Abstract
The novel wake-promoting agent modafinil has been in use for the treatment of several sleep disorders for a few years and is now undergoing clinical trials for its use in the treatment of stimulant addiction, but its primary mechanism of action remains elusive. Previous laboratory studies have shown that modafinil has antioxidative and neuroprotective effects, which have not previously been suggested to be related to its wake-promoting effects. However, recent research indicates that free radicals may be related to sleep induction as well as cellular damage, suggesting that a common target of action may mediate modafinil’s ability to oppose both of these effects. In this review we summarize and discuss previously published research on modafinil’s neural, cytoprotective, and cognitive effects, and we propose possible primary biochemical targets that could underlie the effects of modafinil observed in these studies. We also suggest neurocognitive mechanisms responsible for modafinil’s cognitive enhancing effects and its therapeutic potential in the treatment of stimulant addiction.
Keywords: modafinil, sleep, stimulant, neuroprotective, addiction treatment, free radicals
Introduction
In 1998 a unique drug for the treatment of narcolepsy was approved by the Food and Drug Administration for the narcolepsy armamentarium. Despite several years of pre-clinical research, the mechanism of action of modafinil was unknown. Almost a decade later there is a plethora of evidence showing that it is effective for treating several sleep disorders (Ballon and Feifel 2006), and there are ongoing clinical trials for its use in fatigue, cocaine addiction, attention deficit disorder, depression, seasonal affective disorder, bipolar depression, nicotine addiction, and schizophrenia. Some preclinical evidence also indicates a possible use in the treatment of neurodegenerative diseases. Most research on modafinil’s wake-promoting mechanism has focused on monoaminergic effects showing modafinil stimulates histamine (HA), norepinephrine (NE), serotonin (5-HT), dopamine (DA), and orexin systems in the brain, but researchers have not been able to isolate a single site of action or locate major receptor binding. Modafinil’s mechanism of action (MOA) remains elusive as pointed out in a recent editorial on modafinil entitled, “Modafinil: a drug in search of a mechanism” (Saper and Scammell 2004). There has also been research into the neuroprotective actions of modafinil, which we propose to be related to its alerting effects. We selectively review a number of preclinical and clinical papers relevant to modafinil’s MOA. We conclude with contemplations of MOA, particularly as it pertains to modafinil’s effects in addictive disorders.
Modafinil preclinical studies
General medicine studies
Mignot et al (1994) published one of the first searches to find a receptor to which modafinil was shown to have binding a affinity using binding assays for the following receptors and binding sites: adenosine, dopamine, GABA, serotonin, NMDA, kainite, quisqualate, glycine, benzo-diazepine, phencyclidine, MK-801, angiotensin, Arg-vasopressin, bombesin, cholecystokinin, neuropeptide Y, substance K, substance P, neurotensin, somatostatin, vasoactive intestinal peptide, atrial natriuretic factor 1, epidermal growth factor, nerve growth factor, calcium channels, chloride channels, low conduction K+ channels, and second messenger systems; and the following uptake channels: adenosine, choline, GABA, dopamine, norepinephrine, and serotonin. It was found that modafinil was weakly selective for the dopamine transporter, binding to this cell-membrane protein and not at all to any other receptors tested. They were skeptical that modafinil might act by blocking this transporter, and they pointed out that modafinil has more potent behavioral effects than some molecules that bind with a much greater affinity to the dopamine reuptake transporter.
Simon et al (1995) compared the locomotor effects of modafinil with dexamphetamine in rodents in conjunction with the D2 antagonist haloperidol, the D1 antagonist SCH 23390, alpha-methyl-para-tyrosine, the anti-monoaminergic agent reserpine, and L-DOPA-benserazide. They found that while behavioral effects of amphetamine could be suppressed by haloperidol, SCH 23390, or alpha-methyl-para-tyrosine, modafinil’s behavioral effects were not blocked by these agents at most doses. The administration of a very high dose of SCH 23390 was able to reduce the locomotor effects of modafinil. Amphetamine was able to reverse the akinesia induced by the anti-monoaminergic agent reserpine, while modafinil showed no significant locomotor effect in reserpine-treated animals. A final in vitro study of dopaminergic synaptosomes showed that while amphetamine caused spontaneous dopamine release, modafinil had no such effect.
Tanganelli et al (1995) looked at modafinil’s effects on cortical GABA and monoamine levels through post mortem analysis using high performance liquid chromatography in the brains guinea pigs and rats sacrificed shortly after drug administration. Some were lesioned with the neurotoxin 5,7-dihydroxytryptamine (selective for serotonin neurons) and given the α1 receptor antagonist prazosin. They found that modafinil by itself decreased cortical GABA, but in rats treated with 5,7-dihydroxytryptamine modafinil increased cortical GABA, indicating that modafinil decreases cortical GABA through a serotonin mediated pathway. They also noted that the administration of prazosin in conjunction with 5,7-dihydroxytryptamine could block the increase in GABA, showing that modafinil increases cortical GABA through a norepinephrine mediated pathway. To examine the direct effects of modafinil on GABA uptake and release they administered modafinil to rat brain slices and found that modafinil did not directly affect GABA uptake, GABA release, or glutamate decarboxylase activity.
Lin et al (1996) examined fos immunoreactivity in 26 brain sites of cats after the administration of amphetamine, methylphenidate, or modafinil. They found that modafinil induced very little fos-like immunoreactivity in the cortex, but it did induce fos labeling in the anterior hypothalamus and nearby areas, in contrast to amphetamine and methylphenidate. They also noted no fos labeling in the basal forebrain, thalamus, posterior hypothalamus, or the midbrain tegmentum as a result of modafinil administration.
Ferraro et al (1996) in the first of a series of papers about modafinil’s actions showed using in vivo microdialysis in rats that modafinil decreases GABA in the medial preoptic area of the hypothalamus and the posterior hypothalamus. They found that the 5-HT3 receptor antagonist MDL72222 alone was able to attenuate this effect almost as much as the general serotonin antagonist methysergide, indicating that modafinil worked to decrease GABA partly through a serotonergic pathway mediated primarily by the 5-HT3 receptor.
Bettendorf et al (1996) used high performance liquid chromatography to study cortical glutamate and GABA levels of sacrificed rats after modafinil-induced paradoxical sleep deprivation and non-pharmacological paradoxical sleep deprivation using the platform method, in which the paralysis of REM sleep causes rats to make contact with water and awaken. They found that modafinil did not increase cortical glutamate levels in 2 or in 7 hours of sleep deprivation. They also found that non-pharmacologic sleep deprivation did not increase cortical glutamate in a similar time period (5 hours), but it did increase cortical glutamate after 12 and 24 hours (there were no reports of data collected from modafinil-treated mice after 12 or 24 hours of sleep deprivation). These results suggested that modafinil does not increase cortical glutamate in the first few hours after administration, and modafinil appears to affect cortical glutamate levels no differently than non-pharmacological sleep deprivation in the first few hours.
Ferraro et al (1997b) examined the in vivo dopamine and GABA levels of the nucleus accumbens in rats given modafinil, and they found that modafinil had a very minor effect on nucleus accumbens dopamine, but it led to a substantial reduction in GABA release. That same year, this group published another paper which they described an experiment examining GABA and glutamate in the thalamus and hippocampus, finding that modafinil increased glutamate in these brain areas, but did not alter GABA levels in these locations (Ferraro et al 1997a).
Edgar and Seidel (1997) investigated the effects of modafinil on sleep-wake EEG and locomotor activity in live rats in comparison with the effects of methamphetamine. They found that modafinil increased locomotor activity only slightly unlike methamphetamine which induced profound increases in locomotor activity. They also found that modafinil and methamphetamine increased wake time, but modafinil produced more consolidated periods of wakefulness, and modafinil did not cause rebound hypersomnolence as opposed to methamphetamine. From these results they suggested that modafinil is more effective in inhibiting the sleep drive than methamphetamine.
