was detected by X-gal staining shown in bright field. Insulin (G*), PECAM (H*), and cytokeratin (I*) immunofluorescence was performed on the same sections shown in D, E, and F, respectively. Arrows indicate cells expressing CTGF and the particular marker. Asterisks indicate lacZ+ structures that do not express the cell type-specific marker. Magnification: B and C, ×200; A, and D–H, ×400; I, ×1000.
CTGF mutant embryos have altered proportions of differentiated endocrine cells and disrupted islet morphogenesis
The expression of CTGF in the developing pancreas, and during islet morphogenesis in particular, suggested a role for CTGF in endocrine cell development and islet morphogenesis. To test this hypothesis, we used two models of CTGF inactivation: the previously reported CTGFneo line (31) and the CTGFlacZ mice generated for this study (Fig. 2). The phenotype of CTGFlacZ/lacZ null mice was indistinguishable from the published CTGF null phenotype from the CTGFneo line. CTGFlacZ/+ mice were viable and fertile. CTGFlacZ/lacZ animals were found at the expected Mendelian ratio during embryogenesis, but were not found at P1 or after due to neonatal lethality. For embryonic studies, therefore, the two CTGF null lines were used interchangeably.
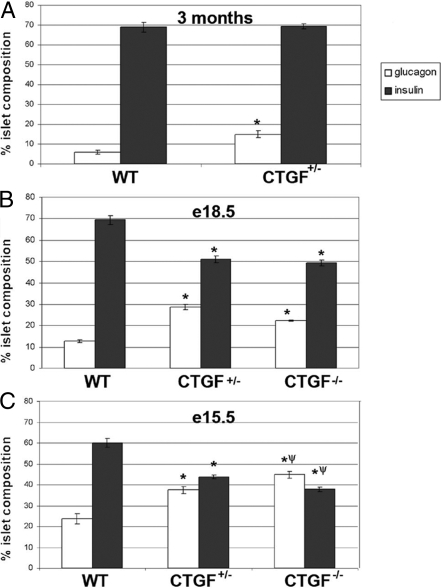
Because CTGF null animals die at birth, studies of endocrine cell development and islet morphogenesis in these animals was limited to embryogenesis. At e18.5, null mutant pancreata showed increased numbers of glucagon-expressing cells compared with controls (Fig. 4B). Morphometric analysis at this stage revealed a disrupted endocrine cell composition: islets from CTGF−/− animals had approximately 25% of their endocrine area occupied by glucagon-producing cells (Fig. 4B) compared with only 12% in wild type (WT). The increase in glucagon+ cells in islets from null embryos was accompanied by a decrease in insulin area compared with WT (49% in CTGF−/− vs. 70% in WT). This corresponds to an α- to β-cell ratio of 1:4 in WT and 1:2 in mutant animals. We observed no difference in average islet size or in the distribution of different size islets in the CTGF null embryos compared with controls (data not shown). Alterations in endocrine composition could be detected in CTGF mutant animals as early as e13.5, at which point there was an abundance of large glucagon clusters in CTGF mutants exceeding those observed in WT controls (Fig. 5). Morphometric analyses performed at e15.5 confirmed early alterations in endocrine composition in CTGF−/− animals (Fig. 4C). At this stage, nearly 45% of the endocrine area in CTGF−/− pancreata was occupied by glucagon+ cells, compared with 23% in WT animals. Insulin+ cells occupied 37% of endocrine area in CTGF−/− embryos at e15.5 compared with 60% in WT controls.
Figure 4.
CTGF mutant animals have an altered islet composition. Morphometric analysis of adult (A), e18.5 (B), and e15.5 (C) insulin and glucagon area. A, Insulin area was unchanged in CTGF+/− adults, whereas glucagon area was increased. At e18.5 (B) and e15.5 (C), insulin area was decreased in CTGF+/− and CTGF−/− pancreata, whereas glucagon area was increased. Animals (n = 5) for each e18.5 genotype, and animals for each e15.5 and adult genotype (n = 3) (see Materials and Methods for full details). Error bars determined by sem. *, P < 0.01 compared with WT. ψ, P < 0.05 compared with CTGF+/− (Student’s t test).
Figure 5.
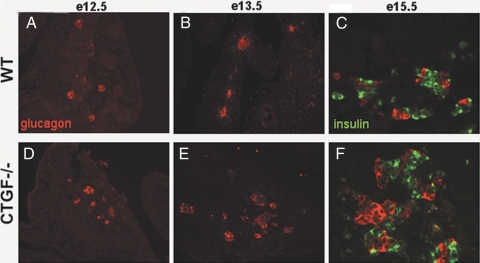
Altered lineage allocation in CTGF null pancreata. When compared with controls (A), CTGF null mutant embryos showed no difference in glucagon area (red) at e12.5 (D). However, an increase in glucagon+ cells was seen in CTGF mutant embryos beginning at e13.5 (compare E with B) and continuing throughout embryonic development. By e15.5. there was clearly a decrease in insulin+ cells (green) in the mutant pancreata (compare C and F). Magnification: × 200 (A, B, D, and E); × 400 (C and F).
We also examined islet cell composition and morphology in CTGF heterozygous embryos. Similar to CTGF−/− embryos, morphometric analysis of pancreata from e18.5 animals confirmed that endocrine cell composition was altered in CTGF+/− animals compared with WT littermates. Whereas WT endocrine clusters contained about 12% glucagon+ cells, islets from CTGF+/− animals had nearly 30% of their endocrine area occupied by glucagon-producing cells (Fig. 4B). This increase in glucagon area was accompanied by a decrease in insulin area: 70% in WT islets vs. 51% in CTGF+/− islets. The alteration in insulin and glucagon area did not affect the average islet size in CTGF+/− animals, and histogram analysis showed no difference in the distribution of islet sizes between the two genotypes (data not shown). At e18.5, both homozygous and heterozygous CTGF mutants showed similar abnormalities in islet organization, including a mixing of α- and β-cells (Fig. 6B), and the presence of small glucagon+ clusters lacking insulin altogether (inset, Fig. 6B). In addition, large glucagon clusters were found associated with islets (Fig. 6C). Morphometric analysis at e15.5 revealed that CTGF+/− islets comprised 37% glucagon-expressing cells compared with 23% in WT controls (Fig. 4C), whereas insulin-producing cells represented 42% of endocrine area in CTGF+/− in contrast with up to 60% in WT pancreata. Insulin/glucagon double-positive cells were not observed at any stage analyzed in CTGF+/− or CTGF−/− animals. Thus, the phenotypes of heterozygous and homozygous CTGF inactivation were very similar, suggesting haploinsufficiency for CTGF during islet development.
Figure 6.
