Abstract
Background
The physiological functions of neurotrophins occur through binding to two different receptors: pan75 neurotrophin receptor (p75NTR) and a family of tropomysin receptor kinases (Trks A, B, and C). Recently, we reported that expression of neurotrophins and TrkB were reduced in brains of suicide subjects. Present study examines whether expression and activation of Trk receptors and expression of p75NTR are altered in brain of these subjects.
Methods
Expression levels of TrkA, B, C, and of p75NTR were measured by quantitative RT-PCR and Western blot in prefrontal cortex (PFC) and hippocampus of suicide and normal control subjects. The activation of Trks was determined by immunoprecipitation followed by Western blotting using phosphotyrosine antibody.
Results
In hippocampus, lower mRNA levels of TrkA and TrkC were observed in suicide subjects. In the PFC, the mRNA level of TrkA was decreased, without any change in TrkC. On the other hand, the mRNA level of p75NTR was increased in both PFC and hippocampus. Immunolabeling studies showed similar results as observed for the mRNAs. In addition, phosphorylation of all Trks was decreased in hippocampus, but in PFC, decreased phosphorylation was noted only for TrkA and B. Increased expression ratios of p75NTR to Trks were also observed in PFC and hippocampus of suicide subjects.
Conclusions
Our results suggest not only reduced functioning of Trks in brains of suicide subjects but that increased ratios of p75NTR to Trks indicate possible activation of pathways that are apoptotic in nature. These findings may be crucial in the pathophysiology of suicide.
Keywords: Trk, p75NTR, depression, suicide, postmortem brain, gene expression
Introduction
Neurotrophins are a family of secreted proteins that include brain-derived neurotrophic factor (BDNF), nerve growth factor (NGF), neurotrophin (NT)-3, and NT-4/5. These neurotrophins are essential for regulating neuronal differentiation in the developing brain but also are crucial for trophic support, maintenance of differentiated neuronal phenotypes, neurogenesis, synaptic formation, and regulation of synaptic connections in adult neurons as well as in activity-dependent plasticity, which is a defining feature of the brain throughout life (1-6). Neurotrophins are unique in using two different classes of cell surface receptors to exert their biological actions: 1) the tropomysin receptor kinase (Trk) and 2) pan75 neurotrophin receptor (p75NTR), a member of the tumor necrosis factor α receptor superfamily (7). The dual receptor system accounts for the diverse effects exerted by neurotrophins.
There are several subtypes of Trks, which are characterized by a specific affinity for the different neurotrophins. For example, NGF binds preferentially to TrkA, whereas BDNF and NT-4/5 show high affinity for TrkB. NT-3, on the other hand, binds to TrkC with high affinity but can also bind to TrkA and TrkB with lower affinity (8,9). Structurally, the extracellular domain of Trk receptors consists of a cysteine-rich cluster, followed by three leucine-rich repeats, another cysteine-rich cluster and two immunoglobulin-like domains, involved in ligand binding. The cytoplasmic domain consists of a tyrosine kinase domain surrounded by several tyrosines. Ligand binding to Trk receptors causes their dimerization and results in receptor autophosphorylation of kinases present in the cytoplasmic domain. Once phosphorylated, Trk receptors become scaffolding structures that recruit adaptor proteins that couple the receptor to downstream signaling pathways, resulting in alterations in gene expression and neuronal functioning (10). Tyrosine kinase activity is thus essential for the vast majority of Trk receptor-mediated responses to neurotrophins (1,11,12). Both Trk receptors and p75NTR are expressed highly in human cortical and hippocampal brain areas (13-16).
p75NTR initially discovered as a low-affinity receptor for NGF, is now known as a class of receptor that can bind to all neurotrophins with equivalent nanomolar affinities (17). The 3.8 kb mRNA for p75NTR encodes a 427 amino acid protein containing a 28 amino acid single peptide, a single transmembrane domain, and a 55 amino acid cytoplasmic domain (18). Although p75NTR receptors do not contain a catalytic motif, they interact with several proteins, including Trk receptors, which causes enhancement of ligand specificity and ligand affinities for Trk receptors (19-21).
Several studies suggest that BDNF may be involved in stress and depressive behavior (22-27), and that the beneficial effects of antidepressants are associated with an upregulation of BDNF expression (28-30). In a previous study, we reported that expression of BDNF is lower in postmortem brains of suicide subjects (31), which was associated with decreased expression of its cognate receptor TrkB (31). In addition, we recently reported altered expression of NGF, NT-3, and NT-4/5 in suicide subjects in a brain region-specific manner (32). The role of neurotrophins in suicide is further substantiated by other investigators who showed altered levels of neurotrophins in suicide brains or in peripheral tissues of suicidal patients (33-35).
Since the physiological functions of neurotrophins require binding to Trk receptors and their successive phosphorylation, examining the expression and functional activation of neurotrophin receptors is an important step in understanding the significance of the role of neurotrophins in a disease state. Therefore, in the present investigation, we examined activation of Trks A, B, and C in the postmortem brains of suicide subjects. In addition, we examined the expression levels of TrkA, TrkC, and p75NTR in these brain areas. Our study provides further insight into the role of neurotrophins and their receptors in the pathophysiologic mechanisms of suicide.
Methods and Materials
Subjects
Brain tissues were obtained from the Maryland Brain Collection at the Maryland Psychiatric Research Center, Baltimore, MD. We used the same brain samples in which we had studied expression of neurotrophins and TrkB (31,32). The study was performed in the PFC (Brodmann’s area 9) and hippocampus obtained from suicide subjects (n = 28) and nonpsychiatric control subjects (n = 21), hereafter referred to as normal controls. The demographic characteristics of suicide subjects and normal controls are provided in supplemental Table 1 and dissection of the brains are described in our earlier publications (31,32). Toxicology and presence of antidepressants were examined by analysis of urine and/or blood samples. pH of the brain was measured in cerebellum (36). All the subjects were diagnosed based on the Diagnostic Evaluation After Death (37) and the Structured Clinical Interview for the DSM-IV (38) as detailed in our earlier publications (31,32,39,40). This study was approved by the Institutional Review Board of the University of Illinois at Chicago.
Determination of mRNA Levels of TrkA, TrkC, and p75NTR
Total RNA was isolated by CsCl2 ultracentrifugation as described earlier (39,40). Samples showing an absorbance ratio (260/280) greater than 1.8 and exhibiting strong 28S and 18S rRNA bands were used. In addition, all the samples showed RNA integrity number >7, which is an excellent value for mRNA studies.
The mRNA levels of TrkA, TrkC, p75NTR, and of housekeeping genes neuron-specific enolase (NSE) and cyclophilin were determined using competitive RT-PCR as described earlier (39,40). The sequences of external and internal primers are given in Table 1. Decreasing concentrations of TrkA (1.5-0.05 pg for PFC and 6.25-0.19 pg for hippocampus), TrkC (200-12.5 pg), or p75NTR (12-0.75 pg for PFC and 6.25-0.39 pg for hippocampus) internal standard cRNAs and 1.5 μCi [32P]dCTP were added to 1 μg of total RNA. The PCR mixture was amplified for 28 cycles. Following amplification, aliquots were digested with Xho I in triplicate and run by 1.5% agarose gel electrophoresis. The results are expressed as attomoles/μg of total RNA.
Table 1.
External and internal primer sequences of Trks and p75NTR for Amplification
| Primer | Primer sequence | GenBankAccession No. | Nucleotide Position (bp) |
|---|---|---|---|
| External | |||
| TrkA | F: 5’GTGGAGAAGAAGGACGAA ACAC | NM_002529 | 1222-1243 |
| R: 5’GTATTGTGGGTTCTCGATGATG | 1467-1488 | ||
| TrkC | F: 5’TACAAGCTTTAACCGGCTCACCACACT CTC | S_76475 | 408-428 |
| R: 5’TACGAATTCCCACCACGT TCTCTGCAA TGC | 851-871 | ||
| p75NTR | F: 5’CTGCAAGCAGAACAAGCAAGGAGC | NM_002507 | 831-854 |
| R: 5’AGGCCTCATGGGTAAAGGAGT | 1121-1141 | ||
| NSE | F: 5’GGGACTGAGAA CAAATCCAAG | NM_001975 | 295-315 |
| R: 5’CTCCAAGGCTTCACTGTTCTC | 655-675 | ||
| Cyclophilin | F: 5’AGCACTGGAGAGA AAGGATTTG | XM_371409 | 118-139 |
| R: 5’CCTCCACAAT ATTCATGCCTTC | 400-421 | ||
| Internal | |||
| TrkA | F: 5’TGGGATCAACCTCGAGGCTGTGC TGG | 1344-1369 | |
| TrkC | F: 5’GTGTGACCTTCTCGAGATCAGCGTG | 618-642 | |
| p75NTR | F: 5’ACGCAGACAGCCTCGAGCCAGGCCCT | 961-990 | |
| NSE | 5’GGCAACAAGCTC GAGATGCAGGAGTTC | 478-504 | |
| Cyclophilin | F:5’GGTGGCAAGTCCATCTAT/AAATGCTGGACCCAACAC | 220-237/303-320 |
F, forward; R, reverse; bold and italicized letters indicate the mutated bases. Underline bases indicate the Xho I cleavage site.
Preparation of Samples for Immunoprecipitation and Western Blot
Proteins from PFC or hippocampal tissues were extracted using RIPA buffer [20 mM Tris-HCL (pH 8), 150 mM NaCl, 1mM EDTA, 50 mM NaF, 1 mM Na2MoO4, 0.5 mM Na3VO4, 5 mM Na2P2O7, 1% Triton X-100, 0.5% Na deoxycholate, 0.1% SDS, 10% glycerol, 10 μg/ml leupeptin, 10 μg/ml aprotinin, 0.01 mM phenylmethyl sulfonyl fluoride (PMSF), 1 mg/ml pepstatin A, and 10 mM benzamidine]. S1 fraction was prepared by centrifugation at 1,000 rpm for 10 min at 4°C. Protein content was determined by the Bradford method (Bio-Rad, CA, USA).