Ferraro et al (1998) studied the effects of modafinil on GABA in the striatum, pallidum, and substantia nigra of conscious rats. They found that modafinil reduced GABA in the pallidum and striatum, but not in the substantia nigra. They also found that modafinil does not increase glutamate except in the substantia nigra at very high doses. They concluded that via GABA reductions, modafinil is able to improve motor activity.
Engber et al (1998) measured glucose utilization with 2-deoxyglucose autoradiography in the brains of rats given modafinil, and they found that modafinil increased glucose utilization in the thalamus, hippocampus, subiculum, and the amygdala, but they noted that much of the glucose utilization in the brain may be in the mitochondria of axons and dendrites rather than cell somas.
Ferraro et al (1999) using in vivo microdialysis and post mortem high performance liquid chromatography found that modafinil increases extracellular glutamate in the medial preoptic and posterior areas of the hypothalamus, but the local application of the GABAA receptor antagonist bicuculline, which raised basal glutamate levels, prevented a further increase in glutamate from modafinil. Administration of the glutamate uptake blocker L-trans-PDC with modafinil was also done, which showed that even after extracellular glutamate levels had been increased by glutamate transport blockade, modafinil was still able to increase extracellular glutamate. These results suggested to the researchers that a reduction in the GABAergic tone of the medial preoptic area and of the posterior hypothalamus mediates modafinil’s glutamatergic effect in these areas.
Perez de la Mora et al (1999), seeking to find the manner in which modafinil could change glutamate and GABA levels of the hypothalamus, studied the effect of modafinil on glutamate and GABA synthesis in ex vivo and in vitro slices of the rat hypothalamus, by measuring tritium incorporation into glutamate and GABA and found no effect of modafinil on the synthesis of these neurotransmitters.
Sebban et al published 2 studies in 1999 using eletroen-cephalography in live rats to test modafinil in conjunction with the general dopamine receptor antagonist clozapine or the selective D2 antagonist raclopride. They found that modafinil bolstered the EEG synchronization caused by raclopride, and it was able to attenuate in both cortices the synchronizing effects of clozapine, which has an α1 adrenergic receptor antagonist properties. However, modafinil by itself causes decreased power of the frequencies in the 6–18 Hz range of the EEG. The α1 agonist cirazoline displayed a very different effect on the EEG spectral powers decreasing power at 3, 5–6, 8, and 13 Hz and increasing power at 1–2 and 19–30 Hz (Sebban et al 1999a, b).
Chemelli et al (1999) examined fos-reactivity in orexin neurons of mice given modafinil before sacrifice and found a substantially greater activation of orexin neurons with modafinil than with placebo.
Scammell et al (2000) administered modafinil to live rats, sacrificed them two hours later, and analyzed the brain slices using immunohistochemistry. They found fos reactivity in the tuberomamillary nucleus and in orexin neurons.
Ferraro et al (2000) studied cortical serotonin release in vivo and vitro in rat brains. They found that modafinil is able to enhance serotonin release, but it does not cause serotonin release or reuptake on its own and suggested that modafinil increased electrosecretory coupling in neurons.
Wisor et al (2001) measured behavioral effects of narcoleptic dogs and of dopamine transporter knockout rats using EEG, EMG, wheel running (for the rats), and in vivo microdialysis in the caudate nucleus (in the dogs). They found that modafinil increased dopamine in the caudate and promoted arousal in the absence of orexin receptors, but modafinil had little effect in dopamine transporter-null rats, who without modafinil already spent substantially more time awake and a little more time wheel running than normal mice.
de Saint Hilaire et al (2001) measured arousal with EEG and local brain monoaminergic levels using microdialysis in the prefrontal cortex and the ventromedial preoptic area of the hypothalamus in rats given modafinil. They found that cortical 5-HT, DA, and NE increased in the hour following modafinil administration, and 5-HT remained high for several hours. In the hypothalamus only NE release was enhanced by modafinil.
Ferraro et al (2001) measured tritiated serotonin efflux from modafinil in vitro on serontonergic synaptosomes and cortical slices and found that modafinil was not able to increase spontaneous 5-HT efflux or K+-evoked 5-HT efflux in synaptosomes, but modafinil was able to increase electrically evoked 5-HT efflux in cortical slices, and this effect was enhanced by serotonin uptake blockade.
Stone et al (2002) showed that the α1A adrenergic receptor antagonist WB4101 and the α1D antagonist BMY7378 had little effect on the increase in motor activity caused by modafinil, but terazosin, which blocks α1A, α1D, and α1B receptors significantly attenuated this effect. Furthermore, modafinil had very small effects on gross movement in α1B receptor knockout mice. Together these results suggest that the α1B adrenergic receptor mediates modafinil’s locomotor effects. They point to a previous study suggesting that α1B relates to movement but is not antisedative, so this pathway is involved in the motor but not the wake-promoting effects of modafinil.
Stone et al (2002) also reported the effects of stress on modafinil’s stimulation of increased gross movement in live rats, some of whom were pretreated with corticosterone or dexamethasone. They noted that stress decreased overall gross movement, an effect attenuated by corticosterone pre-treatment, and stress also decreased the modafinil induced boost in gross movement. However, pretreatment with corticosterone or dexamethasone mitigated the impact of stress on modafinil’s movement effects. The authors comment that these results support the hypothesis that stress desensitizes or inhibits α1 adrenoreceptors and corticosterone pretreatment attenuates this effect, though the exact mechanism of this effect was not clear.
Ferraro et al (2002) measured serotonin levels in rats using in vivo microdialysis in the: frontal cortical, amygdaloid, dorsal raphe, medial preoptic, and posterior hypothalamic areas, and they found that modafinil stimulates the serotonergic system of the cortex, DRN, and amygdala at low doses, but only at high doses did it promote 5-HT release in the hypothalamus.
Ishizuka et al (2003) measured brain histamine release using microdialysis in vivo in rats given modafinil intraperitoneally, intraventricullarlry, or directly into the tuberomamillary nucleus (TMN) and found that modafinil had no effect on HA when administered directly into the TMN neurons, and had the fastest effect on histamine when given ip, indicating that modafinil did not directly target the TMN.
Gallopin et al (2004) recorded VLPO neuron electro-physiology in vivo in rats given modafinil in conjunction with monoamines, clonidine, L-phenylephrine, yohimbine, nisoxetine, carbachol, CNQX, AP-5, bicuculline, or TTX. They found that modafinil promoted wakefulness by inhibiting the VLPO and this was dependent upon noradrenergic inhibition of VLPO neurons via an α2 adrenergic receptor.
Della Marca et al (2004) studied sensory evoked potentials in humans given modafinil and found that modafinil changed the subcortical electrophysiological oscillatory pattern in sensory evoked potentials. They concluded that the cortical effects of modafinil are the result of reduced GABA transmission in the cortex.
Willie et al (2005) studied the effects of modafinil in rats congenitally missing both alleles for orexin and noted that modafinil was actually able to promote wakefulness better in these rats than in wild-type litter mates, but it was not able to promote alertness as well in the orexin-null rats as in wild-type mice. Modafinil was also unable to reduce the number of direct transitions to REM sleep in the orexin-null mice. These results indicate that the orexinergic system is involved in modafinil’s stimulant effects, but it is not the primary center of action or the only pathway through which modafinil works.
Wisor and Eriksson (2005) studied the effects of modafinil in conditions of altered dopamine and norepinephrine levels. They found that DSP-4 administration, which eliminates neuron projections bearing norepinephrine transporters, did not hinder the wake-promoting effects of modafinil in rats, but the α1 adrenergic antagonist terazosin was able to prevent the effects of modafinil in DSP-4 treated mice. They also found that the dopamine autoreceptor agonist quinpirole attenuated the effects of modafinil in DSP-4 treated mice, indicating a role for dopamine in modafinil’s wake-promoting effects. As such, the authors suggested that modafinil worked through an increase in dopamine tone and dopamine’s stimulation of the α1 adrenergic receptor.