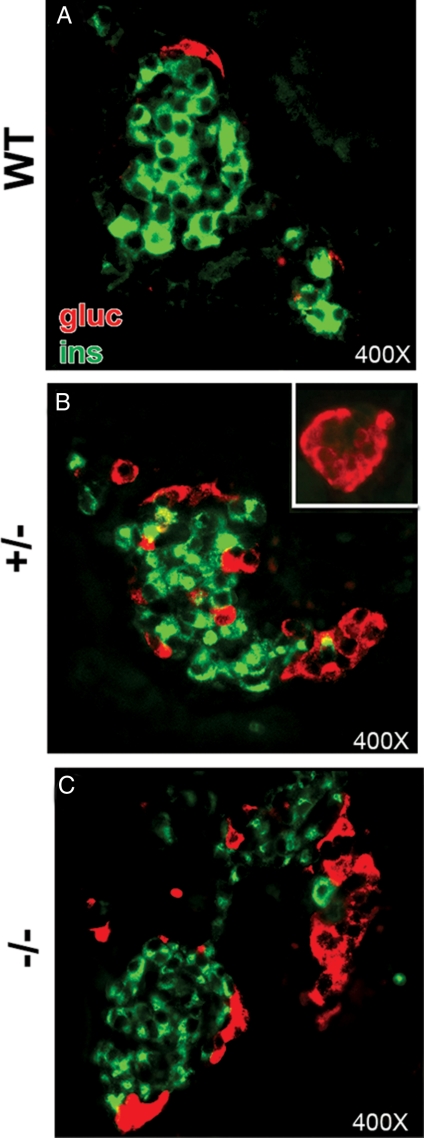
CTGF mutant embryos have altered islet architecture. Insulin (green) and glucagon (red) labeling of islet clusters in WT (A), CTGF+/− (B), and CTGF−/− (C) animals at e18.5. B, Increased glucagon area in CTGF+/− was due to increased glucagon clusters associated with insulin-positive cells and with clusters containing only α-cells (inset). C, Increased glucagon area in CTGF−/− was due to large glucagon clusters located within islets. Gluc, Glucagon; ins, insulin.
CTGF null animals displayed additional defects in pancreas development and islet morphogenesis. Cytokeratin (a pan-keratin marker of ductal structures) and synaptophysin (a general marker of endocrine tissue) colabeling of WT and mutant pancreata revealed that endocrine clusters in CTGF−/− animals were very closely apposed to the ductal epithelium at e18.5 (Fig. 7, compare panels A and C). In addition, the ductal tissue with which the endocrine clusters were associated in CTGF−/− animals had a morphology suggestive of a less mature organ: the lumen and cross-sectional area were larger, resembling the main pancreatic duct from which endocrine progenitors normally arise at an earlier stage in pancreas development, rather than the smaller ducts detected near islets of the same stage in WT (Fig. 7, compare panels B and D).
Figure 7.
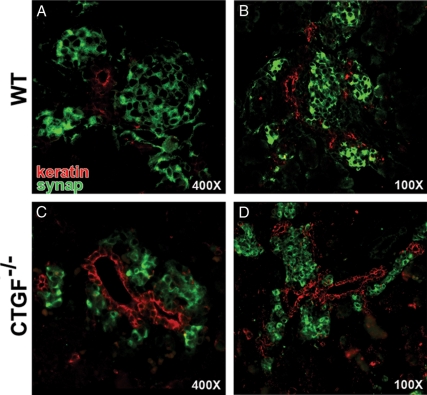
CTGF−/− animals have immature duct morphology at e18.5. Cytokeratin (red) and synaptophysin (green) labeling of e18.5 WT (A and B) and CTGF null (C and D) pancreata. A and B, WT endocrine tissue was organized into spherical islet clusters, not directly apposed to ductal structures. C, CTGF null endocrine clusters remained closely apposed to ducts and did not organize into spherical clusters as in WT (compare C with A). D, CTGF null ducts had a larger lumen and cross-sectional area than WT ducts at e18.5 (compare D with B; also seen in C). synap, Synaptophysin.
To ensure that transcriptional squelching from the promoter used to drive the NeoR cassette still present in the CTGFlacZ/+ construct was not responsible for the observed phenotype we observed in these mice, we crossed them to transgenic mice expressing Cre recombinase under control of a 4.3-kb promoter fragment of the pancreatic gene, pdx1 (33). This allowed for the removal of the loxP-flanked NeoR cassette in pancreatic progenitor cells very early in development. The phenotype of pdx1-Cre; CTGFlacZ/+ mice was indistinguishable from the CTGFlacZ/+ alone, confirming that the alterations seen in these animals were due to the loss of CTGF.
CTGF mutant animals have decreased β cell-proliferation during embryogenesis
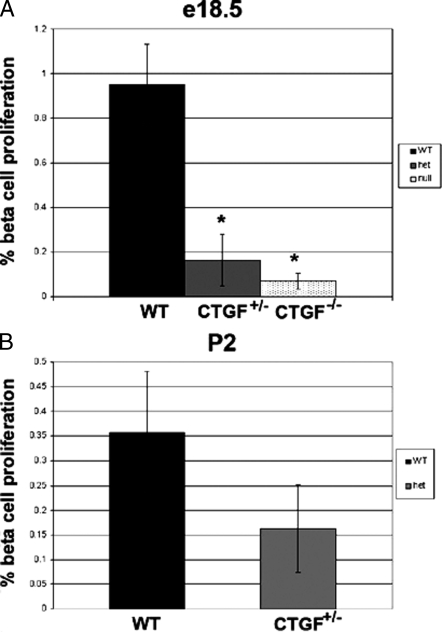
To determine the mechanism leading to the altered endocrine cell distribution, we used morphometric analysis to determine the percentage of α- and β-cells undergoing active proliferation at e13.5, e14.5, e15.5, e16.5, e17.5, and e18.5 in WT and CTGF mutant embryos. We observed no difference in the proportion of α-cells undergoing proliferation between WT or CTGF mutant (+/− or −/−) at any stage analyzed. We also detected no differences in β-cell proliferation between WT and CTGF mutants at e13.5, e14.5, e15.5, e16.5, or e17.5; however, there was a significant decrease in β-cell proliferation in both CTGF+/− and CTGF−/− embryos at e18.5 compared with WT (Fig. 8A). We previously showed that β-cell proliferation is normally occurring at this particular time point (34). Whereas in WT pancreas nearly 1.0% of β-cells were proliferating at e18.5, CTGF+/− and CTGF−/− animals showed less than 0.2% and less than 0.1%, respectively, of β-cells undergoing proliferation. We were unable to detect any β-cells undergoing apoptosis in WT or CTGF mutants at any stage examined. Studies have shown that β-cell proliferation is relatively high at early postnatal stages and contributes significantly to overall β-cell mass (35). Therefore, we also examined β-cell proliferation at P2. Because CTGF−/− animals die at birth, this analysis was restricted to CTGF+/− animals. The number of β-cells proliferating tended to be less in CTGF+/− animals at P2 compared with WT animals but did not reach significance (Fig. 8B).
Figure 8.
CTGF mutant animals have reduced β-cell proliferation. A, β-Cell proliferation at e18.5 in WT (dark bar, n = 3), CTGF heterozygotes (light bar, n = 3), and CTGF null (stippled bar, n = 3) animals. B, β-Cell proliferation at P2 in WT (dark bar, n = 3) and CTGF heterozygotes (light bar, n = 3). Proliferation was assessed using double immunofluorescence for insulin and phosphorylated histone H3 as described in Materials and Methods. Error bars determined by sem. *, P < 0.05 compared with WT (determined by Student’s t test).