Immunoprecipitation of TrkA, TrkB, and TrkC and Immunolabeling with Phosphotyrosine
Supernatant containing 100 μg protein was incubated with antibodies against TrkA, TrkB, or TrkC (100:1 dilution; Santa Cruz Biotechnology, CA, USA) for 2 h at 0°C. The samples were added to a suspension of protein-A sepharose beads (Amersham, NJ, USA) in Tris-buffered saline and incubated at 4°C for 1h. The pellet was collected by centrifugation at 2,500 rpm for 30 s at 4°C and washed four times with TBS containing 0.5 mM Na3VO4 and 0.01 mM PMSF. The pellet was resuspended in 15 ml of 2X sample buffer, boiled for 5 min, and subjected to 10% SDS-polyacrylamide gel electrophoresis as described earlier (39). The blots were incubated overnight at 4°C with anti-mouse phosphotyrosine (1 μg/ml, Chemicon International, Temecula, CA, USA), followed by horseradish-peroxidase-linked secondary anti-mouse IgG (0.3 μg/ml; Bio-Rad) for 5 h at room temperature. The bands on the autoradiograms were quantified using the Loats Image Analysis System (Westminister, MD, USA).
Immunolabeling of TrkA, TrkC, and p75NTR
Equal volumes (20 μl) of samples containing 60 μg of protein were electrophoresed on 10% (w/v) polyacrylamide gel. The blots were incubated overnight at 4°C with primary antibodies for TrkA (1:650), TrkC (1:1,000), or p75NTR (1:200, NeoMarkers, Fremont, CA, USA) followed by horseradish-peroxidase-linked secondary anti-goat IgG (TrkA, 1:1000 dilution), anti-rabbit IgG (TrkC, 1:5,000 dilution) or anti-mouse IgG (p75NTR, 1:800 dilution) for 5 h at room temperature. The membranes were stripped and re-probed with β-actin monoclonal primary (1:5000 for 1 h, Sigma Chemical Co., St. Louis, MO, USA) and anti-mouse secondary antibody (1:5000 for 1 h). The optical density (O.D.) of each protein was corrected by the O.D of the corresponding β-actin band. The antibody for p75NTR has been well characterized in human brain for Western blotting (41,42,43). We characterized TrkA and TrkC antibodies using positive controls (H4 cell lysate, SK-N-SH cell lysate for TrkA; EOC20 whole cell lysate for TrkC). Also, the specificity for TrkA and TrkC was confirmed by pre-incubating the antibodies with the corresponding antigenic peptides (100-fold excess) (Santa Cruz Biotechnology, CA, USA).
Statistical Analysis
Data analyses were performed using the SPSS version 15 (Chicago, IL, USA). All the dependent variables were first subjected to tests of normality. The assumption of normality was tested using the Shapiro-Wilk test. To adjust for multiplicity of testing based on multiple endpoints (i.e., dependent variables), a multiple analysis of covariance (MANCOVA) was applied to the data for each brain area. Age, gender, pH of the brain, race, and postmortem interval (PMI) were used as covariates. The assumption of homogeneity of variance was tested using Box’s test of equality of covariance matrices. In the presence of a significant MANCOVA for a given brain area, ANCOVAs were performed for each dependent variable. For the two-group analysis (normal controls vs. suicide subjects) MANCOVA was followed by ANCOVA. For the three-group analysis (normal controls, depressed suicide subjects, suicide subjects with other psychiatric disorders), if the ANCOVA for that dependent variable was significant, pairwise between-group comparisons were performed for each dependant variable.
The differences in age, gender, pH of the brain, and PMI between suicide subjects and normal controls were analyzed using the independent-sample “t” test. The relationships between Trk receptor activation and their respective mRNA and protein levels; mRNA and protein levels of p75NTR; and measures of Trk receptors and p75NTR with PMI, age, and pH of the brain were determined by Pearson product-moment correlation analyses. The effects of gender and comparison between depressed subjects who showed antidepressant toxicity at the time of death with depressed subjects who did not were determined by an independent sample “t” test.
Results
There were no significant differences in age (t = 0.63, df = 47, P = 0.53), PMI (t = 0.11, df = 47, P = 0.91) or pH of the brain (t = 1.00, df = 47, P = 0.32) between suicide subjects and normal control subjects (supplemental Table 1).
Overall Analysis of Data
All dependent variables in the 2 brain areas were first subjected to tests of normality using the Shapiro-Wilk test. We found non-significant P values (>0.05) for tests of normality for all dependent variables in PFC and hippocampus of both normal control and suicide groups which indicated that we cannot reject the null hypothesis that the data are normally distributed. We used Box’s test of equality of covariance matrices to test the assumption of between-group equality. No significant between-group differences were found for covariance matrices in PFC (P = 0.25) or hippocampus (p = 0.38). The overall MANCOVA for all 11 dependent variables adjusted for covariates was significant for PFC (F = 17.84, df = 11, 32, P <0.001) and hippocampus (F = 40.96, df = 11, 30, P <0.001) when the normal control group was compared with the suicide group. In the following sections, we describe the results of the individual ANCOVAs for each dependent variable for PFC and hippocampus. Also, the percent change in various measures of Trks and p75NTR are summarized in Table 2.
Table 2.
Percent change in various measures of Trks and p75NTR in PFC and hippocampus of suicide subjects
| Brian area | Variables | % Change |
|---|---|---|
| PFC | ||
| mRNA | ||
| TrkA | ↓ 26* | |
| TrkC | (No change) | |
| p75NTR | ↑ 37** | |
| Immunolabeling | ||
| TrkA | ↓ 28** | |
| TrkC | (No change) | |
| p75NTR | ↑ 31** | |
| Phosphorylation | ||
| TrkA | ↓ 29** | |
| TrkB | ↓ 30** | |
| TrkC | (No change) | |
| Hippocampus | ||
| mRNA | ||
| TrkA | ↓ 34** | |
| TrkC | ↓ 39** | |
| p75NTR | ↑ 80** | |
| Immunolabeling | ||
| TrkA | ↓ 29** | |
| TrkC | ↓ 30** | |
| p75NTR | ↑ 30** | |
| Phosphorylation | ||
| TrkA | ↓ 33** | |
| TrkB | ↓ 32** | |
| TrkC | ↓ 34** |
p = 0.001;
p < 0.001.
mRNA Levels of TrkA, TrkC, and p75NTR
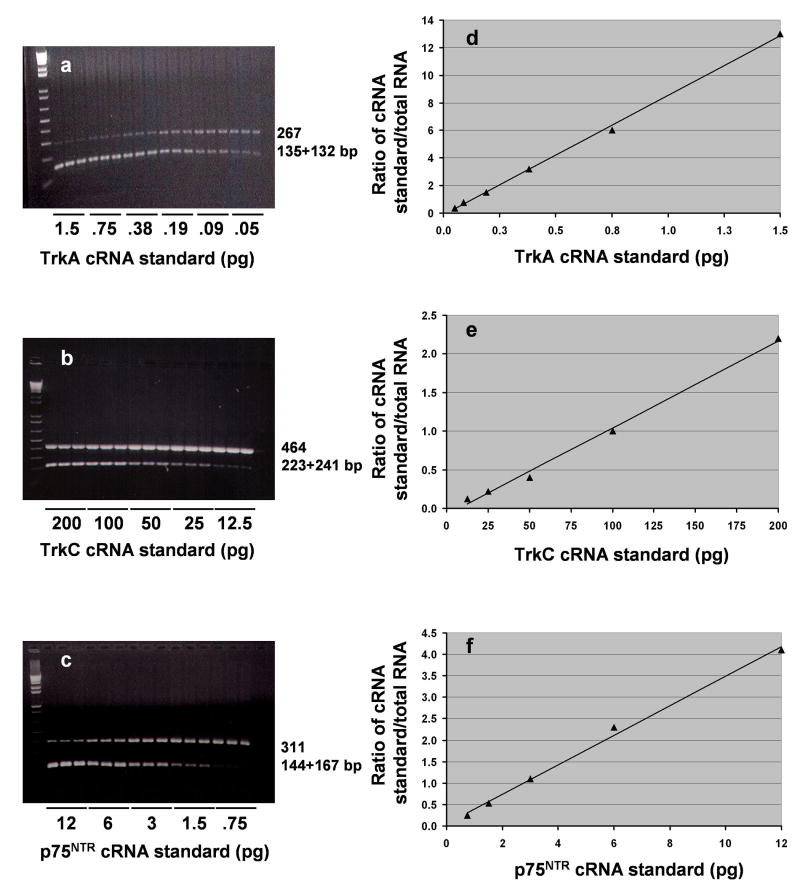
Representative gel electrophoreses of the competitive RT-PCR for TrkA, TrkC, and p75NTR mRNA in the PFC from one control subject are given in Figure 1a, b, and c respectively. We found the amplification product arising from the TrkA, TrkC, and p75NTR mRNA template at 267, 464, and 311 bp respectively, and the corresponding digestion products from the cRNA at 135+132, 223+241, and 144+167 bp respectively. Competitive PCR analyses are presented in Figures 1d, e, and f, where the points of equivalence represent the absolute amounts of TrkA, TrkC, and p75NTR mRNA present. The absolute amounts (attomoles/μg total RNA) of Trk receptor and p75NTR mRNAs in PFC and hippocampus of normal controls were as follows: PFC: TrkA, 2.5 ± 0.6; TrkC, 73.17 ± 12.7; p75NTR, 12.3 ± 2.4; hippocampus: TrkA, 5.1 ± 0.9; TrkC, 98.3 ± 20.9; p75NTR, 4.4 ± 1.4. As can be seen in Figure 2, the expression of TrkC was highest compared with that of TrkA and p75NTR in both PFC and hippocampus. The expression levels of both TrkA and TrkC were greater in hippocampus than PFC. On the other hand, the expression of p75NTR was greater in PFC than hippocampus.