Hou et al (2005) studied the autonomic effects of modafinil in humans. They found that modafinil affects the locus coeruleus, which mediates pupil diameter and arousal, but it does not affect other autonomic functions, which are controlled by noreadrenergic control centers (A1 – A5) located outside of the locus coeruleus.
Ferraro et al (2005) studied the effects of modafinil in vivo in rats and found that by itself it did not increase serotonin transmission, but it did cause an increase in effects of classic serotonin uptake inhibitors given at sub threshold doses.
Madras et al (2006) in a recent paper demonstrated in vivo binding of modafinil to striatal DAT and thalamic NET in rhesus monkeys using PET imaging. The investigators compared binding of the DAT probe [11C]CFT and the NET probe [11C]MeNER in the absence of modafinil with the binding of these probes in the presence of modafinil to calculate modafinil’s occupancy of DAT and NET in vivo. Finding that modafinil occupied these sites, the investigators examined modafinil’s effects compared with those of methylphenidate and benztropine on DAT and NET transporters in vitro. They found that modafinil was a weak inhibtor of the NET and that modafinil’s ability to effect DA reuptake via the DAT was about a one-hundredth that of methylphenidate and about a tenth that of benztropine. The authors conclude that while modafinil probably exerts its effects via more than one mechanism, modafinil’s occupancy of the DAT probably plays a role in its pharmacological effects that should be further investigated.
Discussion of sleep and modafinil’s neurotransmitter effects
Theories regarding the physiology of sleep in recent years have focused on a two-process model of sleep in which the sleep/wake system is governed by both a circadian process affected by exposure to light and a homeostatic process affected by physiologic demand for sleep (Pace-Schott and Hobson 2002). The effect of sleep deprivation to increase the sleep drive is mediated by the homeostatic process, which appears to be largely controlled by the basal forebrain. This region of the brain contains excitatory cholinergic cortical projections and inhibitory GABAergic projections to the sleep-promoting VLPO (Strecker et al 2000; Markov and Goldman 2006). This process is also believed to be regulated by the inhibitory neuromodulator adenosine, which increases during wakefulness and produces sleep pressure by decreasing basal forebrain activity resulting in a disinhibition of VLPO activity and a decrease in ascending cholinergic tone. For reviews see Saper et al (2001), Mignot et al (2002), Pace-Schott and Hobson (2002), Markov and Goldman (2006).
Though it is not fully known which processes cause an animal to be awake or asleep, research has shown that a number of systems are characteristically active during wakefulness and therefore suspected to play a role in maintenance of vigilance. The monoaminergic system, especially, has received attentention for its activity in the sleep wake cycle. It has been observed that histamine, serotonin, and norepinephrine tone is directly related to arousal state, and that neurons releasing these chemicals are almost silent in REM sleep. Relatively recently the peptide orexin was discovered in neurons of the lateral hypothalamus and subsequently shown to play an important role in the maintenance of vigilance (Jones 2005).
Modafinil has shown the ability to increase HA, NE, 5-HT, and DA levels in the brain (Ferraro et al 2000; de Saint Hilaire et al 2001; Ferraro et al 2002; Madras et al 2006), but modafinil almost certainly exerts some of these effects in part via an indirect mechanism or an upstream site of action. Though the recent paper by Madras and colleagues indicates that modafinil has some physiologically significant effect on the DAT and possibly the NET, not all of modafinil’s effect are likely mediated by this particular mechanism, as the investigators themselves suggest. In vitro studies indicate that modafinil does not directly stimulate 5-HT release, but it does enhance 5-HT tone from neurons or synaptosomes stimulated via other methods (Ferraro et al 2000; Ferraro et al 2001; Ferraro et al 2005). In vivo studies show anatomically selective neurochemical effects of modafinil on monoaminergic systems (de Saint Hilaire et al 2001; Ferraro et al 2002), and, notably, while modafinil increases TMN fos expression (Scammell et al 2000) and HAergic tone it is not able to exert this effect when administered directly into the TMN (Ishizuka et al 2003). Additionally, despite the importance of orexin in the maintenance of vigilance, modafinil is capable of promoting wakefulness in the absence of an orexin receptors or orexinergic neurons (Wisor et al 2001; Willie et al 2005).
Modafinil’s effects on glutamate appear to be quite varied by brain region. It was shown that modafinil increased extracellular glutamate in the medial preoptic and posterior hypothalamus and that this effect was due to the reduction in GABAergic tone mentioned previously (Ferraro et al 1996, 1999). In the thalamus and hippocampus modafinil also appeared to increase glutamate levels, but here it did not alter GABA tone (Ferraro et al 1997a). On the other hand it was observed that modafinil did not significantly increase glutamate in the substantia nigra (except at very high doses), in the striatum, or in the pallidum (Ferraro et al 1998). The effect of modafinil on cortical glutamate is unclear, as it has been reported that modafinil increases cortical glutamate and that modafinil does not significantly increase cortical glutamate (Pierard et al 1995; Bettendorf et al 1996). The possibility that modafinil alters GABA and glutamate synthesis rates was explored as possible explanation of modafinil’s effects, and modafinil exhibited no observable effect on these pathways (Perez de la Mora et al 1999).
Modafinil’s effects on GABA appear to be more consistent across brain regions than its effects on glutamate. Modafinil does not appear to have much effect on GABA in the thalamus or hippocampus, but GABA levels were reduced by modafinil in most brain regions studied: the cortex, medial preoptic area of the hypothalamus, posterior hypothalamus, nucleus accumbens, pallidum, and striatum, and this effect generally appears to be mediated by serotonin (Tanganelli et al 1995; Ferraro et al 1996, 1997a, b, 1998a, b, 1999). Interestingly, in one of these studies (Tanganelli et al 1995) destruction of serotonin neurons with a selective neurotoxin, did not simply block modafinil’s GABA inhibiting effects but caused modafinil to increase cortical GABA. It appears that in this study the GABAergic neurons were strongly inhibited by a serotonergic mechanism and weakly stimulated via a noradrenergic pathway. If modafinil enhances neurotransmitter release via increased electrosecretory coupling, then it would be expected that modafinil would enhance GABA release upon removal of the serotonergic inhibitory influence.
Neuroprotective effects of modafinil
Pierard et al (1995) measured the in vivo cortical pool of glutamate-glutamine, aspartate, inositol, and creatine-phosphocreatine using 2D COSY H-NMR. They found that modafinil increased the cortical pool of all of these substances and attributed modafinil’s neuroprotective effects to its ability to increase creatine-phosphocreatine and its wake-promoting actions to the resultant increased metabolic activation.
Antonelli et al (1998) tested modafinil’s neuroprotective effect with regard to glutamate cytotoxicity by measuring GABA release and GABA uptake in cultured rat cortical neurons. They found that unlike glutamate receptor antagonists, modafinil was unable to fully prevent initial reductions in GABA release, but modafinil was able to prevent the further reduction in GABA release over the following half hour that was seen in the cells exposed to glutamate but not modafinil. Modafinil also had no effect on GABA release or uptake in neurons not exposed to glutamate, indicating that modafinil does not simply stimulate additional GABA release; rather it may help cells recover their neurosecretory coupling mechanism after glutamate exposure.
Jenner et al (2000) looked at the neuroprotective and anti-parkinsonian effects of modafinil in monkeys treated with MPTP. In one study they found that the MPTP induced parkinsonism symptoms could be improved with modafinil 11 months after MPTP administration. In a second study they found that modafinil administration with MPTP was unable to prevent initial locomotor effects of MPTP, but was able to restore locomotor activity within two weeks. More nigral neurons survived when modafinil was administered in conjunction with MPTP. They concluded that modafinil stimulates locomotor effects in already injured animals, and modafinil is neuroprotective, but it does not effectively block the DA transporter, for it is not able to prevent the initial effects of MPTP which enters the cell through the dopamine transporter to cause damage.