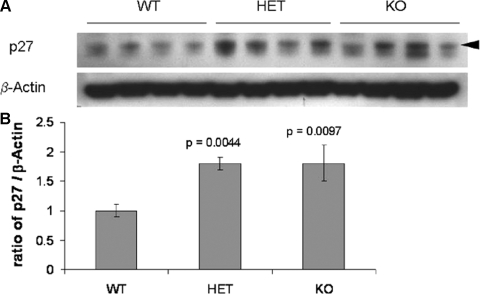
We next examined the expression of the cell cycle inhibitor p27Kip1. Previous studies have shown an increase in β-cell proliferation in p27 null mutant pancreata, thus leading to β-cell hyperplasia (36). Furthermore, several studies have shown that enhanced proliferation in response to exogenous CTGF is due to a reduction in p27. For example, stimulation of human lung fibroblasts with CTGF leads to an increase in proliferation concomitant with a decrease in p27 levels (37). In addition, we have shown increased p27 protein in β-cells in Foxm1 null mutant pancreata in which postnatal β-cell proliferation is dramatically reduced (38). Western blot analysis revealed an increase in p27 in both CTGF+/− and CTGF−/− animals compared with WT (Fig. 9). These data suggest that reduced CTGF expression leads to a decrease in β-cell proliferation, at least in part, via up-regulation/stabilization of p27.
Figure 9.
CTGF mutant animals have an increase in p27Kip1 levels. A, Western blot showing four individual pancreatic samples at e18.5 from each genotype (WT, CTGF+/− and CTGF−/−). B, Densitometric quantitation of p27 levels illustrated as a ratio of p27:β actin, where WT levels were assigned a value of 1.0. Error bars represent sem. P values were calculated using a Student’s t test. Arrowhead indicates band quantitated for densitometry. HET, Heterozygous; KO, knockout.
CTGF heterozygous adults show defects in islet morphogenesis and proportions of differentiated endocrine cells
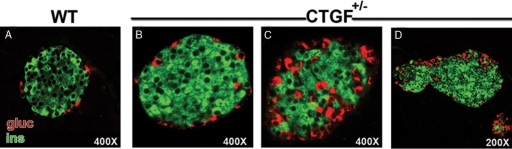
Studies of adult pancreas structure and function were limited to CTGF heterozygous animals (CTGFlacZ/+ were used exclusively). The size and gross morphology of CTGF heterozygous pancreata were normal at all ages examined; however, analysis of pancreatic tissue from adult CTGFlacZ/+ animals revealed alterations in islet architecture. Some islets exhibited a morphology indistinguishable from WT islets (Fig. 10, compare panels A and B); however, 56% of the islets within the CTGFlacZ/+ pancreata had a mixed-islet phenotype, with α-cells found within the islet core (Fig. 10C). This was not observed in WT animals at this age. Additionally, a number of islets were irregularly shaped and appeared to be composed of multiple fused islets that had not separated during islet morphogenesis (Fig. 10D). Morphometric analysis of pancreata from 3-month-old WT and CTGF heterozygotes revealed a disrupted endocrine cell composition in CTGFlacZ/+ animals. In WT animals, only 5% of the islet area was occupied by α-cells, whereas in CTGFlacZ/+ animals, α-cells make up nearly 15% of islet area (Fig. 4A). Despite the increase in α-cell area, the area occupied by β-cells was not different between WT and CTGFlacZ/+ adult animals; thus CTGFlacZ/+ animals showed an overall larger average islet size compared with WT (WT: 5660 μm2 vs. HET: 8609 μm2; P = 0.04). Analysis of the other endocrine cell types revealed no detectable difference in the proportion of δ-cells, and a slight increase in the number of PP in the CTGF+/− animals compared with WT (data not shown).
Figure 10.
CTGF+/− adult islets exhibit a range of phenotypes. Insulin (green) and glucagon (red) labeling of WT (A) and CTGF+/− heterozygous (B–D) islets. B, CTGF+/− islet with normal islet architecture. C, CTGF+/− islet with mixing of α-cells into the β-cell core. D, Two apparently fused islets that failed to separate during islet morphogenesis. gluc, Glucagon; ins, insulin.
CTGF heterozygous adults show compensatory β-cell hypertrophy but no decrease in β-cell function
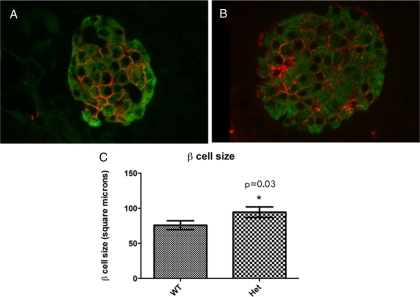
Due to the fact that insulin area was decreased in e18.5 CTGF heterozygous pancreas, but was not different from WT animals during adulthood, we hypothesized that there was some compensatory mechanism occurring between e18.5 and adulthood acting to equalize insulin area in CTGF heterozygous animals. Analysis of β-cell proliferation at 3 months of age revealed no difference between WT and heterozygous animals (data not shown). We therefore examined whether individual β-cell hypertrophy might contribute to the restoration in insulin+ area in adult islets. Using two different methods to assess cell size (see Materials and Methods), the β-cells from CTGF heterozygotes were statistically larger than β-cells in WT animals (Fig. 11), suggesting that β-cell hypertrophy contributes to the increase in β-cell area that occurs between e18.5 and 3 months of age. These results do not exclude the possibility that β-cell proliferation also contributes to the restoration of β-cell mass after birth in CTGF heterozygous animals. Despite the fact that CTGFlacZ/+ heterozygous adults had larger individual β-cells, they had a lower total pancreatic insulin content compared with WT littermates of the same age (83.67 ng/mg pancreas vs. 67.44 ng/mg pancreas; P < 0.05 at 8 wk of age), suggesting that that these larger β-cells do not have the ability to produce more insulin than WT β-cells.
Figure 11.
β-Cell hypertrophy in CTGF+/− adult islets. β-Cell size was measured by labeling for Glut2 (red), which specifically outlines the membranes of β-cells (insulin, green) in both wild type (A) and CTGF heterozygous (B) adults. C, There was a statistically significant increase in average β-cell size in the CTGF heterozygous animals. See Materials and Methods for details regarding measurement of β-cell size. Het, Heterozygous.
To determine whether heterozygosity for CTGF resulted in susceptibility to diabetes under certain conditions, CTGFlacZ/+ animals were challenged with 12 wk on high-fat diet. CTGFlacZ/+ animals showed normal fasting blood glucose levels and glucose clearance (data not shown). Thus, by this assay, the decrease in total pancreatic insulin content in CTGF heterozygous animals does not translate into defects in β-cell function.
Discussion
The present study represents the first report on the expression pattern and function of CTGF in the developing pancreas. CTGF expression is elevated in pancreatic diseases such as cancer and pancreatitis and contributes to pancreatic tumor growth and metastasis (30,39), but before this study, nothing was known about its potential role in pancreas development and function. Here we demonstrate that CTGF is expressed in multiple cell types in the developing pancreas and is involved in endocrine lineage allocation, islet morphogenesis, and embryonic β-cell proliferation.
We first became interested in the role of CTGF in the developing pancreas after a microarray analysis revealed a 2-fold decrease in CTGF expression in a transgenic model of islet dysmorphogenesis and diabetes studied in our laboratory (pdx1PBHNF6) (32,40,41). Recently, the CTGF promoter was shown to be directly repressed by the proendocrine transcription factor, Ngn3 (42). Ngn3 expression is increased in pdx1PBHNF6 animals, which may partially account for the decrease in CTGF expression in these transgenic mice. Interestingly, our analysis of CTGF mutant animals showed an islet phenotype strikingly similar to the hepatocyte nuclear factor (HNF)6 transgenic phenotype. The increase in α-cell number and mixed islets observed in the CTGF mutant animals at e18.5 is nearly identical to what was described in HNF6 transgenic animals at the same developmental stage (supplemental Fig. 2 and Ref. 39). In addition, HNF6 transgenic pancreata have endocrine tissue closely apposed to ducts, and larger, misshapen islets, some of which appear to be fused, characteristics also observed in CTGF null animals (Figs. 7 and 10D). These phenotypic similarities, in conjunction with the fact that CTGF is down-regulated in HNF6 transgenic pancreata, suggest that the lack of CTGF may be at least partially responsible for the defects in islet morphogenesis observed in this mouse model of diabetes.