Figure 1.
Representative gel electrophoreses showing competitive PCR analysis for TrkA (a), TrkC (b), or p75NTR (c) mRNA contents in PFC obtained from one normal control subject. Decreasing concentrations of internal standard cRNA (TrkA, 1.5-0.05 pg; TrkC, 200-12.5 pg; p75NTR, 12-0.75) were added to a constant amount (1 μg) of total RNA. The mixtures were reverse transcribed and PCR-amplified in the presence of trace amounts of [32P]dCTP; aliquots were electrophoresed on 1.5% agarose gel. The higher molecular size band corresponds to the amplification product arising from the mRNA, whereas the lower bands arise from cRNA generated from the internal standard. Data derived from the agarose gel are plotted as the counts incorporated into the amplified TrkA (d), TrkC (e), or p75NTR (f) cRNA standard divided by the counts incorporated into the corresponding mRNA amplification product versus the known amount of internal standard cRNA added to the test sample. The point of equivalence represents the amount of the respective mRNA.
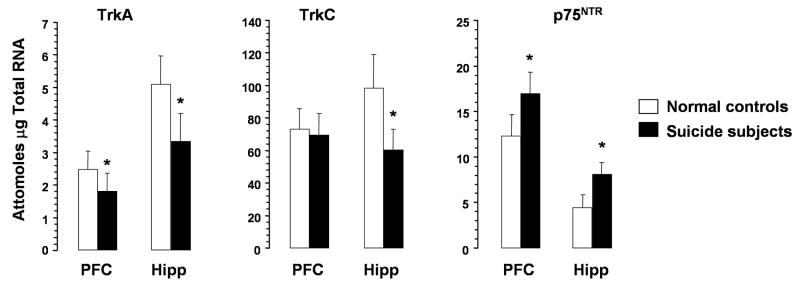
Figure 2.
mRNA levels TrkA, TrkC, and p75NTR in PFC and hippocampus of suicide subjects and normal controls. Data are the mean ± S.D. PFC samples were from 21 normal controls and 28 suicide subjects; hippocampus samples were from 21 normal controls and 26 suicide subjects. Hip, hippocampus. Overall ANCOVA in PFC and hippocampus were as follows: PFC: TrkA, df = 1,40, F = 37, P <0.001; TrkC, df = 1,42, F = 0.5, P = 0.47; p75NTR (df = 1,42, F = 33.6, P <0.001); hippocampus: TrkA, df = 1,40, F = 37, P <0.001; TrkC, df = 1,40, F = 52.1, P <0.001; p75NTR, df = 1,40, F = 72.4, P <0.001). * P <0.001.
When compared between normal controls and suicide subjects, the mRNA expression of TrkA was significantly decreased in PFC of suicide subjects without any change in mRNA level of TrkC. On the other hand, mRNA levels of TrkA and TrkC were significantly lower in hippocampus of suicide subjects. In contrast, the mRNA level of p75NTR was significantly increased in both PFC and hippocampus of suicide subjects compared with normal controls (Figure 2).
We used NSE and cyclophilin as housekeeping genes. As reported earlier (31,32,39), we did not find a significant difference in mRNA levels (attomoles/μg total RNA) of cyclophilin between normal controls and suicide subjects, either in PFC (controls: 776.6 ± 112.5, suicide: 801.5 ± 117.34; df = 1,42, F = 0.3, P = 0.57) or in hippocampus (controls: 783.5 ± 110.1, suicide: 768.3 ± 102.8; df = 1,40, F = 0.001, P = 0.98). Similarly, no significant differences were observed in mRNA levels of NSE in PFC (controls: 360.2 ± 47.7, suicide: 344.0 ± 43.9; df = 1,42, F = 0.7, P = 0.39) or hippocampus (controls: 349.8 ± 38.2, suicide: 345.9 ± 81.4; df = 1,40, F = 0.2, P = 0.62) between normal controls and suicide subjects. We found similar results when the changes in mRNA levels of TrkA, TrkC, and p75NTR were calculated as ratios to cyclophilin or NSE.
Protein Levels of TrkA, TrkC, and p75NTR
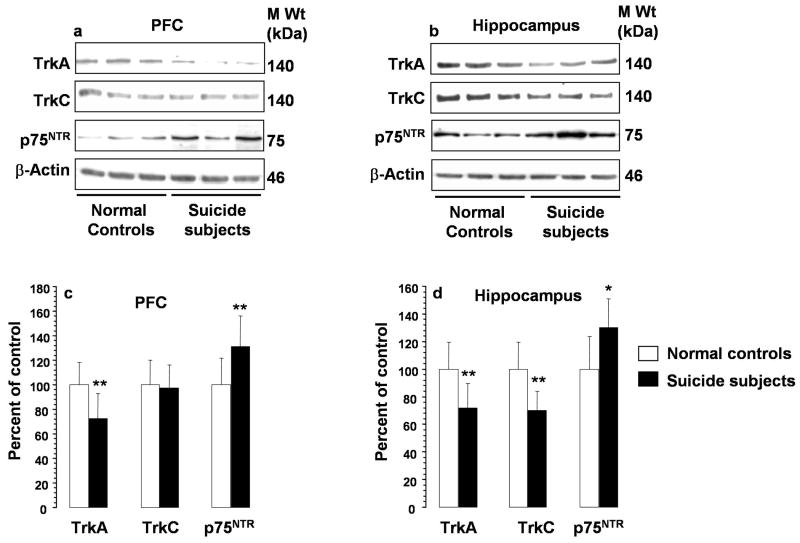
Western blot revealed that TrkA and TrkC migrated to 140 kDa, whereas p75NTR migrated to 75 kDa (Figure 3a and 3b). β-Actin was used as a housekeeping protein, and ratios of TrkA, TrkC, and p75NTR vs. β-actin were calculated. Immunolabeling of β-actin was not significantly different in suicide subjects (1.3 ± 0.3 AU) compared with normal controls (1.2 ± 0.3 AU). Bar diagrams showing ratios of protein levels of neurotrophin receptors and β-actin in PFC and hippocampus are shown in Figure 3c and 3d, respectively. Comparison analysis revealed that immunolabeling of TrkA was significantly decreased in both PFC and hippocampus of suicide subjects. Protein levels of TrkC were significantly decreased in hippocampus but not changed in the PFC of suicide subjects. In contrast, the levels of p75NTR were increased in both PFC and hippocampus of suicide subjects compared with normal controls.
Figure 3.
Western blots showing the immunolabeling of TrkA, TrkC, p75NTR and β-actin in PFC (a) and hippocampus (b) of 3 normal controls and 3 suicide subjects and the mean ± S.D. of immunolabeling of TrkA, TrkC, or p75NTR in PFC (c) and hippocampus (d) from normal controls and suicide subjects. Protein samples were subjected to 10% polyacrylamide gel electrophoresis and transferred to ECL-nitrocellulose membranes, which were then incubated with primary antibody specific for TrkA, TrkC, p75NTR, or β-actin and corresponding secondary antibody. The bands were quantified as described in Methods. Ratios of the optical density of TrkA, TrkC, or p75NTR to that of β-actin were calculated. PFC samples were from 21 normal controls and 28 suicide subjects; hippocampus samples were from 21 normal controls and 26 suicide subjects. Suicide group was compared with control group.* Overall ANCOVA: PFC: TrkA, df = 1,42, F = 20.1, P <0.001; TrkC, df = 1,42, F = 0.001, P = 0.98; p75NTR, df = 1,42, F = 18.9, P <0.001; hippocampus: TrkA, df = 1,40, F = 21.7, P <0.001; TrkC, df = 1,40, F = 40.3, P <0.001; p75NTR, df = 1,40, F = 13.9, P = 0.001. *P = 0.001, ** P <0.001.
Correlations between mRNA and Protein Levels of TrkA, TrkC, and p75NTR
To examine whether the altered protein levels of neurotrophin receptors were associated with their respective mRNAs, we examined the correlations between the mRNA and the protein levels of the neurotrophic receptors in the combined normal control and suicide groups. We observed significant correlations between mRNA and protein levels of TrkA in PFC (r = 0.39, P = 0.006) and hippocampus (r = 0.42, P = 0.003). Significant correlations were also observed between mRNA and protein levels of TrkC in hippocampus (r = 0.52, P<0.001), and of p75NTR in both PFC (r = 0.42, P = 0.002), and hippocampus (r = 0.47, P = 0.001).
TrkA, TrkB, and TrkC Phosphorylation
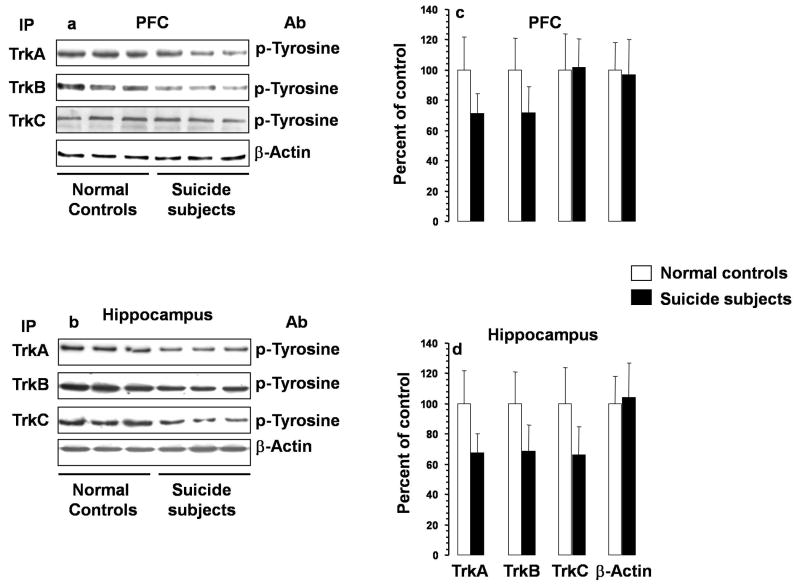
Phosphorylation states of Trks were determined by immunoprecipitation with specific antibodies followed by immunoblotting with phosphotyrosine antibody. Autoradiograms showing the phosphorylation in PFC and hippocampus are given in Figure 4a and 4b respectively and diagrammatically presented in Figures 4c and d respectively. MANCOVA followed by ANCOVA tests showed that there were significant decreases in the phosphorylation of TrkA and TrkB in both PFC and hippocampus of suicide subjects, whereas the phosphorylation of TrkC was decreased only in hippocampus without any change in PFC.