Xiao et al (2004) used post mortem examination of the brains of MPTP treated mice. They found that modafinil reduced striatal GABA, increased the levels of reduced glutathione in MPTP damaged neurons, and reduced levels of the lipid peroxidation product malodialdehyde. These results suggest that modafinil exerts a neuroprotective effect through its ability to attenuate or prevent oxidative damage.
Discussion of modafinil’s neuroprotective effects
In addition to modafinil showing potent effects on the sleep/wake system, it is clear that modafinil has noteworthy neuroprotective effects as well that involve some sort of antioxidative process. While these effects may be coincidental to modafinil’s wake-promoting effects, the role of the ATP breakdown product adenosine in homeostatic sleep regulation is at least suggestive that modafinil’s neuroprotective effects are not irrelevant to the consideration of modafinil’s wake-promoting effects. Because the primary site of action of modafinil’s antioxidant effects remains elusive, we discuss some possible targets for future investigation here.
It is clearly a possibility that modafinil could directly act on enzymes in the brain’s free-radical scavenging system (eg, glutathione peroxidase or superoxide dismutase) to directly reduce free-radical levels. Because, reactive oxygen species feed back positively on the mitochondrion to reduce ATP production and possibly enhance free radical production (Echtay et al 2002; Brookes et al 2004), such a mechanism could also account for modafinil’s ability to increase the cortical creatine-phosphocreatine pool (Pierard et al 1995). It would be worth examining whether other known free-radical reducing compounds have a similar effect on the creatine pool of the brain.
The mitochondrion is the biggest producer of reactive oxygen species in the cell, and as such modafinil may target this organelle to directly inhibit free-radical production and promote ATP production, which would tend to promote increases in creatine-phosphocreatine production. One good candidate for a site of action of modafinil in the mitochondrion is cytochrome c or an enzyme that reacts with it. Cytochrome c functions in the mitochondrial electron transport chain normally to move electrons from complex III to complex IV to make water, but it is also capable of being released from the inner mitochondrial membrane and accepting electrons from hydrogen peroxide in the intermembrane space or superoxide generated by complex I (see Skulachev [1998] for review). Modafinil may enhance cytochrome c’s ability to accept and donate electrons by allosteric modification or a catalytic mechanism. Such a mechanism would directly reduce net hydrogen peroxide levels and superoxide production and increase ATP production. The ability to accept electrons from superoxide at complex I would provide a direct mechanism for modafinil’s ability to reduce MPTP-induced neuron death, which appears to be mediated by promoting superoxide production in complex I and inhibiting its normal activity. This mechanism would also involve reduced activity of the inhibitory KATP-channels that suppress neurotransmitter release and thereby account for increased neurotransmitter release.
Also noteworthy is the action of modafinil on other cytochromes, particularly those of the cytochrome P450 system, which is responsible for drug metabolism in the liver and appears to have a role in the brain (McFadyen et al 1998; Klose et al 1999; Voirol et al 2000; Gervasini et al 2001; Llerena et al 2003; Gervasini et al 2004). Modafinil inhibits CYP2C19, and is a potent suppressor in hepatocytes of CYP2C9 (Robertson et al 2000), which itself has not yet been found to be present in the brain, but other cytochrome P450 enzymes including CYP2C enzymes have been found in the brain, and there is evidence for a role of brain CYP 2C9 specifically (Llerena et al 2003; Gervasini et al 2004). This particular member of the cytochrome P450 family has been shown to be a functionally relevant source of reactive oxygen species in coronary artery ischemia and reperfusion injury, and inhibition of cytochrome P450 enzymes has been shown to reduce damage in coronary artery ischemia and reperfusion (Fleming et al 2001; Granville et al 2004). As such CYP2C9 would likely produce physiologically relevant levels of reactive oxygen species in the brain if it is located there. It has also been proposed that CYP2C enzymes are involved in the metabolism of arachidonic acid in the brain and in altering the effects of neurotransmitters (Gervasini et al 2004), and the potential importance of CYP2C9 activity in brain function is further supported by the observation that CYP2C9 genotypes may affect a person’s susceptibility to major depressive disorder (Llerena et al 2003). From these studies it is clear that modafinil’s effect on cytochrome P450 enzymes in the brain, especially CYP2C9, which modafinil is already known to suppress, is worthy of further study.
Modafinil’s suppression of brain CYP2C9 could explain modafinil’s ability to reduce reactive oxygen species production. There is also the question of how modafinil would suppress or inhibit CYP2C9 activity in the brain. It is possible that modafinil could work through a direct intracellular site of action to suppress CYP2C9, but it should also be mentioned that serotonin, which modafinil has been shown to enhance or require the release of (Tanganelli et al 1995; Ferraro et al 1996, 2000, 2001, 2005), and epinephrine are inhibitors of CYP2C9 activity in hepatocytes (Gervasini et al 2001). Therefore, modafinil could intracellularly inhibit CYP2C9 in the brain, thereby reducing reactive oxygen species levels and promoting better mitochondrial function. This could enhance serotonin release through greater availability of metabolic substrates, which would further inhibit CYP2C9, and modafinil would exert its powerful wakening effects through this positive feedback loop potentiating its antioxidative and serotonergic effects. We chose to focus specifically on a potential mechanism of modafinil involving CYP2C9 because of the tested cytochrome P450 enzymes, modafinil has been shown to have the greatest effect on this particular enzyme (Robertson et al 2000), but this does not rule out the possibility of an effect mediated by other P450 enzymes.
Anatomically specific regions of activation rather than neurochemical effects of modafinil have also been explored in some studies (Lin et al 1996; Engber et al 1998; Chemelli et al 1999; Scammell et al 2000), but a particular brain region of action for modafinil has not yet been determined. The anti-oxidative basis of modafinil’s stimulant effects proposed here would likely act in neurons throughout the brain, but there may be particular brain regions where this anti-oxidative effect most strongly exerts its wake-promoting influence. The basal forebrain is perhaps such a region, for it is here particularly that adenosine exerts its sleep promoting effects (Porkka-Heiskanen et al 1997; Alam et al 1999; Porkka-Heiskanen et al 2000; Strecker et al 2000). Adenosine appears to be an endogenous sleep factor that increases while awake and induces sleepiness as its levels increase (Huston et al 1996; Strecker et al 2000), and the sleep-inducing effects of free radicals have been attributed at least in part to the consequent increases in extracellular adenosine (Ikeda et al 2005). As such, modafinil may play an antioxidant role throughout the entire brain and modulate adenosine levels throughout the entire brain, but it is in the basal forebrain that a reduction in adenosine resulting from reduced reactive oxygen species concentrations would have its greatest wake-promoting effects. In a previous study it was shown that modafinil does not show fos-immunoreactivity in the basal forebrain (Lin et al 1996), and this is consistent with reduced levels of the inhibitory neuromodulator adenosine in this region of the brain, for adenosine increases c-fos expression in the basal forebrain (Basheer et al 1999).
Modafinil human neurocognitive studies
EEG studies
EEG band definitions can vary somewhat among studies, and research indicates that alpha bands vary among individuals and with age. These EEG band definitions are specific to humans and are different in lower mammals (Klimesch 1999).