Based on reports in the literature describing the role of CTGF in many of the biological processes involved in islet morphogenesis (cell adhesion, cell migration, and ECM remodeling), and the decrease in CTGF expression observed in a mouse model of islet dysmorphogenesis, we chose to investigate CTGF as a potential factor involved in normal islet development. The novel CTGFlacZ null allele described in this study facilitated studies of CTGF gene expression and provided important information about the dynamics of CTGF gene activity during normal mouse development.
Morphometric analysis revealed alterations in endocrine cell composition at e13.5, but not before, in both CTGF heterozygous and homozygous null animals without a concomitant increase in islet size. These data suggest that CTGF functions to affect endocrine diversification specifically during the secondary transition. The secondary transition is a wave of differentiation that begins around e13.5 and continues postnatally (6,43). The endocrine cells that arise during this period are thought to contribute to the mature, functional endocrine mass of the animal.
To understand the mechanism(s) leading to this alteration in endocrine cell distribution, α- and β-cell proliferation and apoptosis were quantitated at multiple embryonic stages. These analyses revealed a decrease in β-cell proliferation in CTGF mutants at e18.5, but showed no alterations in α-cell proliferation or β-cell death at any stage examined. Although a decrease in embryonic β-cell proliferation may explain the decrease in islet β-cell area, the mechanism for the increased number of α-cells has not yet been uncovered. We hypothesize that the expanded α-cell population may be due to an increased allocation of endocrine progenitors to the α-cell fate. Interestingly, the insulin+ cell area normalizes in CTGFlacZ/+ islets by 3 months of age due to β-cell hypertrophy. The hypertrophy seen in CTGF heterozygous animals in the face of an absolute decrease in β-cell number is consistent with what we have observed in another mouse model of decreased β-cell proliferation (38).
During development, CTGF is expressed in pancreatic ducts and vasculature in addition to β-cells. Global inactivation of CTGF results in a loss of CTGF from all of the cell types within the pancreas from the very earliest developmental stages. Therefore, at this point in our analysis, we are unable to determine which cell type(s) is responsible for the defects in endocrine lineage allocation, β-cell proliferation, and islet morphogenesis displayed in CTGF mutant animals. It remains possible that the endocrine phenotype is secondary to the loss of CTGF from the ducts or the vasculature of the developing pancreas. Early pancreatic endocrine cells delaminate from the ductal epithelium in the pancreas, and previous studies have shown that blood vessel endothelial cells are a source of developmental signals promoting endocrine differentiation during pancreas development (44,45). Cell type-specific inactivation of CTGF will ultimately reveal which cellular source of CTGF functions to promote proper lineage allocation, β-cell proliferation, and/or islet morphogenesis.
The modular nature of the CTGF protein allows it to interact with multiple growth factor signaling pathways. It is currently unclear whether loss of CTGF in the developing pancreas primarily impairs TGF-β signaling, results in enhanced BMP or Wnt signaling, or a combination of all three. Preliminary studies in our laboratory indicate that loss of CTGF causes a dose-dependent decrease in membrane-localized β-catenin (supplemental Fig. 3A), although there is no change in total β-catenin protein (supplemental Fig. 3, B and C), providing a link with the Wnt signaling pathway. Both chemical and genetic inhibition of TGF-β signaling in the pancreas affect islet morphogenesis and pancreatic function (10,46). The finding, that inhibition of TGF-β signaling and inactivation of CTGF lead to similar abnormalities in islet development, suggests that some aspects of the phenotype we observed in CTGF mutant animals may be due to decreased TGF-β signaling. Future studies will further explore the undoubtedly complex interactions between CTGF, TGF-β, BMP, and Wnt signaling in the establishment of normal islet morphogenesis.
Our findings show that CTGF is expressed in developing β-cells and suggest that CTGF is important for β-cell proliferation during development. This is consistent with reports that CTGF plays a role in cell proliferation in other systems (47,48,49,50). Until recently, β-cells were viewed as a nonproliferating cell population. Recent data, however, demonstrate that β-cells actually undergo slow turnover in adults and that under normal circumstances, the majority of new β-cells arise via proliferation of existing β-cells (51,52). There have been several factors described as having a specific role in postnatal β-cell proliferation, including known cell cycle regulators the inactivation of which has no effect on embryonic β-cell proliferation (reviewed in Refs. 43,53, and 54). Currently, there are few genes known to affect the proliferation of embryonic β-cells (34,55). Efforts in many laboratories are focused on identifying genes involved in β-cell replication, with the hopes of altering/increasing β-cell mass in vivo or proliferation of in vitro-derived β-cells to increase insulin production and potentially treat diabetes. Because many of these studies aim to direct the differentiation and proliferation of a precursor or progenitor population, it is critical to identify factors that can cause an expansion of an immature cell population. Because CTGF can affect embryonic β-cell proliferation, we propose that it is an interesting candidate to investigate for its potential in studies involving expansion of the β-cell population. Although heterozygosity for CTGF did not result in high-fat diet-induced diabetes, conditional deletion of CTGF in adult β-cells will allow us to explore whether decreased CTGF expression may be a contributing factor to type 2 diabetes or gestational diabetes in combination with metabolic and/or physiological stresses on the β-cell (i.e. increased body mass, increased insulin resistance).
Materials and Methods
Animals
Generation of a CTGF null allele (CTGFneo) and genotyping of mice were previously described by Ivkovic et al. (31). The CTGFlacZ null allele (Fig. 2) was generated as follows. Targeted embryonic stem (ES) cells harboring a lacZ-targeted null allele of CTGF were generated using Velocigene technology, essentially as described (56). Briefly, a bacterial artificial chromosome (BAC) containing mouse genomic DNA encompassing CTGF was selected by PCR-screen from a BAC library of 129/Svj mouse genomic DNA (Incyte). The CTGF BAC (identification no. 460d11) contains approximately 170 kb of genomic DNA. To generate the targeting vector, the coding sequence of CTGF contained within exon 3 [from the start of its von Willebrand factor type C (VWC) domain] to the end of the coding sequence in exon 5 was replaced with a transmembrane domain-lacZ/Neomycin phosphotransferase (TM-lacZ/ NeoR) cassette using bacterial homologous recombination (BHR) (57,58,59,60). The NeoR selection minigene was flanked with LoxP sites to allow its removal using Cre (61). Using restriction mapping, it was determined that the resulting modified BAC had homology arms of approximately 120 and 40 kb flanking the TM-lacZ/ NeoR cassette. This modified BAC was used as a targeting vector to target the CTGF gene in an F1 (C57BL/6-129SvJ) hybrid ES line. Ninety six ES cell clones were picked and genotyped using a loss of allele (LOA) assay. Of 96 clones, eight were targeted, indicating a targeting frequency of 8.3%. Two of these clones (286A-B8 and 286A-F9) were used to generate mice. CTGF−/− mice resulting from F1 CTGF+/− intercrosses (from mice generated from both of these clones) displayed a phenotype identical to that published elsewhere (31) (data not shown).