Figure 4.
Activation of TrkA, TrkB, and TrkC in PFC and hippocampus of suicide subjects and normal controls. Autoradiograms showing immunolabeling of phosphotyrosine in PFC (a) and hippocampus (b) determined after immunoprecipitation using TrkA, TrkB, or TrkC antibody. Mean ± S.D. of O.D. of bands of phosphotyrosine depicting activation of TrkA, TrkB, or TrkC in PFC (c) and hippocampus (d) of suicide subjects and normal controls. PFC samples were from 21 normal controls and 28 suicide subjects and hippocampus were from 21 normal controls and 26 suicide subjects. Overall ANCOVA: PFC: TrkA, df = 1,42, F = 26.5, p <0.001; TrkB: df = 1,42, F = 38.5, p <0.001; TrkC, df = 1,42, F = 0.03, p = 0.86; hippocampus: TrkA, df = 1,40, F = 33.7, p <0.001; TrkB, df = 1,40, F = 28.2, p <0.001; TrkC, df = 1,40, F = 27.7, p <0.001. * P <0.001. Ab, antibody; IP, immunoprecipitation.
Ratios of p75NTR vs. mRNA and protein levels and phosphorylation of TrkA, TrkB, and TrkC
To examine whether there is an imbalance in the expression and activation of TrkA, B, and C in relation to p75NTR expression, we determined the mRNA and protein expression ratios of p75NTR vs. TrkA, TrkB, and TrkC. In addition, we also determined the ratios of protein expression of p75NTR vs. phosphorylation levels of TrkA, TrkB, and TrkC. As shown in Table 3, we observed significant increase in expression ratios of p75NTR vs. TrkA, B, and C. Similarly, ratios of protein expression of p75NTR vs. phosphorylation of TrkA, TrkB, and TrkC were also increased.
Table 3.
Ratios of p75NTR/Trks in PFC and hippocampus of normal controls and suicide subjects
| Group | Mean | SD | |
|---|---|---|---|
| PFC | |||
| mRNA | |||
| TrkA | Control | 5.18 | 1.62 |
| Suicide | 9.84 | 2.74** | |
| TrkB | Control | 0.02 | 0.008 |
| Suicide | 0.05 | 0.013** | |
| TrkC | Control | 0.17 | 0.04 |
| Suicide | 0.25 | 0.05** | |
| Immunolabeling | |||
| TrkA | Control | 1.03 | 0.31 |
| Suicide | 1.95 | 0.59** | |
| TrkB | Control | 1.05 | 0.36 |
| Suicide | 2.12 | 0.47** | |
| TrkC | Control | 1.03 | 0.25 |
| Suicide | 1.42 | 0.40** | |
| Phosphorylation | |||
| TrkA | Control | 1.04 | 0.31 |
| Suicide | 2.02 | 0.69** | |
| TrkB | Control | 1.04 | 0.32 |
| Suicide | 1.97 | 0.64** | |
| TrkC | Control | 1.07 | 0.42 |
| Suicide | 1.38 | 0.43* | |
| Hippocampus | |||
| mRNA | |||
| TrkA | Control | 0.91 | 0.35 |
| Suicide | 2.55 | 0.74** | |
| TrkB | Control | 0.002 | 0.001 |
| Suicide | 0.007 | 0.002** | |
| TrkC | Control | 0.05 | 0.02 |
| Suicide | 0.14 | 0.04** | |
| Immunolabeling | |||
| TrkA | Control | 1.05 | 0.37 |
| Suicide | 1.93 | 0.60** | |
| TrkB | Control | 1.02 | 0.24 |
| Suicide | 2.31 | 0.61** | |
| TrkC | Control | 1.03 | 0.29 |
| Suicide | 1.91 | 0.41** | |
| Phosphorylation | |||
| TrkA | Control | 1.05 | 0.34 |
| Suicide | 2.00 | 0.51** | |
| TrkB | Control | 1.03 | 0.30 |
| Suicide | 1.99 | 0.57** | |
| TrkC | Control | 1.04 | 0.29 |
| Suicide | 2.10 | 0.60** |
p = 0.013,
p < 0.001,
SD = standard deviation
Effects of age, PMI, pH, Means of Death and Antidepressant Toxicology (provided as supplemental material)
We found no significant effects of age, PMI, or pH of the brain on any of the measures in which we found significant differences between normal controls and suicide subjects (supplemental Table 2). We also did not find significant effects of means of suicide (violent vs. nonviolent) or the presence of antidepressant toxicology at the time of death on various measures.
Effects of Major Depression
We next examined whether the differences in the mRNA and protein levels of neurotrophin receptors and in the phopsphorylation states of Trk receptors were related to depression or were present in all suicide subjects. ANCOVA followed by pairwise between-group comparisons revealed that the mRNA and protein levels of TrkA, TrkC, and p75NTR, as well as the activation of TrkA, TrkB, and TrkC, were not different between suicide subjects with major depression (n = 12) and suicide subjects with other psychiatric disorders (n = 16) in both PFC (Table 4) and hippocampus (Table 5). However, the groups of suicide subjects with major depression and of suicide subjects with other psychiatric disorders both showed significant differences in these measures in both PFC (Table 4) and hippocampus (Table 5) when compared separately with normal control subjects.
Table 4.
Effect of major depression on neurotrophin receptors in PFC of suicide subjects
| Variable | Normal Controls (n=21) 1 | Suicide Subjects (n=28) |
Overall ANCOVA | Multiple Comparison | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| With a History of MDD (n=12) 2 | With a History of Other Psychiatric Disorders (n=16) 3 | F | P | 1 VS 2 | 1 VS 3 | 2 VS 3 | |||||
| Mean | SD | Mean | SD | Mean | SD | ||||||
| Phosphorylationa | |||||||||||
| TrkA | 100 | 20 | 69 | 19 | 72 | 14 | 13 | <0.001 | 0.001 | <0.001 | 0.89 |
| TrkB | 100 | 21 | 65 | 15 | 77 | 17 | 21 | <0.001 | <0.001 | <0.001 | 0.12 |
| TrkC | 100 | 25 | 107 | 26 | 98 | 19 | 0.6 | 0.55 | 0.38 | 0.71 | 0.28 |
| mRNA Levelsb | |||||||||||
| TrkA | 2.5 | 0.5 | 1.6 | 0.5 | 1.9 | 0.6 | 8 | 0.001 | <0.001 | 0.02 | 0.08 |
| TrkC | 73.2 | 12.7 | 70.9 | 11.8 | 68.2 | 14 | 0.8 | 0.47 | 0.87 | 0.27 | 0.32 |
| p75NTR | 12.3 | 2.4 | 16.9 | 2.4 | 16.9 | 2.45 | 16.6 | <0.001 | <0.001 | <0.001 | 0.65 |
| Immunolabelinga | |||||||||||
| TrkA | 100 | 18 | 74 | 19 | 71 | 22 | 10 | <0.001 | 0.006 | <0.001 | 0.65 |
| TrkC | 100 | 20 | 97 | 20 | 98 | 19 | 0.3 | 0.97 | 0.84 | 0.92 | 0.79 |
| p75NTR | 100 | 22 | 126 | 26 | 134 | 25 | 9.3 | <0.001 | 0.005 | <0.001 | 0.83 |
= percent of control;
= attomoles/μg total RNA.
Data were analyzed using multivariate analysis of covariance (MANCOVA). Overall MANCOVA (Pillai’s Trace test) was found to be statistically significant (F = 2.73, df = 22, 64, P <0.001). The data were then subjected to analysis of covariance (ANCOVA) followed by pairwise between-group comparisons. A total of 11 dependent variables (Activation of TrkA, TrkB, TrkC; mRNA levels of TrkA, TrkC, p75NTR, NSE, and cyclophilin; and protein levels of TrkA, TrkC, p75NTR) were considered during multivariate analysis. Age, gender, pH of the brain, race, and postmortem interval were used as covariates. df = 2,41
Table 5.
Effect of major depression on neurotrophin receptors in hippocampus of suicide subjects
| Variable | Normal Controls (n=21) 1 | Suicide Subjects (n=26) |
Overall ANCOVA | Multiple Comparison | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| With a History of MDD (n=10) 2 | With a History of Other Psychiatric Disorders (n=16) 3 | F | P | 1 VS 2 | 1 VS 3 | 2 VS 3 | |||||
| Mean | SD | Mean | SD | Mean | SD | ||||||
| Phosphorylationa | |||||||||||
| TrkA | 100 | 22 | 60 | 7 | 72 | 14 | 18.8 | <0.001 | <0.001 | <0.001 | 0.11 |
| TrkB | 100 | 21 | 71 | 19 | 67 | 16 | 13.7 | <0.001 | 0.001 | <0.001 | 0.87 |
| TrkC | 100 | 24 | 72 | 22 | 63 | 16 | 13.9 | <0.001 | 0.003 | <0.001 | 0.48 |
| mRNA Levelsb | |||||||||||
| TrkA | 5.1 | 0.9 | 2.9 | 0.6 | 3.6 | 0.9 | 19.7 | <0.001 | <0.001 | <0.001 | 0.19 |
| TrkC | 98.2 | 20.9 | 62.2 | 12.6 | 58.9 | 13.1 | 25.7 | <0.001 | <0.001 | <0.001 | 0.59 |
| p75NTR | 4.4 | 1.4 | 7.9 | 1.5 | 8.2 | 1.2 | 35.3 | <0.001 | <0.001 | <0.001 | 0.81 |
| Immunolabelinga | |||||||||||
| TrkA | 100 | 19 | 69 | 18 | 73 | 18 | 10.6 | <0.001 | 0.004 | <0.001 | 0.86 |
| TrkC | 100 | 19 | 71 | 13 | 69 | 14 | 19.8 | <0.001 | <0.001 | <0.001 | 0.67 |
| p75NTR | 100 | 23 | 137 | 23 | 125 | 18 | 8.1 | 0.001 | 0.001 | 0.01 | 0.16 |
= percent of control;
= attomoles/μg total RNA.