Delta: 1–4 Hz
Theta: 4–7 Hz
-
Alpha: 7.5–12.5 Hz
Alpha 1: 7.5–10 Hz
Alpha 2: 10–12.5 Hz
Beta: 13–20 Hz
The sources, functions, and behavior of alpha and theta rhythms have been the subject of much theoretical and empirical research, but the detailed mechanics of these observed findings remain far from being understood or agreed upon by researchers (Sadato et al 1998; Klimesch 1999; Liley et al 1999; Cantero et al 2000; Nunez 2000; Nunez et al 2001). Alpha and theta EEG bands are probably the most extensively researched EEG spectrums in humans, and regardless of the confusion over the physiological brain events underlying these rhythms a few phenomenological properties of alpha and theta EEG rhythms have been well established. The alpha band power is the prominent EEG band of the normal awake human resting EEG and diminishes in amplitude with drowsiness and sleep onset (see Klimesch [1999] and Nunez et al [2001] for reviews). Theta rhythms also exhibit resting differences corresponding to arousal level, showing increased synchrony in states of decreased vigilance and diminished cognitive performance (Paus et al 1997; Smit et al 2004). Upon mental exertion (as opposed to resting conditions) alpha rhythms desynchronize (reduce power), and theta rhythms synchronize, and it is thought that the magnitude of these changes is positively correlated with amount of mental exertion required of an individual in completing a mental task (Gevins et al 1997, 1998). It has been shown that more intelligent individuals display less alpha desynchronization in novel tasks than less gifted individuals, supporting the Neural Efficiency Hypothesis, which states that more efficient information processing in the brains of more intelligent subjects results in the need for less mental effort than their average counterparts in solving the same problem (Jausovec 1996, 2000; Neubauer et al 2002; Grabner et al 2003). It has also been observed that in human adults intelligence is positively correlated with EEG alpha power in a simple awake resting condition (Jausovec 1996, 2000; Doppelmayr et al 2002).
Caldwell et al (2000) studied the effects of modafinil in six helicopter pilots kept awake for two 40-hour periods; in one period they received three 200-mg doses of modafinil, and in the other they received placebo. Modafinil treatment kept flight simulation performance near baseline, while flight simulation performance in the placebo condition was decreased by roughly 10%–20%. Modafinil also showed decreased power in the delta and theta EEG bands under modafinil versus placebo. There was little reported effect from modafinil on alpha and beta band powers.
Chapotot et al (2003) studied the EEG effects of modafinil and d-amphetamine compared to placebo on 64 hours of sustained wakefulness in 41 healthy volunteers. It was found that both modafinil and d-amphetamine decreased power in the delta and theta 2 bands. D-amphetamine was shown to decrease power in the alpha 1 band, and modafinil was shown to increase power in the alpha 1 band.
Saletu and colleagues published two papers examining EEG differences in narcoleptics and normal controls and the effects of modafinil on local EEG differences of narcoleptics in a double-blind, placebo-controlled, crossover trial. Both studies compared EEG spectral power differences for 16 narcoleptics and 16 normal controls in resting EEG. The second part of both studies involved placing the narcoleptic patients in a double-blind, placebo-controlled, crossover study of modafinil consisting of two treatment periods each of three weeks separated by a 1-week washout phase and a measurement of EEG activity at the beginning and end of each treatment phase. Vigilant EEG was measured in the first study but showed few differences between any of the groups, so it was not measured in the second study. The resting EEG, however, did show differences in the alpha 2, beta 1, beta 2, and beta 3 bands in both studies, with normal controls showing greater power in these bands than the narcoleptic patients, and the modafinil-treated narcoleptic group showing greater power in these bands than the placebo-treated group. These results indicate that narcolepsy causes decreased alpha and beta activity, and modafinil increases the activity seen in these bands (Saletu et al 2004, 2005).
Functional magnetic resonance imaging studies
Spence et al (2005) examined the acute effects of 100 mg of modafinil on short term memory and cerebral blood flow (with fMRI) in 17 medication controlled schizophrenic patients using a double-blind, placebo-controlled, crossover design consisting of 2 trial days separated by one week. Subjects identified the numbers 1–4 by pushing 1 of 4 buttons, and color coding told subjects whether they should identify the number currently on the screen or the number on the screen 2 numbers previously. They found that anterior cingulate activation increased in most subjects, and working memory improved in a minority of subjects, but no subjects with reduced anterior cingulated activation demonstrated improved working memory. A post-hoc analysis of the data also showed that those who improved on modafinil had low baseline scores. These results indicated to the authors that low dose modafinil may have an anterior cingulate cortex mediated effect on working memory in impaired schizophrenics with particular characteristics.
Ellis et al (1999) used fMRI to examine auditory and visual cortical activation levels in 12 normal subjects and in 12 narcoleptic subjects (not exposed to amphetamine for at least 4 days) and the effects of 400 mg of modafinil versus placebo in these two groups. They found no significant differences in mean group cortical activations for narcoleptic subjects versus normal subjects. After administering modafinil to 8 subjects in each group and placebo to 4 in each group and waiting 13.25 or 18.25 hours, they observed cortical activation in response to visual and auditory stimuli with fMRI again. They found no significant change in the mean activation due to modafinil or placebo, but they found a strong negative correlation (auditory r = −0.74; visual r = −0.76) between cortical activation before modafinil and cortical activation after modafinil for individual subjects. The fact that modafinil increased cortical activation in subjects with low cortical activation and decreased it in subjects with high cortical activation indicates that its effects are not unilateral but are a function of baseline cortical activation and its effects are modulatory and regulatory rather than augmentative.
Discussion of neurocognitive studies
The tendency of modafinil to increase alpha power and decrease theta power (Caldwell et al 2000; Chapotot et al 2003; Saletu et al 2004, 2005) in human subjects is both consistent with modafinil’s stimulant properties and suggestive that modafinil improves brain function, an effect shown in the helicopter pilot study (Caldwell et al 2000) and in the cognitive performance studies discussed below.
None of the studies regarding EEG changes from modafinil that we found measured modafinil’s effects on event-related EEG changes in instances of mental exertion, but modafinil’s resting EEG profile and stimulant properties do suggest that it would enhance mental performance, at least in individuals in the condition of sleep-deprivation, a common factor in stimulant abusers. A number of studies testing modafinil’s effects on neurocognitive functioning tend to confirm that modafinil mildly enhances cognitive performance in healthy volunteers, especially with regards to executive function. These results are summarized in Tables 1–3. There were two studies published by Randall et al that showed little or no significant effect of modafinil on neurocognitive test performance in healthy individuals (Randall et al 2003, 2004), but a later review done by this group on their own research showed that modafinil did improve neurocognitive performance in average IQ subjects but not high IQ subjects (Randall et al 2005). The authors concluded that this indicates that modafinil has limited cognitive enhancing effects in already high-performing well-rested individuals, but they did not consider ceiling effects in neurocognitive tests designed to measure cognitive impairment as some of the other studies did (Turner et al 2003; Muller et al 2004).
Table 1.
Summary of modafinil effects
| Neurocognitive aspects | + Accuracy | − Accuracy | + Speed | − Speed | No effect | May help in cocaine abuse |
|---|---|---|---|---|---|---|
| Working memory | 1,2,8,9 | 11b | 1,8,9,11 | 1,2,4,6,10c,12 | ||
| Memory | 1,2,7 | 2 | 1,9 | 1,3,4,8,10c,11 | ||
| Attention/impule control | 1,5a,6,7,9b,11,13,14 | 4,5a,7,9b,11,14b | 1,9 | 1,2,3,4,10c,11 | ■ | |
| Reasoning, learning, high level function | 8,9,14b | 4 | 1,3,4,6,10c,11 | ■ |
1) Turner et al (2003), 2) Muller et al (2004), 3) Randall et al (2003), 4) Randall et al (2004), 5) Randall et al (2005), 6) Walsh et al (2004), 7) Hart et al (2005), 8) Turner et al (2004b), 9) Turner et al (2004a), 10) Sevy et al (2005), 11) Randall et al (2005), 12) Chan et al (2006), 13) Gill et al (2006), 14) Killgore et al (2006).
improvement for lower IQ subjects,
400 mg dose showed effect,
Schizophrenic subjects controlled with atypical antipsychotics.
Table 3.