Genotyping was performed by PCR amplification of a portion of CTGF exon 4. This exon is missing in the null allele. Amplification of a 193-bp fragment from the endogenous allele was performed using the following primers: CTGFlacZfor, 5′-aagacacatttggcccagac-3′; and CTGFlacZrev, 5′-ttttcctccaggtcagcttc-3′. CTGFlacZ/+ animals were further distinguished from WT using X-gal staining of ear punches (adult) or ribcage (embryo).
The pdx1-Cre mice were generously provided by Guoqiang Gu (Vanderbilt University). The generation and genotyping of these mice have been previously described (33,38).
Tissue preparation, X-gal staining, and histology
Digestive organs or pancreata from embryonic and adult stages were dissected in PBS and fixed immediately in 4% paraformaldehyde (3–5 h). Tissues were dehydrated using an increasing ethanol series, followed by two xylene washes, infiltrated with xylene-paraffin (1:1; vol/vol) and two changes of paraffin at 56 C, and embedded in paraffin for sectioning. Pancreata for frozen sectioning were dissected and fixed as described above and then placed in a 30% sucrose solution overnight. Tissue was then embedded and frozen in Tissue-Tek O.C.T. compound and sectioned at 5 μm.
β-gal activity was detected using X-gal as previously described (62). Tissues were postfixed (4% paraformaldehyde in PBS; 1 h at 4 C) and then dehydrated for embedding as above, with isopropanol replacing xylene to minimize leaching of the blue precipitate. For colabeling on X-gal-stained tissue, antibodies were added directly to sections of pancreata that had been stained with X-gal in whole mount.
Serial 5-μm sections were deparaffinized and rehydrated using an increasing ethanol series to distilled water. Indirect protein localization was obtained by incubation of tissue with the following primary antibodies: guinea pig antiinsulin (1:1000; Linco Research, Inc., St. Charles, MO), rabbit anti-CTGF raised against residues 81–94 (1:500; this antibody did not react with tissue from CTGF null animals, demonstrating its specificity; data not shown) (63); guinea pig antiglucagon (1:1000; Linco); rabbit antiphosphorylated histone H3 (pH3; Upstate Biotechnology, Inc., Lake Placid, NY); rabbit anticytokeratin (1:1000; DAKO Corp., Carpinteria, CA), mouse anti-PECAM (PharMingen, San Diego, CA; 1:200), mouse antisynaptophysin (Upstate, 1:500), mouse anti-β-catenin (BD Transduction Laboratories, Lexington, KY; 1:50). All primary antibodies were incubated overnight at 4 C in a humid chamber. Detection of CTGF, and pH3, and synaptophysin required antigen retrieval. For CTGF, sections were microwaved in TEG (1 mm Tris, 0.5 mm EGTA; pH 9.0) buffer on 10% power until lightly boiling. For pH3, synaptophysin, and β-catenin, slides were microwaved in 10 mm sodium citrate on full power for 3 min, followed by a 1 min cool and another 1 min on full power. Chicken anti-β-gal antibody (1:1000; Abcam, Inc., Cambridge, MA) was used on cryosections to detect β-gal protein expression.
Primary antibodies were detected by species-specific donkey secondary antibodies conjugated to Cy2 or Cy3 fluorophores (1:500; Jackson ImmunoResearch Laboratories, Inc., West Grove, PA). In the case of CTGF, amplification was needed such that antirabbit biotin was added for 1 h at room temperature, followed by fluorescent-conjugated streptavidin (1:1000; Vector Laboratories, Inc., Burlingame, CA). Fluorophores were excited using either an Olympus BX41 research microscope or a Zeiss LSM 510 confocal microscope (Carl Zeiss, Thornwood, NY). Tiff images were captured using either MagnaFire software (Optronics Engineering, Goleta, CA) or LSM Viewer (Zeiss), and total range and brightness were equivalently adjusted by histogram using Adobe Photoshop (Adobe Systems, Inc., San Jose, CA).
Insulin and glucagon area
Entire paraffin blocks containing e18.5 digestive organs or adult pancreas were serially sectioned at 5 μm. Every tenth slide of pancreas tissue was immmunolabeled for insulin and glucagon (described above). Every islet from one section on each slide was photographed using an Olympus BX41 microscope and MagnaFire software. Insulin and glucagon area in each islet was calculated using Metamorph 6.1 morphometric analysis software. Islets were considered to be mixed in adult animals if glucagon-expressing cells were found beyond the fourth cell layer from the outermost edge.
α-Cell and β-cell proliferation
Entire paraffin blocks containing e15.5-P2 digestive organs were serially sectioned at 5 μm. Every sixth (for e14.5 and e16.5) or tenth (for e17.5-e18.5) slide was immunolabeled for pH3 and insulin or glucagon (described above). For adult tissue, blocks containing pancreata was serially sectioned at 5 μm. One hundred slides containing five sections per slide were made. From these, every twentieth slide was immunolabeled for pH3 and insulin as previously described. Every insulin or glucagon cell on one section per slide was photographed using an Olympus BX41 microscope and MagnaFire software. Using Metamorph 6.1 software, the total number of cells positive for insulin or glucagon only was counted, as were the number of cells colabeled for both insulin or glucagon and pH3. The percentage of β- or α-cells proliferating (colabeled for pH3 and insulin or glucagon) was determined.
β-Cell apoptosis
β-Cell apoptosis was quantitated using the ApoAlert DNA fragmentation assay kit (CLONTECH Laboratories, Inc.) according to the manufacturer’s instructions. Slides were colabeled with insulin (described above). Using Metamorph 6.1 software, the total number of apoptotic cells was counted, as were the number of cells colabeled for both insulin and apoptosis. The percentage of β-cells undergoing apoptosis (colabeled for Apo and insulin) was determined.
β-Cell size
Entire paraffin blocks containing adult pancreas were serially sectioned at 5 μm. Every thirtieth slide of pancreas tissue was immmunolabeled for insulin (described above). Every islet from one section on each slide was photographed using an Olympus BX41 microscope. Using Metamorph 6.1 software, the total β-cell area for each islet was determined. The number of β-cells in each islet was counted, and average β-cell size for each islet was determined by dividing the total β-cell area by the number of β-cell nuclei. β-Cells from at least 125 islets from three animals per genotype were used to determine the average β-cell size. As an alternative method to measure β-cell size, rabbit antimouse Glut2 (from Bernard Thorens, 1:500) was used to visualize the plasma membrane of β-cells. Using Metamorph 6.1 software, the area of 100 individual β-cells was determined in at least five different islets per mouse. Three mice per genotype were analyzed to determine the average β-cell size.
In vivo analysis of glucose homeostasis
Intraperitoneal glucose tolerance tests (IP-GTTs) were performed as previously described (39). Briefly, 4- to 12-wk-old mice fasted for 16 h were given ip injection of filter-sterilized glucose in PBS (2.0 mg dextrose/g body weight). Glucose concentrations were measured in tail vein blood using the Freestyle glucose meter and test strips (TheraSense, Inc., Alameda, CA) before injection (time 0) and 15, 30, 60, 90, and 120 min after injection.