Data were analyzed using multivariate analysis of covariance (MANCOVA). Overall MANCOVA (Pillai’s Trace test) was found to be statistically significant (F = 3.65, df = 22, 60, P <0.001). The data were then subjected to analysis of covariance (ANCOVA) followed by pairwise between-group comparisons. A total of 11 dependent variables (Activation of TrkA, TrkB, TrkC; mRNA levels of TrkA, TrkC, p75NTR, NSE, and cyclophilin; and protein levels of TrkA, TrkC, p75NTR) were considered during multivariate analysis. Age, gender, pH of the brain, race, and postmortem interval were used as covariates. df = 2,39
Discussion
In the present study, we found reduced expression of TrkA, C, and increased expression of p75NTR in hippocampus and reduced expression of TrkA and increased expression of p75NTR in PFC of suicide subjects. Decreased phosphorylation of TrkA and B in PFC and TrkA, B, and C in hippocampus was also noted. Our present study provides evidence for the first time that not only Trk receptors are less expressed but also that their activation is compromised in postmortem brains of suicide subjects. In our earlier studies, we found that expression levels of neurotrophins, i.e., BDNF, NGF, NT-3, and NT4/5, as well as TrkB were decreased in the postmortem brains of suicide subjects (31,32). Our previous studies together with the present results clearly demonstrate that there is an overall decrease in expression and functioning of neurotrophins in the brains of suicide subjects.
Our study also indicates that the levels of the receptors for neurotrophins are regulated at the level of transcription, as significant correlations between mRNA and protein levels of these receptors were noted. Promoter sequences for all neurotrophin receptors have been identified, and multiple transcription factors are implicated in the regulation of the expression of these receptors (44,45). For example, many putative transcription factor binding sites within the 5’flanking region of the human TrkA gene have been identified. These include Sp1 and AP-1. Interestingly, the AP-1 site is bound by c-Jun homodimers, which is blocked by methylation. In many cell lines, it has been shown that activation of Trk A expression is caused by direct interference with c-Jun binding to the negative AP-1-like sequence and that the AP-1 binding site plays a crucial epigenetic role in activating TrkA expression. In addition, the 138-bp region located upstream of the transcription initiation site is also crucial for the human TrkA gene. On the other hand, p75NTR transcription is regulated by transcription factor Egr-1. The TrkC gene is regulated by transcription factors AP-1, AP-2, GC, ATF, and Brn2, AML1, and Nkx2.5. Whether these transcription factors and/or epigenetic regulation are involved in the altered expression of neurotrophin receptor genes in the brains of suicide subjects needs to be studied.
Functionally, in contrast to Trk receptors, which contain autophosphorylation sites and are involved in cell survival, p75NTR lacks intrinsic enzymatic activity and can transmit both positive and negative signals (46). It has been shown that p75NTR acts as a positive regulator of TrkA activity in a number of neuronal cell lines (20,47-49). Coexpression of p75NTR and TrkA receptors increases TrkA high affinity binding sites for NGF (19,50) and NGF-mediated TrkA activation (47,49,50). Ligand binding to p75NTR can potentiate TrkA autophosphorylation at a sub-saturating concentration of NGF; this depends upon the relative levels of p75NTR and TrkA (47,50,51). Also, in the presence of p75NTR, NT-3 is less effective in activating TrkA, and NT-3 and NT-4 are much less effective in activating TrkB, which thus enhances the affinity for NGF and BDNF to bind to TrkA and TrkB respectively (20,52-54). In contrast to these positive actions, p75NTR can mediate neuronal apoptosis when the cognate Trk receptor is less activated or not activated. For example, in neuronal cell lines, expression of p75NTR in the absence of TrkA receptors induces cell death (55). Similarly, p75NTR can cause developing hippocampal neuronal death induced by any of the neurotrophins in the absence of a Trk receptor (56-58). On the other hand, mice lacking p75NTR show an increased number of cholinergic neurons in the basal forebrain (59). In adult CNS, it has been shown that excitotoxin-induced neuronal apoptosis is accompanied by the induction of p75NTR in the dying neurons (60), which suggests that p75NTR may represent a general stress-induced apoptotic mechanism (44). However, it is pertinent to note that apoptotic mechanisms of p75NTR are active only when Trk receptors are less expressed or less active. Moreover, ectopic expression of the appropriate Trk receptor can convert a proapoptotic neurotrophin to a pro-survival neurotrophin. Thus it appears that the ratio of expression levels and/or activation states of Trk receptors and p75NTR is quite relevant in neurotrophin-mediated functions. Given that many physiological functions are associated with Trk receptor activation, including cell survival and enhancement of the efficacy of synaptic neurotransmission, and, therefore, neural plasticity, and the strong evidence of a role of p75NTR in the mediation of cell death, our findings of increased expression ratios of p75NTR to Trks appear to be of great relevance to the pathophysiology of mood disorders and suicide. The PFC plays a major role in mood regulation and has been implicated in the pathophysiology of affective disorders and suicide (61). On the other hand, the hippocampus is involved in cognition (62) and is the primary brain area affected by stress (63), one of the major factors in suicidal behavior (64,65). Interestingly, structural abnormalities in cortical and hippocampal brain areas and reduced hippocampal plasticity have been demonstrated in affective disorder patients and during stress (66-72). Some studies even suggest structural abnormalities in the brains of suicide subjects (73,74). Our previously observed reduced expression of neurotrophins (31,32) together with the present findings of reduced expression and activation of Trks and concomitant increased expression p75NTR indicate that the possible consequences is a tipping of the balance away from cell survival, which could be associated with structural abnormalities and reduced neuronal plasticity in suicide brains. In addition to the modulation of hippocampal plasticity (75), recently, Greferath et al (76) and Hennigan et al (77) showed that p75NTR is involved in negative regulation of plasticity, such that mice lacking p75NTR display intact long-term potentiation but impairment in long-term depression. It is pertinent to mention that recently Saarelainen et al (78) demonstrated that normal TrkB signaling is required for antidepressant action and that the phosphorylation of TrkB in response to antidepressants is greater in cortical and hippocampal brain areas after chronic treatment, suggesting that TrkB activation is required to produce the effects of antidepressants. Moreover, stress, a major risk factor of suicide (79-81) causes a decrease in the expression of TrkA, TrkB, and TrkC in rat brain (27). A recent genetic study suggests that the S205L polymorphism, which substitutes serine with leucine residue, of the p75NTR gene is associated with attempted suicide (82), revealing the crucial role of p75NTR in suicide.
The physiological relevance of Trk receptors is further substantiated by the fact that Trk receptors and p75NTR cross talk to each other at the level of the signal transduction mechanisms that they activate transautophosphorylation of tyrosine leads to the recruitment of proteins containing PTB and SH2 domains. The two major signaling pathways, activated by Trks through these domains, are Ras-Raf-extracellular signal regulated kinase (ERK) and phosphoinositide 3-kinase (PI 3-kinase)-Akt. In addition, phospholipase Cγ binds to activated Trk receptors and initiates an intracellular signaling cascade that results in the activation of protein kinase C. On the other hand, p75NTR stimulates several proapoptotic pathways, which include Jun kinase signaling, sphingolipid turnover, and association with adaptor proteins, such as neurotrophin receptor-interacting MAGE homolog (NRAGE) and p75NTR-associated cell death executor (NADE), that directly promote cell cycle arrest and apoptosis (83-86). Trk receptors suppress the major proapoptotic signaling pathway, c-Jun kinase, initiated by p75NTR (87). In sympathetic neurons, Ras-mediated activation of PI3-kinase is required to suppress this signaling pathway (88). Activation of Trk receptors completely suppresses the activation by p75NTR of sphingomyelinase through the association of activated PI3-kinase with acidic sphingomyelinase (89,90). Sphingomyelinase activation results in generation of ceramide which promotes apoptosis by inactivating ERK and PI3-kinase pathways (91-93). Contrary to their proapoptotic action, p75NTR enhances cell survival by activating NF-κB signaling in the presence of Trk receptor activation. Thus, p75NTR acts as a switch between pro- and antiapoptotic actions in neurons. Interestingly, we have reported less-activated ERK1/2 (39), B-Raf (94), and PI-3 kinase (95) in both PFC and the hippocampus of suicide subjects. These findings could be associated with less activation/expression of Trks. These findings also indicate suboptimal activation of prosurvival pathways. Conversely, if p75NTR is more abundantly expressed, this may lead to proapoptotic signaling. Further studies are required to determine whether proapoptotic pathways are activated in brains of suicide subjects and how Trk - and p75NTR-mediated signal transduction pathways interplay in the pathophysiology of suicide.