Modafinil’s effects on working memory and memory
| Neurocognitive measures | Modafinil effect | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Test name | Abbrev | Working memory | Attention/impulse control | Memory | Frontal lobe function | Speed | + Accuracy | −Accuracy | + Speed | − Speed | No Effect |
| Working memory tests | 1,2,8,9 | 11b | 1,8,9,11 | 1,2,4,6,10c,2 | |||||||
| Digit Span | ■ | 1,8,9 | 12 | ||||||||
| Spatial Working memory | SWM | ■ | 1 | ||||||||
| Tower of London | TOL | ■ | ■ | 1 | 11b | 1,8,9,11 | 3,4 | ||||
| Numeric Working Memory | NWM | ■ | 2 | ||||||||
| Letter Number Sequencing | LNS | ■ | 6,10c | ||||||||
| Occulomotor Delayed Response | ODR | ■ | 10° | ||||||||
| MemoryTests | 1,2,7 | 2 | 1,9 | 1,3,4,8,10c,1 | |||||||
| Digit Recall | DRT | ■ | 7 | ||||||||
| Repeated Acquisition | RAT | ■ | 7 | ||||||||
| Pattern Recognition Memory | PRM | ■ | 1 | 8 | |||||||
| Paired Associates Learning | PAL | ■ | |||||||||
| Delayed Matching to Sample | DMS | ■ | ■ | 2 | 2 | 1,9 | 3,10c,11 | ||||
| Spatial Span | SSP | ■ | 1 | ||||||||
| Visuospatial Delayed Matching | VSD | ■ | |||||||||
| Logical memory | LMT | ■ | 3,4,11 | ||||||||
| Digit Symbol Substitution | DSS | ■ | 7 | 6 | |||||||
1) Turner et al (2003), 2) Muller et al (2004), 3) Randall et al (2003), 4) Randall et al (2004), 5) Randall et al (2005), 6) Walsh et al (2004), 7) Hart et al (2005), 8) Turner et al (2004b), 9) Turner et al (2004a), 10) Sevy et al (2005), 11) Randall et al (2005), 12) Chan et al (2006), 13) Gill et al (2006), 14) Killgore et al (2006)
improvement for lower IQ subjects,
400 mg dose showed effect,
Schizophrenic subjects controlled with atypical antipsychotics.
The effects of modafinil on response latency as well as accuracy are also particularly telling. Modafinil showed increased response latency in some cases, especially in TOL spatial planning task (Turner et al 2003, 2004a, b; Randall et al 2005), and modafinil generally caused decreased response latency in tests of attention and impulse control and improvements in tests of attention (Randall et al 2004, 2005a, b; Turner et al 2004a; Walsh et al 2004; Hart et al 2005; Gill et al 2006; Killgore et al 2006). Only one of the studies showing slowed response time in the TOL also showed an accuracy improvement due to modafinil in this task (Turner et al 2003), but this may be due to ceiling effects as mentioned previously. These results indicate that modafinil promotes impulse control and improves attention. Both of these effects are of value in stimulant abuse and addiction treatment. In all tasks in which a study showed that modafinil increased speed of response, there was an observed increase in accuracy by at least one (possibly different) study and no observed decreases in accuracy, with the exception of the Stroop test for which total errors were near zero or equal to zero for all groups in the data shown. This shows that modafinil did not increase speed of response at the cost of accuracy, but it increased accuracy while reducing information processing and response time, and this suggests that modafinil may also enhance neural efficiency.
It should also be noted that a number of studies examined the effects of modafinil in patients with underlying neurocognitive health deficits and found no significant effects in these populations. A double-blind, placebo-controlled trial testing the cognitive enhancing effects of 100 mg modafinil in 10 medication stabilized schizophrenic patients versus placebo in 10 other medication stabilized schizophrenic patients showed almost no effect of modafinil (Sevy et al 2005). Two small independent studies of fatigued patients showed mixed neurocognitive effects of modafinil and an inability of subjects to reliably distinguish between modafinil and placebo (Randall et al 2005a; Chan et al 2006). All of these studies had major limitations, especially small sample size, and the 100 mg dose used in the study by Sevy et al may have been too low to have any effect. Nevertheless, future research endeavors may wish to investigate if there is a physiologic reason for the relative lack of effect of modafinil in these patient populations.
Although only one study with significant limitations tested the effects of modafinil on humor appreciation (Killgore et al 2006), this topic deserves particular attention, because humor appreciation is a very complex neural task requiring frontal lobe function and integrative information processing between numerous cortical and subcortical brain regions (Shammi and Stuss 1999; Goel and Dolan 2001; Mobbs et al 2003; Moran et al 2004). This test compared the effects of modafinil to caffeine and amphetamine in not only humor appreciation, but also PVT performance and Stanford Sleepiness Test Score. While the modafinil group had only the second best PVT scores and the worst Stanford Sleepiness Test scores, they had the best humor appreciation scores. This suggests that modafinil’s mechanism is not limited to actions on wake-promoting brain regions, because caffeine and amphetamine must have stimulated those regions even more potently in this study than modafinil while producing less effect on humor appreciation. The results of this study combined with studies of the brain regions mediating humor (Shammi and Stuss 1999; Goel and Dolan 2001; Mobbs et al 2003; Moran et al 2004) provide further support to the idea that modafinil improves whole-brain function.
We found only two neuroimaging studies examining the effects of modafinil (Ellis et al 1999; Spence et al 2005) both of which used BOLD fMRI to observe event-related circulatory changes in the brain. These two studies are very different in their procedure and population, but they both showed that modafinil appears to modulate rather than unilaterally alter event-related cortical blood flow changes, for in both studies modafinil’s effect on event-related cortical blood flow changes is negatively correlated to baseline event-related cortical blood flow change. Notably, the study involving schizophrenic subjects measured event related changes in a working memory task, while the study comparing narcoleptic and normal subjects measured event-related changes during sensory stimulation. Modafinil’s effects on regional activation appear to be dependent on baseline activation in both paradigms, increasing BOLD signal in those with low baseline event related BOLD changes and decreasing BOLD signal in those with high baseline event related BOLD changes. In contrast to this, the stimulant amphetamine simply increases blood flow changes in cortical activation (Uftring et al 2001). Thus, these studies provide further evidence that modafinil’s stimulant properties are the result of enhanced whole brain function rather than localized neural excitation.
Unexplored mechanisms of modafinil
The current body of research presented above appears to be focused on investigating only extracellular localized sites of action for modafinil in the brain, despite the fact that there is little evidence that modafinil’s primary mechanism of action would be limited to an extracellular site or a particular single brain region. In fact many of these studies provide evidence to the contrary, showing that modafinil does not act on the extracellular targets that would be most plausible in mediating the effects of modafinil in the diseases and conditions studied. There are, however, a few studies that investigate effects of modafinil on processes that are possibly or even likely intracellularly mediated, and in each of these studies an effect due to modafinil is found (Pierard et al 1995; Antonelli et al 1998; Ferraro et al 2000, 2001; Jenner et al 2000; Xiao et al 2004). Though an extracellular mechanism of action cannot be ruled out, these studies taken together suggest that perhaps modafinil targets an intracellular protein or receptor rather than an extracellular site.
A number of plausible but uninvestigated sites of action for modafinil, both intracellular and extracellular, remain to be studied to explain its stimulant effects and its neuroprotective effects. While modafinil has been shown to have no binding affinity to a number of ion channels (Mignot et al 1994), we found no reports examining modafinil’s affinity for sodium channels or P/Q or R calcium channels. Modafinil’s ability to enhance neurotransmitter release without actually stimulating neurons has led to the suggestion of enhanced neuroelectrosecretory coupling as a mechanism of modafinil (Ferraro et al 2000), and the ion channels above have a potential here as a direct target of the action of modafinil. Altered depolarization requirements of neurons via changes in sodium homeostasis, or enhanced calcium influx could explain increased neurotransmitter release (which is calcium dependent) when a neuron is stimulated.