Pancreatic extracts and measurement of insulin content
Pancreata from e17.5 embryos and 4-wk-old mice were dissected, weighed, and homogenized in acid alcohol for extraction of insulin (64). Insulin content from acid alcohol-extracted pancreas was measured by solid-phase RIA (125I-insulin, Diagnostic Products Corp., Los Angeles, CA) for mouse antiinsulin (MP Biomedicals, Irvine, CA). Average insulin concentration was calculated as a function of total pancreatic wet weight.
Protein extraction and Western blotting
Pancreata from e18.5 embryos were dissected in ice-cold PBS and immediately placed into extraction buffer containing protease inhibitors (0.5 mg/liter N-α-tosyl-l-phenylalanylchloromethylketone, 0.5 mg/liter N-α-tosyl-L-lysine chloromethyl kefone hydrochloride (TLCK), 0.6 μm leupeptin, and 2 μm pepstatin), 0.5 m dithiothreitol, and 0.1 m phenylmethylsulfonyl fluoride. Samples were homogenized and centrifuged briefly, and the supernatant was frozen at −80 C. Protein was quantitated using the Bio-Rad DC protein assay according to manufacturer’s instructions (Bio-Rad Laboratories, Hercules, CA). Western blot analysis was performed on individual pancreatic protein extracts. Protein was electrophoresed on 4–12% Bis Tris gels under denaturing conditions, and blotted to polyvinylidenedifluoride membrane using the NuPAGE Western blotting system (Invitrogen, Carlsbad, CA). Blots were blocked in 5% nonfat milk in Tris-buffered saline (TBS) (pH 7.6) for 1 h at room temperature and probed with primary antibodies diluted in 3% nonfat milk in TBS overnight at 4 C: mouse anti-β actin (1:5000; Santa Cruz Biotechnology, Inc., Santa Cruz, CA), mouse anti-p27 (1:2500; BD Biosciences, Palo Alto, CA), mouse anti-β-catenin (1:500; BD Biosciences), and washed in 0.05% Tween 20 in TBS for 30 min at room temperature with three changes of buffer. Horseradish peroxidase-conjugated species-specific secondary antibodies were diluted to 1:3000 (antimouse IgG; Promega Corp., Madison, WI) in 1% nonfat milk in TBS and incubated for 1 h at room temperature. After washes, protein detection was performed using the ECL detection system (Amersham Pharmacia Biotech, Arlington, IL) per manufacturer’s instructions using Kodak X-Omat Blue film (Eastman Kodak, Rochester, NY). Protein levels in individual pancreata were quantitated on a Molecular Imager FX densitometer (Bio-Rad) using Quantity One 4.6 software (Bio-Rad) and normalized to the quantity of β-actin obtained for each sample. Protein levels were represented as a ratio of p27:β-actin or β-catenin:β-actin, where WT levels were assigned a value of 1.0. Error bars were determined using the sem. P values were calculated using Student’s t test.
High-fat diet feeding
At weaning (21 d after birth) animals were assayed by IP-GTT, and subsequently placed on high-fat diet (58.7% fat, 26.7% carbohydrate, 14.8% protein; Bio-Serv) or normal (control) mouse chow (24% fat, 57% carbohydrates, 19% protein; TestDiet) and fed ad libitum. Every 4 wk for 3 months, animals were subjected to IP-GTT as above.
Supplementary Material
Acknowledgments
We thank Dr. Elizabeth Tweedie Ables and other members of the Gannon laboratory for helpful discussions and suggestions. A special thanks to Christine Pope and David Lowe for technical assistance. We thank Jami Day and Andre Boustani for help with adult morphometric analyses, and Dr. Shubhada Jagasia for helpful discussions. Thanks also to Dr. Guoqiang Gu for generously providing the pdx1-Cre mice and Dr. Bernard Thorens for the Glut2 antibody. Imaging was performed, in part, through the use of the Vanderbilt University Medical Center Cell Imaging Shared Resource.
Footnotes
This work was supported by National Institutes of Health Grants CA68485, DK20593, DK58404, HD15052, DK59637, and EY08126 (to the VUMC Cell Imaging Shared Resource), NIH Grant DK065131, and JDRF Career Development Award, Grant 2-2002-583 (to M.G.), and the Vanderbilt Molecular Endocrinology Training Program, Grant 5-T32-DK07563 (to L.C. and M.A.G.).
Disclosure Statement: L.A.C., M.A.G., Y.A.O., A.D., K.M.L., D.R.B., and M.G. have nothing to declare. D.M.V., A.J.M., G.D.Y., and A.E. are employees of Regeneron Pharmaceuticals.
First Published Online January 8, 2009
Abbreviations: BAC, Bacterial artificial chromosome; BHR, bacterial homologous recombination; BMP, bone morphogenetic protein; CTGF, connective tissue growth factor; e9.5, embryonic d 9.5; ECM, extracellular matrix; ES, embryonic stem; β-gal, β-galactosidase; HNF, hepatocyte nuclear factor; IP-GTT, ip glucose tolerance test; P1, postnatal d 1; PECAM, platelet-endothelial cell adhesion molecule; TBS, Tris-buffered saline; VWC, von Willebrand factor type C; WT, wild type.
References
- Meissner HP 1976 Electrophysiological evidence for coupling between β cells of pancreatic islets. Nature 262:502–504 [DOI] [PubMed] [Google Scholar]
- Bennett MV, Goodenough DA 1978 Gap junctions, electrotonic coupling, and intercellular communication. Neurosci Res Program Bull 16:1–486 [PubMed] [Google Scholar]
- Halban PA, Wollheim CB, Blondel B, Meda P, Niesor EN, Mintz DH 1982 The possible importance of contact between pancreatic islet cells for the control of insulin release. Endocrinology 111:86–94 [DOI] [PubMed] [Google Scholar]
- Samols E, Stagner JI, Ewart RB, Marks V 1988 The order of islet microvascular cellular perfusion is B—A—D in the perfused rat pancreas. J Clin Invest 82:350–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosco D, Orci L, Meda P 1989 Homologous but not heterologous contact increases the insulin secretion of individual pancreatic β-cells. Exp Cell Res 184:72–80 [DOI] [PubMed] [Google Scholar]
- Pictet RL, Clark WR, Williams RH, Rutter WJ 1972 An ultrastructural analysis of the developing embryonic pancreas. Dev Biol 29:436–467 [DOI] [PubMed] [Google Scholar]
- Teitelman G, Lee J, Reis DJ 1987 Differentiation of prospective mouse pancreatic islet cells during development in vitro and during regeneration. Dev Biol 120:425–433 [DOI] [PubMed] [Google Scholar]
- Bonner-Weir S, Baxter LA, Schuppin GT, Smith FE 1993 A second pathway for regeneration of adult exocrine and endocrine pancreas. A possible recapitulation of embryonic development. Diabetes 42:1715–1720 [DOI] [PubMed] [Google Scholar]
- Gu D, Sarvetnick N 1993 Epithelial cell proliferation and islet neogenesis in IFN-γ transgenic mice. Development 118:33–46 [DOI] [PubMed] [Google Scholar]