In the present study, we observed that the changes in Trk and p75NTR were present in all suicide subjects regardless of psychiatric diagnosis, suggesting that these changes could be associated with suicide. However, one should be cautious to draw such a conclusion. One of the limitations of the present study is that the study population did not have subjects who had psychiatric disorders and died naturally. In addition, a majority of suicide subjects had some form of mental illness. As mentioned in Introduction, several studies demonstrate alterations in expression of neurotrophic factors and Trk receptors in mood disorders and during stress. For example, decreased expression of neurotrophins or TrkB receptors in depressed patients as well as in animals subjected to several types of stresses have been reported (22-27). Likewise, several genetic studies indicate a linkage of BDNF to bipolar disorder (96-98). Thus, whether the observed changes in Trks and p75NTR are specifically related to suicide or are associated with mental disorders, need to be further clarified.
Supplementary Material
Acknowledgments
This research was supported by grants from NIMH (R0168777, NIMH R21MH081099), NARSAD, Marshall Reynolds Foundation, and the American Foundation for Suicide Prevention to Dr. Y. Dwivedi; NIMH RO1MH48153 to Dr. G.N. Pandey; and MH60744 and MH66123 to Dr. R. Roberts. We thank Miljana Petkovic and Barbara Brown and Joy K. Roche for their help in organizing the brain tissues. We also thank the members of the Maryland Brain Collection for their efforts, particularly in family interviews and dissection. We are grateful for the cooperation of the Office of the Chief Medical Examiner in Baltimore, Maryland. The authors report no biomedical financial interests or potential conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Huang EJ, Reichardt LF. Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci. 2001;24:677–736. doi: 10.1146/annurev.neuro.24.1.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sofroniew MV, Galletly NP, Isacson O, Svendsen CN. Survival of adult basal forebrain cholinergic neurons after loss of target neurons. Science. 1990;247:338–342. doi: 10.1126/science.1688664. [DOI] [PubMed] [Google Scholar]
- 3.McAllister T, Saykin A, Flashman L. Brain activation during working memory 1 month after mild traumatic brain injury: A functional MRI study. Neurology. 1999;53:1300–1308. doi: 10.1212/wnl.53.6.1300. [DOI] [PubMed] [Google Scholar]
- 4.Poo M. Neurotrophins as synaptic modulators. Nature Reviews Neurosci. 2001;2:24–32. doi: 10.1038/35049004. [DOI] [PubMed] [Google Scholar]
- 5.Lessmann V, Gottmann K, Malcangio M. Neurotrophin secretion: current facts and future prospects. Prog Neurobiol. 2003;69:341–374. doi: 10.1016/s0301-0082(03)00019-4. [DOI] [PubMed] [Google Scholar]
- 6.Lu B. BDNF and activity-dependent synaptic modulation. Learn Mem. 2003;10:86–98. doi: 10.1101/lm.54603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang EJ, Reichardt LF. Trk receptors: role in signal transduction. Annu Rev Biochem. 2003;72:609–642. doi: 10.1146/annurev.biochem.72.121801.161629. [DOI] [PubMed] [Google Scholar]
- 8.Barbacid M. The trk family of neurotrophin receptors. J Neurobiol. 1994;25:1386–1403. doi: 10.1002/neu.480251107. [DOI] [PubMed] [Google Scholar]
- 9.Chao MV. The p75 neurotrophin receptor. J Neurobiol. 1994;25:1373–1385. doi: 10.1002/neu.480251106. [DOI] [PubMed] [Google Scholar]
- 10.Reichardt LF. Neurotrophin-regulated signaling pathways. Phil Trans R Soc B. 2006;361:1545–1564. doi: 10.1098/rstb.2006.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bibel M, Barde YA. Neurotrophins: Key regulators of cell fate and cell shape in the vertebrate nervous system. Genes Dev. 2000;14:2919–2937. doi: 10.1101/gad.841400. [DOI] [PubMed] [Google Scholar]
- 12.Kaplan D, Miller F. Neurotrophin signal transduction in the nervous system. Curr Opin Neurobiol. 2000;10:381–391. doi: 10.1016/s0959-4388(00)00092-1. [DOI] [PubMed] [Google Scholar]
- 13.Hock C, Heese K, Müller-Spahn F, Hulette C, Rosenberg C, Otten U. Decreased trkA neurotrophin receptor expression in the parietal cortex of patients with Alzheimer’s disease. Neurosci Lett. 1998;241:151–154. doi: 10.1016/s0304-3940(98)00019-6. [DOI] [PubMed] [Google Scholar]
- 14.Counts SE, Nadeem M, Wuu J, Ginsberg SD, Saragovi HU, Mufson EJ. Reduction of cortical TrkA but not p75(NTR) protein in early-stage Alzheimer’s disease. Ann Neurol. 2004;56:520–531. doi: 10.1002/ana.20233. [DOI] [PubMed] [Google Scholar]
- 15.Ozbas-Gerceker F, Gorter JA, Redeker S, Ramkema M, van der Valk P. Neurotrophin receptor immunoreactivity in the hippocampus of patients with mesial temporal lobe epilepsy. Neuropathol Appl neurobiol. 2004;30:651–664. doi: 10.1111/j.1365-2990.2004.00582.x. [DOI] [PubMed] [Google Scholar]
- 16.Dechant G, Neumann H. Neurotrophins. Adv Exp Med Biol. 2002;513:303–334. doi: 10.1007/978-1-4615-0123-7_11. [DOI] [PubMed] [Google Scholar]
- 17.Frade JM, Barde YA. Nerve growth factor: two receptors, multiple functions. BioEssays. 1998;20:137–145. doi: 10.1002/(SICI)1521-1878(199802)20:2<137::AID-BIES6>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 18.Hasegawa Y, Yamagishi S, Fujitani M, Yamashita T. p75 neurotrophin receptor signaling in the nervous system. Biotechnol Annu Rev. 2004;10:123–149. doi: 10.1016/S1387-2656(04)10005-7. [DOI] [PubMed] [Google Scholar]
- 19.Hempstead BL, Martin-Zanca D, Kaplan DR, Parada LF, Chao MV. High-affinity NGF binding requires co-expression of the trk proto-oncogene and the low-affinity NGF receptor. Nature. 1991;350:678–683. doi: 10.1038/350678a0. [DOI] [PubMed] [Google Scholar]
- 20.Benedetti M, Levi A, Chao MV. Differential expression of nerve growth factor receptors leads to altered binding affinity and neurotrophin responsiveness. Proc Natl Acad Sci USA. 1993;90:7859–7863. doi: 10.1073/pnas.90.16.7859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Esposito D, Patel P, Stephens RM, Perez P, Chao MV, Kaplan DR, et al. The cytoplasmic and transmembrane domains of the p75 and Trk A receptors regulate high affinity binding to nerve growth factor. J Biol Chem. 2001;276:32687–32695. doi: 10.1074/jbc.M011674200. [DOI] [PubMed] [Google Scholar]
- 22.Barbany G, Persson H. Regulation of neurotrophin mRNA expression in the rat brain by glucocorticoids. Eur J Neurosci. 1992;4:396–403. doi: 10.1111/j.1460-9568.1992.tb00888.x. [DOI] [PubMed] [Google Scholar]
- 23.Smith MA, Makino S, Kvetnanský R, Post RM. Effects of stress on neurotrophic factor expression in the rat brain. Ann N Y Acad Sci. 1995;771:234–239. doi: 10.1111/j.1749-6632.1995.tb44684.x. [DOI] [PubMed] [Google Scholar]
- 24.Shimizu E, Hashimoto K, Okamura N, Koike K, Komatsu N, Kumakiri C, et al. Alterations of serum levels of brain-derived neurotrphic factor (BDNF) in depressed patients with or without antidepressants. Biol Psychiatry. 2003;54:70–75. doi: 10.1016/s0006-3223(03)00181-1. [DOI] [PubMed] [Google Scholar]
- 25.Karege F, Perret G, Bondolfi G, Schwald M, Bertschy G, Aubry J-M. Decreased serum brain-derived neurotrophic factor levels in major depressed patients. Psychiatry Res. 2002;109:143–148. doi: 10.1016/s0165-1781(02)00005-7. [DOI] [PubMed] [Google Scholar]
- 26.Siuciak JA, Lewis DR, Wiegand SJ, Lindsay R. Antidepressant-like effect of brain derived neurotrophic factor (BDNF) Pharmacol Biochem Beh. 1996;56:131–137. doi: 10.1016/S0091-3057(96)00169-4. [DOI] [PubMed] [Google Scholar]
- 27.Ueyama T, Kawai Y, Nemoto K, Sekimoto M, Tone S, Senba E. Immobilization stress reduced the expression of neurotrophins and their receptors in the rat brain. Neurosci Res. 1997;28:103–110. doi: 10.1016/s0168-0102(97)00030-8. [DOI] [PubMed] [Google Scholar]
- 28.Shirayama Y, Chen ACH Nakagawa S, Russell DS, Duman RS. Brain-derived neurotrophic factor produces antidepressant effects in behavioral models of depression. J Neurosci. 2002;22:3251–3261. doi: 10.1523/JNEUROSCI.22-08-03251.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nibuya M, Morinobu S, Duman RS. Regulation of BDNF and trkB mRNA in rat brain by chronic electroconvulsive seizure and antidepressant drug treatments. J Neurosci. 1995;15:7539–7547. doi: 10.1523/JNEUROSCI.15-11-07539.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lindefors N, Brodin E, Metsis M. Spatiotemporal selective effects on brain-derived neurotrophic factor and trkB messenger RNA in rat hippocampus by electroconvulsive shock. Neuroscience. 1995;65:661–670. doi: 10.1016/0306-4522(94)00550-o. [DOI] [PubMed] [Google Scholar]