It is also worth noting that while modafinil is chiefly thought of as a stimulant, it has clearly demonstrated both wake-promoting and neuroprotective effects in preclinical studies, yet no previous papers to our knowledge have reported any attempt to integrate these findings or to find a common site of action that could mediate both of these effects. If modafinil works through either of the first two mechanisms mentioned above (ie, via alterations in sodium or calcium channel function), this could explain modafinil’s stimulant effects, but these mechanisms do not lend themselves well to explaining its neuroprotective effects. The neuroprotective and wake-promoting effects may be the result of different mechanisms of action, but recent research shows that sleep induction and neurodegeneration may have common or related pathways, which would indicate the potential for a single site of action to be responsible for a drug’s ability to inhibit both processes.
It has been suspected for a long time, and it is generally agreed now that cellular mitochondria, calcium homeostasis, and oxidative stress play important roles in neurodegeneration. Research also suggests that oxidative stress and neural metabolic function, such as the availability of high energy metabolic substrates including creatine, are important mediators of arousal state and cognitive functions (McMorris et al 2006). A report showing that reactive oxygen species increased adenosine levels and induced slow-wave sleep suggests that sleep may function in part to allow the reactive oxygen species scavenging system to restore neurochemical redox states (Ikeda et al 2005). There has also been research showing that neurons of the neocortex and substantia nigra have ATP-sensitive potassium channels (KATP-channels) that suppress neuron firing and neurotransmitter release in states of reduced ATP or elevated H2O2. The effect of these channels on neuron firing rate in nigral dopamine neurons is such that administration of the KATP-channel antagonist glibenclamide at a 100 nM concentration was able to increase neuron firing rate by 34% (Garcia de Arriba et al 1999; Avshalumov et al 2005). KATP-channel activity also appears to be increased by extracellular adenosine via adenosine A1 receptor stimulation (Heurteaux et al 1995). Therefore, enhanced mitochondrial ATP production, reduced production of H2O2, or reduced reactive oxygen species production would be expected to increase neurotransmitter release upon neuron stimulation via reduction in KATP-channel activity.
Any mechanism involving improved mitochondrial function or free-radical scavenging could, therefore, explain how modafinil enhances neurocognitive function and bolsters serotonin release without stimulating serotonin release on its own (Ferraro et al 2000, 2001, 2005). While no antioxidant or mitochondrial effects of modafinil have been reported in the context of its ability to promote wakefulness or enhance neurotransmitter release, it has been shown that modafinil does have an antioxidant effect that appears to mediate its neuroprotective actions in MPTP-induced neurodegeneration (Xiao et al 2004). The site of action mediating this effect has not yet been elucidated, and there are a number of plausible intracellular targets which we explore here that would explain both modafinil’s stimulant effects, neuroprotective effects, and perhaps its effects as a therapeutic tool in addiction.
In summary, the bulk of research into modafinil’s wake-promoting mechanism has focused mostly on possible extracellular activities of modafinil. We propose that more work be done on examining potential intracellular mechanisms of modafinil and finding a point of convergence of modafinil’s stimulant and neuroprotective effects. It is likely that modafinil both enhances cellular metabolism and reduces free-radicals in neurons (Pierard et al 1995; Xiao et al 2004). Reduction in brain oxidation or an increase in cortical creatine could promote vigilance (Ikeda et al 2005; McMorris et al 2006), and each effect can increase neurotransmitter release by reducing inhibitory KATP-channel activity. Thus, through any disruption in the positive feedback loop of increased free-radical production and reduced ATP production modafinil could potentially exert its neuroprotective and wake-promoting effects.
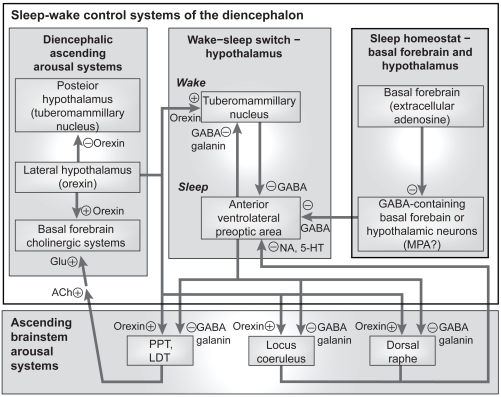
Figure 1.
Simplified sleep circuit. Reprinted with permission from Pace-Schott E, Hobson JA. 2002. Nat Rev Neurosci, 3:591–605. Copyright © 2002 Macmillan Publishers Ltd.
Table 2.
Neurocognitive improvements useful in the treatment of addiction
| Neurocognitive measures | Modafinil effect | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Test name | Abbrev | Working Memory | Attention/Impulse Control | Memory | Frontal lobe function | Speed | + Accuracy | − Accuracy | + Speed | − Speed | No effect |
| Attention and impulse control tasks | 1,5a,6,7,9b, 11,13,14 | 4,5a,7,9b, 11,14b | 1,9 | 1,2,3,4, 10c,11 | |||||||
| Divided Attention | DAT | ■ | ■ | 7 | 7 | ||||||
| Repeated Information | RIT | ■ | 7 | ||||||||
| Stop Signal | Stop | ■ | ■ | 1,9b | 9b | 1 | 8 | ||||
| Gamble | Gamble | ■ | 1,9 | ||||||||
| Continuous Performance | CPT | ■ | 5a,11b,13 | 11 | 1,3,4,10c | ||||||
| d2 | d2 | ■ | 2 | ||||||||
| Trail Making | TMT | ■ | 2,3,4 | ||||||||
| Stroop | Stroop | ■ | ■ | 4,5a | 3,11 | ||||||
| Psychomotor Vigilance | PVT | ■ | 6,14 | 14b | |||||||
| Auditory Learning | ALT | ■ | 10c | ||||||||
| Reasoning, learning, and higher level function | 8,9,14b | 4 | 1,3,4,6, 10c,11 | ||||||||
| Intra Extra Dimensional Set Shift | IED | ■ | 8,9 | 4 | 1,3,4 | ||||||
| Controlled Oral Word Association | COWAT | ■ | 3,4,10c,11 | ||||||||
| Torrance Test of Creative Thinking | TTCT | ■ | 6 | ||||||||
| Wisconsin Card Sorting | WCST | ■ | 6 | ||||||||
| Anagram | ANG | ■ | 6 | ||||||||
| Word Fluency | WFT | ■ | 6 | ||||||||
| Category | CAT | ■ | 6 | ||||||||
| Sentence Completion | SCT | ■ | 6 | ||||||||
| University of Pennsylvania Humor Appreciation Test | HAT | ■ | 14b | ||||||||
1) Turner et al (2003), 2) Muller et al (2004), 3) Randall et al (2003), 4) Randall et al. (2004), 5) Randall et al (2005), 6) Walsh et al (2004), 7) Hart et al (2005), 8) Turner et al (2004b), 9) Turner et al (2004a), 10) Sevy et al (2005), 11) Randall et al (2005), 12) Chan et al (2006), 13) Gill et al (2006), 14) Killgore et al (2006)
improvement for lower IQ subjects,
400 mg dose showed effect,
Schizophrenic subjects controlled with atypical antipsychotics.
Acknowledgments
We would like to thank Dr. Steven LaRowe and Dr. Patrick Mulholland for helpful consultation in the preparation of this manuscript and Evans Jenkins for technical assistance.