- Miralles F, Battelino T, Czernichow P, Scharfmann R 1998 TGF-β plays a key role in morphogenesis of the pancreatic islets of Langerhans by controlling the activity of the matrix metalloproteinase MMP-2. J Cell Biol 143:827–836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirulli V, Beattie GM, Klier G, Ellisman M, Ricordi C, Quaranta V, Frasier F, Ishii JK, Hayek A, Salomon DR 2000 Expression and function of α(v) β(3) and α(v) β(5) integrins in the developing pancreas: roles in the adhesion and migration of putative endocrine progenitor cells. J Cell Biol 150:1445–1460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu G, Brown JR, Melton DA 2003 Direct lineage tracing reveals the ontogeny of pancreatic cell fates during mouse embryogenesis. Mech Dev 120:35–43 [DOI] [PubMed] [Google Scholar]
- Chang C, Holtzman DA, Chau S, Chickering T, Woolf EA, Holmgren LM, Bodorova J, Gearing DP, Holmes WE, Brivanlou AH 2001 Twisted gastrulation can function as a BMP antagonist. Nature 410:483–487 [DOI] [PubMed] [Google Scholar]
- Oelgeschlager M, Larrain J, Geissert D, De Robertis EM 2000 The evolutionarily conserved BMP-binding protein Twisted gastrulation promotes BMP signalling. Nature 405:757–763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt LT, Barker WC 1987 von Willebrand factor shares a distinctive cysteine-rich domain with thrombospondin and procollagen. Biochem Biophys Res Commun 144:876–882 [DOI] [PubMed] [Google Scholar]
- Sasai Y, Lu B, Steinbeisser H, Geissert D, Gont LK, De Robertis EM 1994 Xenopus chordin: a novel dorsalizing factor activated by organizer-specific homeobox genes. Cell 79:779–790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abreu JG, Ketpura NI, Reversade B, De Robertis EM 2002 Connective-tissue growth factor (CTGF) modulates cell signalling by BMP and TGF-β. Nat Cell Biol 4:599–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams JC 2001 Thrombospondins: multifunctional regulators of cell interactions. Annu Rev Cell Dev Biol 17:25–51 [DOI] [PubMed] [Google Scholar]
- Rothberg JM, Jacobs JR, Goodman CS, Artavanis-Tsakonas S 1990 slit: an extracellular protein necessary for development of midline glia and commissural axon pathways contains both EGF and LRR domains. Genes Dev 4:2169–2187 [DOI] [PubMed] [Google Scholar]
- Brose K, Tessier-Lavigne M 2000 Slit proteins: key regulators of axon guidance, axonal branching, and cell migration. Curr Opin Neurobiol 10:95–102 [DOI] [PubMed] [Google Scholar]
- Moussad EE, Brigstock DR 2000 Connective tissue growth factor: what’s in a name? Mol Genet Metab 71:276–292 [DOI] [PubMed] [Google Scholar]
- Kireeva ML, Latinkic BV, Kolesnikova TV, Chen CC, Yang GP, Abler AS, Lau LF 1997 Cyr61 and Fisp12 are both ECM-associated signaling molecules: activities, metabolism, and localization during development. Exp Cell Res 233:63–77 [DOI] [PubMed] [Google Scholar]
- Igarashi A, Okochi H, Bradham DM, Grotendorst GR 1993 Regulation of connective tissue growth factor gene expression in human skin fibroblasts and during wound repair. Mol Biol Cell 4:637–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blom IE, Goldschmeding R, Leask A 2002 Gene regulation of connective tissue growth factor: new targets for antifibrotic therapy? Matrix Biol 21:473–482 [DOI] [PubMed] [Google Scholar]
- Mercurio S, Latinkic B, Itasaki N, Krumlauf R, Smith JC 2004 Connective-tissue growth factor modulates WNT signalling and interacts with the WNT receptor complex. Development 131:2137–2147 [DOI] [PubMed] [Google Scholar]
- Babic AM, Chen CC, Lau LF 1999 Fisp12/mouse connective tissue growth factor mediates endothelial cell adhesion and migration through integrin αvβ3, promotes endothelial cell survival, and induces angiogenesis in vivo. Mol Cell Biol 19:2958–2966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CC, Chen N, Lau LF 2001 The angiogenic factors Cyr61 and connective tissue growth factor induce adhesive signaling in primary human skin fibroblasts. J Biol Chem 276:10443–10452 [DOI] [PubMed] [Google Scholar]
- Fan WH, Karnovsky MJ 2002 Increased MMP-2 expression in connective tissue growth factor over-expression vascular smooth muscle cells. J Biol Chem 277:9800–9805 [DOI] [PubMed] [Google Scholar]
- di Mola FF, Friess H, Martignoni ME, Di Sebastiano P, Zimmermann A, Innocenti P, Graber H, Gold LI, Korc M, Buchler MW 1999 Connective tissue growth factor is a regulator for fibrosis in human chronic pancreatitis. Ann Surg 230:63–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dornhofer N, Spong S, Bennewith K, Salim A, Klaus S, Kambham N, Wong C, Kaper F, Sutphin P, Nacalumi R, Hockel M, Le Q, Longaker M, Yang G, Koong A, Giaccia A 2006 Connective tissue growth factor-specific monoclonal antibody therapy inhibits pancreatic tumor growth and metastasis. Cancer Res 66:5816–5827 [DOI] [PubMed] [Google Scholar]
- Ivkovic S, Yoon BS, Popoff SN, Safadi FF, Libuda DE, Stephenson RC, Daluiski A, Lyons KM 2003 Connective tissue growth factor coordinates chondrogenesis and angiogenesis during skeletal development. Development 130:2779–2791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilding Crawford L, Tweedie Ables E, Oh YA, Boone B, Levy S, Gannon M 2008 Gene expression profiling of a mouse model of pancreatic islet dysmorphogenesis. PLoS One 3:e1611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu G, Dubauskaite J, Melton DA 2002 Direct evidence for the pancreatic lineage: NGN3+ cells are islet progenitors and are distinct from duct progenitors. Development 129:2447–2457 [DOI] [PubMed] [Google Scholar]
- Gannon M, Tweedie Ables E, Crawford L, Lowe D, Offield MF, Magnuson MA, Wright CV 2008 pdx-1 function is specifically required in embryonic β cells to generate appropriate numbers of endocrine cell types and maintain glucose homeostasis. Dev Biol 314:406–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes CJ 2005 Type 2 diabetes—a matter of β-cell life and death? Science 307:380–384 [DOI] [PubMed] [Google Scholar]
- Uchida T, Nakamura T, Hashimoto N, Matsuda T, Kotani K, Sakaue H, Kido Y, Hayashi Y, Nakayama KI, White MF, Kasuga M 2005 Deletion of Cdkn1b ameliorates hyperglycemia by maintaining compensatory hyperinsulinemia in diabetic mice. Nat Med 11:175–182 [DOI] [PubMed] [Google Scholar]
- Wu SH, Wu XH, Lu C, Dong L, Chen ZQ 2006 Lipoxin A4 inhibits proliferation of human lung fibroblasts induced by connective tissue growth factor. Am J Respir Cell Mol Biol 34:65–72 [DOI] [PubMed] [Google Scholar]
- Zhang H, Ackermann AM, Gusarova GA, Lowe D, Feng X, Kopsombut UG, Costa RH, Gannon M 2006 The Foxm1 transcription factor is required to maintain pancreatic β cell mass. Mol Endocrinol 20:1853–1866 [DOI] [PubMed] [Google Scholar]