- 31.Dwivedi Y, Rizavi HS, Conley RR, Roberts RC, Tamminga CA, Pandey GN. Altered gene expression of brain-derived neurotrophic factor and receptor tyrosine kinase B in postmortem brain of suicide subjects. Arch Gen Psychiatry. 2003;60:804–815. doi: 10.1001/archpsyc.60.8.804. [DOI] [PubMed] [Google Scholar]
- 32.Dwivedi Y, Mondal AC, Rizavi HS, Conley RR. Suicide brain is associated with decreased expression of neurotrophins. Biol Psychiatry. 2005;58:315–324. doi: 10.1016/j.biopsych.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 33.Karege F, Perret G, Bondolfi G, Schwald M, Bertschy G, Aubry JM. Decreased serum brain-derived neurotrophic factor levels in major depressed patients. Psychiatry Res. 2002;109:143–148. doi: 10.1016/s0165-1781(02)00005-7. [DOI] [PubMed] [Google Scholar]
- 34.Tsai P. Predictors of distress and depression in elders with arthritic pain. J of AdvNursing. 2005;51:158–165. doi: 10.1111/j.1365-2648.2005.03481.x. [DOI] [PubMed] [Google Scholar]
- 35.Kim YK, Lee HP, Won SD, Park EY, Lee HY, Lee BH, et al. Low plasma BDNF is associated with suicidal behavior in major depression. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:78–85. doi: 10.1016/j.pnpbp.2006.06.024. [DOI] [PubMed] [Google Scholar]
- 36.Harrison PJ, Heath PR, Eastwood SL, Burnet PW, McDonald B, Pearson RC. The relative importance of premortem acidosis and postmortem interval for human brain gene expression studies: selective mRNA vulnerability and comparison with their encoded proteins. Neurosci Lett. 1995;200:151–154. doi: 10.1016/0304-3940(95)12102-a. [DOI] [PubMed] [Google Scholar]
- 37.Salzman S, Endicott J, Clayton P, Winokur G. Diagnostic Evaluation After Death (DEAD) National Institute of Mental Health, Neuroscience Research Branch; Rockville, MD: 1983. [Google Scholar]
- 38.Spitzer RL, Williams JBW, Gibbon M, First MD. Structural Clinical Interview for DSM-IV (SCID) New York State Psychiatric Institute Biometrics Research; New York, NY: 1995. [Google Scholar]
- 39.Dwivedi Y, Rizavi HS, Roberts RC, Conley RC, Tamminga CA, Pandey GN. Reduced activation and expression of ERK1/2 MAP kinase in the postmortem brain of depressed suicide subjects. J Neurochem. 2001;77:916–928. doi: 10.1046/j.1471-4159.2001.00300.x. [DOI] [PubMed] [Google Scholar]
- 40.Dwivedi Y, Rizavi H, Conley RR, Roberts RC, Tamminga CA, Pandey GN. mRNA and protein expression of selective alpha subunits of G proteins are abnormal in prefrontal cortex of suicide victims. Neuropsychopharmacology. 2002;27:499–517. doi: 10.1016/S0893-133X(02)00335-4. [DOI] [PubMed] [Google Scholar]
- 41.Mufson EJ, Bothwell M, Hersh LB, Kordower JH. Nerve growth factor receptor immunoreactive profiles in the normal, aged human basal forebrain: colocalization with cholinergic. J Comp Neurol. 1989;285:196–217. doi: 10.1002/cne.902850204. [DOI] [PubMed] [Google Scholar]
- 42.Mufson EJ, Ma SY, Dills J, Cochran EJ, Leurgans S, Wuu J, et al. Loss of basal forebrain P75(NTR) immunoreactivity in subjects with mild cognitive impairment and Alzheimer’s disease. J Comp Neurol. 2002;443:136–153. doi: 10.1002/cne.10122. [DOI] [PubMed] [Google Scholar]
- 43.Counts SE, Mufson EJ. The role of nerve growth factor receptors in cholinergic basal forebrain degeneration in prodromal Alzheimer disease. J Neuropathol Exp Neurol. 2005;64:263–72. doi: 10.1093/jnen/64.4.263. [DOI] [PubMed] [Google Scholar]
- 44.Miller FD, Kaplan DR. Neurotrophin signaling pathways regulating neuronal apoptosis. Cell Mol Life Sci. 2001;58:1045–1053. doi: 10.1007/PL00000919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lei L, Parada LF. Transcriptional regulation of Trk family neurotrophin receptors. Cell Mol Life Sci. 2007;64:522–523. doi: 10.1007/s00018-006-6328-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Meldolesi J, Sciorati C, Clementi E. The p75 receptor: first insights into the transduction mechanisms leading to either cell death or survival. Trends Pharmacol Sci. 2000;21:242–243. doi: 10.1016/s0165-6147(00)01497-8. [DOI] [PubMed] [Google Scholar]
- 47.Verdi JM, Birren SJ, Ibanez CF, Persson H, Kaplin DR, Benedetti M, et al. p75LNGFR regulates Trk signal transduction and NGF-induced neuronal differentiation in MAH cells. Neuron. 1994;12:733–745. doi: 10.1016/0896-6273(94)90327-1. [DOI] [PubMed] [Google Scholar]
- 48.Ip NY, Stitt TN, Tapley P, Klein R, Glass DJ, Fandl J, Greene LA, et al. Similarities and differences in the way neurotrophins interact with the Trk receptors in neuronal and nonneuronal cells. Neuron. 1993;10:137–149. doi: 10.1016/0896-6273(93)90306-c. [DOI] [PubMed] [Google Scholar]
- 49.Baker PA, Shooter EM. Disruption of NGF binding to the low affinity neurotrophin receptor p75 LNTR reduces NGF binding to TrkA on PC12 cells. Neuron. 1994;13:203–215. doi: 10.1016/0896-6273(94)90470-7. [DOI] [PubMed] [Google Scholar]
- 50.Mahadeo D, Kaplan D, Chao MV, Hempstead BL. High affinity nerve growth factor binding displays a faster rate of association than p140trk binding: implications for multi-subunit polypeptide receptors. J Biol Chem. 1994;269:6884–6891. [PubMed] [Google Scholar]
- 51.Twiss JL, Wada HG, Fok KS, Chan SDH, Verity AN, Baxter GT, et al. Duration and magnitude of nerve growth factor signaling depend on the ratio of p75LNTR to TrkA. J Neurosci Res. 1998;51:442–453. doi: 10.1002/(SICI)1097-4547(19980215)51:4<442::AID-JNR4>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 52.Clary DO, Weskamp G, Austin LA, Reichardt LF. TrkA cross-linking mimics neuronal responses to nerve growth factor. Mol Biol Cell. 1994;5:549–563. doi: 10.1091/mbc.5.5.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mischel PS, Smith SG, Vining ER, Valletta JS, Mobley WC, Reichardt LF. The extracellular domain of p75NTR is necessary to inhibit neurotrophin-3 signaling through TrkA. J Bio Chem. 2001;276:11294–11301. doi: 10.1074/jbc.M005132200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brennen T, Martinussen M, Hansen BO, Hjemdal O. Arctic cognition: a study of cognitive performance in summer and winter at 69 degrees N. Appl Cogn Psychol. 1999;13:561–580. doi: 10.1002/(SICI)1099-0720(199912)13:6<561::AID-ACP661>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 55.Rabizadeh S, Oh J, Zhong L, Yang J, Bitler CM, Butcher LL. Induction of apoptosis by the low-affinity NGF receptor. Science. 1993;261:345–358. doi: 10.1126/science.8332899. [DOI] [PubMed] [Google Scholar]
- 56.Friedman WJ, Grossman J. Neurotrophins induce death of cultured hippocampal neurons. Soc Neurosci Abstr. 1999;25:767. [Google Scholar]
- 57.Friedman W. Neurotrophins induce death of hippocampal neurons via the p75 receptor. J Neurosci. 2000;28:6340–6346. doi: 10.1523/JNEUROSCI.20-17-06340.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Casaccia-Bonnefil P, Carter BD, Dobrowsky RT, Chao MV. Death of oligodendrocytes mediated by the interaction of nerve growth factor with its receptor p75. Nature. 1996;383:716–719. doi: 10.1038/383716a0. [DOI] [PubMed] [Google Scholar]
- 59.Van der Zee CEE, Ross GM, Ripelle RJ, Hagg T. Survival of cholinergic forebrain neurons in developing p75 NGFR-deficient mice. Science. 1996;274:1729–1732. doi: 10.1126/science.274.5293.1729. [DOI] [PubMed] [Google Scholar]
- 60.Roux PP, Colicos MA, Barker PA, Kennedy TE. p75 neurotrophin receptor expression is induced in apoptotic neurons after seizure. J Neurosci. 1999;19:6887–6896. doi: 10.1523/JNEUROSCI.19-16-06887.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.George MS, Ketter TA, Post RM. Prefrontal cortex dysfunction in clinical depression. Depression. 1994;2:59–72. [Google Scholar]
- 62.Sweatt JD. Hippocampal function in cognition. Psychopharmacology. 2004;174:99–110. doi: 10.1007/s00213-004-1795-9. [DOI] [PubMed] [Google Scholar]
- 63.Sala M, Perez J, Soloff P, Ucelli di Nemi S, Caverzasi E, Sores JC, et al. Stress and hippocampal abnormalities in psychiatric disorders. Eur Neuropsychopharmacol. 2004;14:393–405. doi: 10.1016/j.euroneuro.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 64.Clayton PJ. Suicide. Psychiatr Clin North Am. 1985;8:203–214. [PubMed] [Google Scholar]
- 65.Monk M. Epidemiology of suicide. Epidemiol Rev. 1987;9:51–69. doi: 10.1093/oxfordjournals.epirev.a036308. [DOI] [PubMed] [Google Scholar]
- 66.Benes FM, Kwok EW, Vincent SL, Todtankopf MS. A reduction of nonpyramidal cells in sector CA2 of schizophrenics and manic depressives. Biol Psychiatry. 1998;15:88–97. doi: 10.1016/s0006-3223(98)00138-3. [DOI] [PubMed] [Google Scholar]
- 67.Drevets WC, Price JL, Simpson JR, Jr, Todd RD, Reich T, Vannier M, et al. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386:824–827. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- 68.MacQueen GM, Campbell S, McEwen BS, Macdonald K, Amano S, Joffe RT, et al. Course of illness, hippocampal function, and hippocampal volume in major depression. Proc Natl Acad Sci USA. 2003;100:1387–1392. doi: 10.1073/pnas.0337481100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Miguel-Hidalgo JJ, Rajkowska G. Morphological brain changes in depression: can antidepressants reverse them? CNS Drugs. 2002;16:361–372. doi: 10.2165/00023210-200216060-00001. [DOI] [PubMed] [Google Scholar]
- 70.Sheline YI, Gado MH, Kraemer HC. Untreated depression and hippocampal volume loss. Am J Psychiatry. 2003;160:1516–1518. doi: 10.1176/appi.ajp.160.8.1516. [DOI] [PubMed] [Google Scholar]
- 71.McEwen BS. Stress and hippocampal plasticity. Ann Rev Neurosci. 1999;22:105–122. doi: 10.1146/annurev.neuro.22.1.105. [DOI] [PubMed] [Google Scholar]
- 72.Sapolsky RM. Why stress is bad for your brain. Science. 1996;273:749–750. doi: 10.1126/science.273.5276.749. [DOI] [PubMed] [Google Scholar]
- 73.Altschuler DL, Casanova MF, Goldberg TE, Kleinman JE. The hippocampus and parahippocampus in schizophrenia, suicide and control brains. Arch Gen Psychiatry. 1990;47:1029–1034. doi: 10.1001/archpsyc.1990.01810230045008. [DOI] [PubMed] [Google Scholar]
- 74.Rajkowska G. Morphometric methods for studying the prefrontal cortex in suicide victims and psychiatric patients. Ann NY Acad Sci USA. 1997;836:253–268. doi: 10.1111/j.1749-6632.1997.tb52364.x. [DOI] [PubMed] [Google Scholar]
- 75.Zakharenko SS, Patterson SL, Dragatsis I, Zeitlin SO, Siegelbaum SA, Kandel ER, et al. Presynaptic BDNF required for a presynaptic but not postsynaptic component of LTP at hippocampal CA1-CA3 synapses. Neuron. 2003;39:957–990. doi: 10.1016/s0896-6273(03)00543-9. [DOI] [PubMed] [Google Scholar]
- 76.Greferath U, Bennie A, Kourakis A, Barrett GL. Impaired spatial learning in aged rats is associated with loss of p75-positive neurons in the basal forebrain. Neurosci. 2000;100:363–370. doi: 10.1016/s0306-4522(00)00260-8. [DOI] [PubMed] [Google Scholar]
- 77.Hennigan A, Trotter C, Kelly AM. Lipopolysaccharide impairs long-term potentiation and recognition memory and increases p75NTR expression in the rat dentate gyrus. Brain Res. 2007;1130:158–166. doi: 10.1016/j.brainres.2006.10.066. [DOI] [PubMed] [Google Scholar]
- 78.Saarelainen T, Hendolin P, Lucas G, Koponen E, Sairanen M, MacDonald E, et al. Activation of the TrkB neurotrophin receptor is induced by antidepressant drugs and is required for antidepressant-induced behavioral effects. J Neurosci. 2003;23:349–357. doi: 10.1523/JNEUROSCI.23-01-00349.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lopez JF, Vazquez DM, Chalmers DT, Watson SJ. Regulation of 5-HT receptors and the hypothalamic-pituitary-adrenal axis. Implications for the neurobiology of suicide. Ann N Y Acad Sci. 1997;836:106–34. doi: 10.1111/j.1749-6632.1997.tb52357.x. [DOI] [PubMed] [Google Scholar]
- 80.Arato M, Banki CM, Bissette G, Nemeroff CB. Elevated CSF CRH in suicide victims. Biol Psychiatry. 1989;25:355–359. doi: 10.1016/0006-3223(89)90183-2. [DOI] [PubMed] [Google Scholar]
- 81.Nemeroff CB, Owens MJ, Bissette G. Reduced corticotropin releasing factor binding sites in the frontal cortex of suicide victims. Arch Gen Psychiatry. 1988;45:577–579. doi: 10.1001/archpsyc.1988.01800300075009. [DOI] [PubMed] [Google Scholar]
- 82.Kunugi H, Hashimoto R, Yoshida M, Tatsumi M, Kamijima K. A missense polymorphism (S205L) of the low-affinity neurotrophin receptor p75NTR gene is associated with depressive disorder and attempted suicide. Am J Med Gene Part B (Neuropsych Genet) 2004;129B:44–46. doi: 10.1002/ajmg.b.30062. [DOI] [PubMed] [Google Scholar]
- 83.Mukai J, Hachiya T, Shoji-Hoshino S, Kimura MT, Nadano D, Suvanto P, et al. NADE, a p75NTR -associated cell death executor, is involved in signal transduction mediated by the common neurotrophin receptor p75NTR. J Biol Chem. 2000;275:17566–17570. doi: 10.1074/jbc.C000140200. [DOI] [PubMed] [Google Scholar]
- 84.Salehi AH, Roux PP, Kubu CJ, Zeindler C, Bhakar C, Tannis LL, et al. NRAGE, a novel MAGE protein, interacts with the p75 neurotrophin receptor and facilitates nerve growth factor-dependent apoptosis. Neuron. 2000;27:279–288. doi: 10.1016/s0896-6273(00)00036-2. [DOI] [PubMed] [Google Scholar]
- 85.Jordan BW, Dinev D, LeMellay V, Troppmair J, Gotz R, Wixler L, et al. Neurotrophin receptor-interacting mage homologue is an inducible inhibitor of apoptosis protein-interacting protein that augments cell death. J Biol Chem. 2001;276:39985–39989. doi: 10.1074/jbc.C100171200. [DOI] [PubMed] [Google Scholar]
- 86.Whitfield J, Neame SJ, Paquet L, Bernard O, Ham J. Dominant-negative c-Jun promotes neuronal survival by reducing BIM expression and inhibiting mitochondrial cytochrome c release. Neuron. 2001;29:629–643. doi: 10.1016/s0896-6273(01)00239-2. [DOI] [PubMed] [Google Scholar]
- 87.Yoon SO, Cassaccia-Bonnefil P, Carter B, Chao MV. Competitive signaling between TrkA and p75 nerve growth factor receptors determines cell survival. J Neurosci. 1998;18:3273–3281. doi: 10.1523/JNEUROSCI.18-09-03273.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mazzoni IE, Said FA, Aloyz R, Miller FD, Kaplan D. Ras regulates sympathetic neuron survival by suppressing the p53-mediated cell death pathway. J Neurosci. 1999;19:9716–9727. doi: 10.1523/JNEUROSCI.19-22-09716.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dobrowsky RT, Jenkins GM, Hannun YA. Neurotrophins induce sphingomyelin hydrolysis-modulation by co-expression of p75NTR with trk receptors. J Biol Chem. 1995;270:22135–22142. doi: 10.1074/jbc.270.38.22135. [DOI] [PubMed] [Google Scholar]
- 90.Bilderback TR, Gazula VR, Dobrowsky RT. Phosphoinositide 3-kinase crosstalk between Trk A tyrosine kinase and p75NTR dependent sphingolipid signaling pathways. Neurochem. 2001;76:1540–1551. doi: 10.1046/j.1471-4159.2001.00171.x. [DOI] [PubMed] [Google Scholar]
- 91.Müller G, Storz P, Bourteele S, Döppler H, Pfizenmaier K, Mischak H. Regulation of Raf-1 kinase by TNF via its second messenger ceramide and cross-talk with mitogenic signalling. EMBO J. 1998;17:732–742. doi: 10.1093/emboj/17.3.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhou H, Summers SA, Birnbaum MJ, Pittman RN. Inhibition of Akt kinase by cell-permeable ceramide and its implications for ceramide-induced apoptosis. J Biol Chem. 1998;273:16568–16575. doi: 10.1074/jbc.273.26.16568. [DOI] [PubMed] [Google Scholar]
- 93.Zundel W, Schindler C, Haas-Kogan D, Koong A, Kaper F, Chen E, et al. Loss of PTEN facilitates HIF-1-mediated gene expression. Genes & Dev. 2000;14:391–396. [PMC free article] [PubMed] [Google Scholar]
- 94.Dwivedi Y, Rizavi HS, Conley RR, Pandey GN. ERK MAP kinase signaling in post-mortem brain of suicide subjects: differential regulation of upstream Raf kinases Raf-1 and B-Raf. Mol Psychiatry. 2006;11:86–98. doi: 10.1038/sj.mp.4001744. [DOI] [PubMed] [Google Scholar]
- 95.Dwivedi Y, Rizavi HS, Teppen T, Zhang H, Mondal A, Roberts RC, et al. Lower phosphoinositide 3-kinase (PI 3-kinase) activity and differential expression levels of selective catalytic and regulatory PI 3-kinase subunit isoforms in prefrontal cortex and hippocampus of suicide subjects. Neuropsychopharmacol. 2008 doi: 10.1038/sj.npp.1301641. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 96.Neves-Pereira M, Mundo E, Muglia P, King N, Macciardi F, Kennedy JL. The brain-derived neurotrophic factor gene confers susceptibility to bipolar disorder: evidence from a family-based association study. Am J Hum Genet. 2002;71:651–655. doi: 10.1086/342288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sklar P, Gabriel SB, McInnis MG, Bennett P, Lim YM, Tsan G, et al. Family-based association study of 76 candidate genes in bipolar disorder: BDNF is a potential risk locus. Brain-derived neutrophic factor. Mol Psychiatry. 2002;7:579–593. doi: 10.1038/sj.mp.4001058. [DOI] [PubMed] [Google Scholar]
- 98.Nakata K, Ujike H, Sakai A, Uchida N, Nomura A, Imamura T, et al. Association study of the brain-derived neurotrophic factor (BDNF) gene with bipolar disorder. Neurosci Lett. 2003;337:17–20. doi: 10.1016/s0304-3940(02)01292-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.