- Wenger C, Ellenrieder V, Alber B, Lacher U, Menke A, Hameister H, Wilda M, Iwamura T, Beger HG, Adler G, Gress TM 1999 Expression and differential regulation of connective tissue growth factor in pancreatic cancer cells. Oncogene 18:1073–1080 [DOI] [PubMed] [Google Scholar]
- Gannon M, Ray MK, Van Zee K, Rausa F, Costa RH, Wright CV 2000 Persistent expression of HNF6 in islet endocrine cells causes disrupted islet architecture and loss of β cell function. Development 127:2883–2895 [DOI] [PubMed] [Google Scholar]
- Tweedie E, Artner I, Crawford L, Poffenberger G, Thorens B, Stein R, Powers AC, Gannon M 2006 Maintenance of hepatic nuclear factor 6 in postnatal islets impairs terminal differentiation and function of β-cells. Diabetes 55:3264–3270 [DOI] [PubMed] [Google Scholar]
- Kapasa M, Serafimidis I, Gavalas A, Kossida S 2008 Identification of phylogenetically conserved enhancer elements implicated in pancreas development in the WISP1 and CTGF orthologs. Genomics 92:301–308 [DOI] [PubMed] [Google Scholar]
- Murtaugh L, Melton DA 2003 Genes, signals, and lineages in pancreas development. Annu Rev Cell Dev Biol 19:71–89 [DOI] [PubMed] [Google Scholar]
- Lammert E, Cleaver O, Melton D 2001 Induction of pancreatic differentiation by signals from blood vessels. Science 294:564–567 [DOI] [PubMed] [Google Scholar]
- Cleaver O, Melton DA 2003 Endothelial signaling during development. Nat Med 9:661–668 [DOI] [PubMed] [Google Scholar]
- Smart NG, Apelqvist AA, Gu X, Harmon EB, Topper JN, MacDonald RJ, Kim SK 2006 Conditional expression of Smad7 in pancreatic β cells disrupts TGF-β signaling and induces reversible diabetes mellitus. PLoS Biol 4:e39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi T, Nishida T, Shimo T, Kobayashi K, Kubo T, Tamatani T, Tezuka K, Takigawa M 2000 Effects of CTGF/Hcs24, a product of a hypertrophic chondrocyte-specific gene, on the proliferation and differentiation of chondrocytes in culture. Endocrinology 141:264–273 [DOI] [PubMed] [Google Scholar]
- Safadi FF, Xu J, Smock SL, Kanaan RA, Selim AH, Odgren PR, Marks Jr SC, Owen TA, Popoff SN 2003 Expression of connective tissue growth factor in bone: its role in osteoblast proliferation and differentiation in vitro and bone formation in vivo. J Cell Physiol 196:51–62 [DOI] [PubMed] [Google Scholar]
- Pi L, Oh SH, Shupe T, Petersen BE 2005 Role of connective tissue growth factor in oval cell response during liver regeneration after 2-AAF/PHx in rats. Gastroenterology 128:2077–2088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asano M, Kubota S, Nakanishi T, Nishida T, Yamaai T, Yosimichi G, Ohyama K, Sugimoto T, Murayama Y, Takigawa M 2005 Effect of connective tissue growth factor (CCN2/CTGF) on proliferation and differentiation of mouse periodontal ligament-derived cells. Cell Commun Signal 3:11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dor Y, Brown J, Martinez OI, Melton DA 2004 Adult pancreatic β-cells are formed by self-duplication rather than stem-cell differentiation. Nature 429:41–46 [DOI] [PubMed] [Google Scholar]
- Teta M, Long SY, Wartschow LM, Rankin MM, Kushner JA 2005 Very slow turnover of β-cells in aged adult mice. Diabetes 54:2557–2567 [DOI] [PubMed] [Google Scholar]
- Ackermann AM, Gannon M 2007 Molecular regulation of pancreatic β-cell mass development, maintenance, and expansion. J Mol Endocrinol 38:193–206 [DOI] [PubMed] [Google Scholar]
- Jiang Y, Nishimura W, Devor-Henneman D, Kusewitt D, Wang H, Holloway MP, Dohi T, Sabo E, Robinson ML, Altieri DC, Sharma A, Altura RA 2008 Postnatal expansion of the pancreatic β-cell mass is dependent on survivin. Diabetes 57:2718–2727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang FD, Li Y, Iida K, McGrath B, Cavener DR 2006 PERK EIF2AK3 control of pancreatic β cell differentiation and proliferation is required for postnatal glucose homeostasis. Cell Metab 4:491–497 [DOI] [PubMed] [Google Scholar]
- Valenzuela DM, Murphy AJ, Frendewey D, Gale NW, Economides AN, Auerbach W, Poueymirou WT, Adams NC, Rojas J, Yasenchak J, Chernomorsky R, Boucher M, Elsasser AL, Esau L, Zheng J, Griffiths JA, Wang X, Su H, Xue Y, Dominguez MG, Noguera I, Torres R, Macdonald LE, Stewart AF, DeChiara TM, Yancopoulos GD 2003 High-throughput engineering of the mouse genome coupled with high-resolution expression analysis. Nat Biotechnol 21:652–659 [DOI] [PubMed] [Google Scholar]
- Yang XW, Model P, Heintz N 1997 Homologous recombination based modification in Escherichia coli and germline transmission in transgenic mice of a bacterial artificial chromosome. Nat Biotechnol 15:859–865 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Buchholz F, Muyrers JP, Stewart AF 1998 A new logic for DNA engineering using recombination in Escherichia coli. Nat Genet 20:123–128 [DOI] [PubMed] [Google Scholar]
- Liu YT, Chao DC, Lee F, Chen CG, Ji DD 1998 Molecular characterization of Gluconobacter oxydans recA gene and its inhibitory effect on the function of the host wild-type recA gene. Can J Microbiol 44:149–156 [PubMed] [Google Scholar]
- Narayanan K, Williamson R, Zhang Y, Stewart AF, Ioannou PA 1999 Efficient and precise engineering of a 200 kb β-globin human/bacterial artificial chromosome in E. coli DH10B using an inducible homologous recombination system. Gene Ther 6:442–447 [DOI] [PubMed] [Google Scholar]
- Sauer B, Henderson N 1988 Site-specific DNA recombination in mammalian cells by the Cre recombinase of bacteriophage P1. Proc Natl Acad Sci USA 85:5166–5170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu KL, Gannon M, Peshavaria M, Offield MF, Henderson E, Ray M, Marks A, Gamer LW, Wright CV, Stein R 1997 Hepatocyte nuclear factor 3β is involved in pancreatic β-cell-specific transcription of the pdx-1 gene. Mol Cell Biol 17:6002–6013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffen CL, Ball-Mirth DK, Harding PA, Bhattacharyya N, Pillai S, Brigstock DR 1998 Characterization of cell-associated and soluble forms of connective tissue growth factor (CTGF) produced by fibroblast cells in vitro. Growth Factors 15:199–213 [DOI] [PubMed] [Google Scholar]
- Brissova M, Shiota M, Nicholson WE, Gannon M, Knobel SM, Piston DW, Wright CV, Powers AC 2002 Reduction in pancreatic transcription factor PDX-1 impairs glucose-stimulated insulin secretion. J Biol Chem 277:11225–11232 [DOI] [PubMed] [Google Scholar]
- Thimmappaya B, Zain BS, Dhar R, Weissman SM 1978 Nucleotide sequence of DNA template for the 3′ ends of SV40 mRNA. II. The sequence of the DNA fragment EcorII-F and a part of EcorII-H. J Biol Chem 253:1613–1618 [PubMed] [Google Scholar]
- Adra CN, Boer PH, McBurney MW 1987 Cloning and expression of the mouse pgk-1 gene and the nucleotide sequence of its promoter. Gene 60:65–74 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.