References
- Alam MN, Szymusiak R, et al. Adenosinergic modulation of rat basal forebrain neurons during sleep and waking:neuronal recording with microdialysis. J Physiol. 1999;521:679–90. doi: 10.1111/j.1469-7793.1999.00679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonelli T, Ferraro L, et al. Modafinil prevents glutamate cytotoxicity in cultured cortical neurons. Neuroreport. 1998;9:4209–13. doi: 10.1097/00001756-199812210-00038. [DOI] [PubMed] [Google Scholar]
- Avshalumov MV, Chen BT, et al. Endogenous hydrogen peroxide regulates the excitability of midbrain dopamine neurons via ATP-sensitive potassium channels. J Neurosci. 2005;25:4222–31. doi: 10.1523/JNEUROSCI.4701-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballon JS, Feifel D. A systematic review of modafinil: potential clinical uses and mechanisms of action. J Clin Psychiatry. 2006;67:554–66. doi: 10.4088/jcp.v67n0406. [DOI] [PubMed] [Google Scholar]
- Basheer R, Porkka-Heiskanen T, et al. Adenosine and behavioral state control:adenosine increases C-Fos protein and AP-1 binding in basal forebrain neurons. Brain Res Mol Brain Res. 1999;73:1–10. doi: 10.1016/s0169-328x(99)00219-3. [DOI] [PubMed] [Google Scholar]
- Bettendorf L, Sallanon-Moulin M, et al. Paradoxical sleep deprivation increases the content of glutamate and glutamine in rat cerebral cortex. Sleep. 1996;19:65–71. doi: 10.1093/sleep/19.1.65. [DOI] [PubMed] [Google Scholar]
- Brookes PS, Yoon Y, et al. Calcium, ATP, and ROS: a mitochondrial love-hate triangle. Am J Physiol Cell Physiol. 2004;287:C817–33. doi: 10.1152/ajpcell.00139.2004. [DOI] [PubMed] [Google Scholar]
- Caldwell JA, Caldwell JL, et al. A double-blind, placebo-controlled investigation of the efficacy of modafinil for sustaining the alertness and performance of aviators:a helicopter simulator study. Psychopharmacology (Berl) 2000;150:272–82. doi: 10.1007/s002130000450. [DOI] [PubMed] [Google Scholar]
- Cantero JL, Atienza M, et al. State-modulation of cortico-cortical connections underlying normal EEG alpha variants. Physiol Behav. 2000;71:107–15. doi: 10.1016/s0031-9384(00)00334-6. [DOI] [PubMed] [Google Scholar]
- Chan KM, Strohschein FJ, et al. Randomized controlled trial of modafinil for the treatment of fatigue in postpolio patients. Muscle Nerve. 2006;33:138–41. doi: 10.1002/mus.20437. [DOI] [PubMed] [Google Scholar]
- Chapotot F, Pigeau R, et al. Distinctive effects of modafinil and d-amphetamine on the homeostatic and circadian modulation of the human waking EEG. Psychopharmacology (Berl) 2003;166(2):127–138. doi: 10.1007/s00213-002-1315-8. [DOI] [PubMed] [Google Scholar]
- Chemelli RM, Willie JT, et al. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell. 1999;98:437–51. doi: 10.1016/s0092-8674(00)81973-x. [DOI] [PubMed] [Google Scholar]
- de Saint Hilaire Z, Orosco M, et al. Variations in extracellular mono-amines in the prefrontal cortex and medial hypothalamus after modafinil administration:a microdialysis study in rats. Neuroreport. 2001;12:3533–7. doi: 10.1097/00001756-200111160-00032. [DOI] [PubMed] [Google Scholar]
- Della Marca G, Restuccia D, et al. Influence of modafinil on somato-sensory input processing in the human brain-stem. Clin Neurophysiol. 2004;115:919–26. doi: 10.1016/j.clinph.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Doppelmayr M, Klimesch W, et al. EEG alpha power and intelligence. Intelligence. 2002;30:289–302. [Google Scholar]
- Echtay KS, Roussel D, et al. Superoxide activates mitochondrial uncoupling proteins. Nature. 2002;415:96–9. doi: 10.1038/415096a. [DOI] [PubMed] [Google Scholar]
- Edgar DM, Seidel WF. Modafinil induces wakefulness without intensifying motor activity or subsequent rebound hypersomnolence in the rat. J Pharmacol Exp Ther. 1997;283:757–69. [PubMed] [Google Scholar]
- Ellis CM, Monk C, et al. Functional magnetic resonance imaging neuroactivation studies in normal subjects and subjects with the narcoleptic syndrome. Actions of modafinil. J Sleep Res. 1999;8:85–93. doi: 10.1046/j.1365-2869.1999.00142.x. [DOI] [PubMed] [Google Scholar]
- Engber TM, Dennis SA, et al. Brain regional substrates for the actions of the novel wake-promoting agent modafinil in the rat: comparison with amphetamine. Neuroscience. 1998;87:905–11. doi: 10.1016/s0306-4522(98)00015-3. [DOI] [PubMed] [Google Scholar]
- Ferraro L, Antonelli T, et al. The antinarcoleptic drug modafinil increases glutamate release in thalamic areas and hippocampus. Neuroreport. 1997a;8:2883–7. doi: 10.1097/00001756-199709080-00016. [DOI] [PubMed] [Google Scholar]
- Ferraro L, Antonelli T, et al. Modafinil:an antinarcoleptic drug with a different neurochemical profile to d-amphetamine and dopamine uptake blockers. Biol Psychiatry. 1997b;42:1181–3. doi: 10.1016/s0006-3223(97)00353-3. [DOI] [PubMed] [Google Scholar]
- Ferraro L, Antonelli T, et al. The effects of modafinil on striatal, pallidal and nigral GABA and glutamate release in the conscious rat: evidence for a preferential inhibition of striato-pallidal GABA transmission. Neurosci Lett. 1998;253:135–8. doi: 10.1016/s0304-3940(98)00629-6. [DOI] [PubMed] [Google Scholar]
- Ferraro L, Antonelli T, et al. The vigilance promoting drug modafinil increases extracellular glutamate levels in the medial preoptic area and the posterior hypothalamus of the conscious rat: prevention by local GABA A receptor blockade. Neuropsychopharmacology. 1999;20:346–56. doi: 10.1016/S0893-133X(98)00085-2. [DOI] [PubMed] [Google Scholar]
- Ferraro L, Fuxe K, et al. Amplification of cortical serotonin release:a further neurochemical action of the vigilance-promoting drug modafinil. Neuropharmacology. 2000;39:1974–83. doi: 10.1016/s0028-3908(00)00019-8. [DOI] [PubMed] [Google Scholar]
- Ferraro L, Fuxe K, et al. Differential enhancement of dialysate serotonin levels in distinct brain regions of the awake rat by modafinil: possible relevance for wakefulness and depression. J Neurosci Res. 2002;68:107–12. doi: 10.1002/jnr.10196. [DOI] [PubMed] [Google Scholar]
- Ferraro L, Fuxe K, et al. Modafinil enhances the increase of extracellular serotonin levels induced by the antidepressant drugs fluoxetine and imipramine: a dual probe microdialysis study in awake rat. Synapse. 2005;55:230–41. doi: 10.1002/syn.20111. [DOI] [PubMed] [Google Scholar]
- Ferraro L, Tanganelli S, et al. The vigilance promoting drug modafinil decreases GABA release in the medial preoptic area and in the posterior hypothalamus of the awake rat: possible involvement of the serotonergic 5-HT3 receptor. Neurosci Lett. 1996;220:5–8. doi: 10.1016/s0304-3940(96)13212-2. [DOI] [PubMed] [Google Scholar]
- Ferraro L, Tanganelli S, et al. Modafinil does not affect serotonin efflux from rat frontal cortex synaptosomes: comparison with known serotonergic drugs. Brain Res. 2001;894:307–10. doi: 10.1016/s0006-8993(01)02000-5. [DOI] [PubMed] [Google Scholar]
- Fleming I, Michaelis UR, et al. Endothelium-derived hyperpolarizing factor synthase (cytochrome P450 2C9) is a functionally significant source of reactive oxygen species in coronary arteries. Circ Res. 2001;88:44–51. doi: 10.1161/01.res.88.1.44. [DOI] [PubMed] [Google Scholar]
- Gallopin T, Luppi P-H, et al. Effect of the wake-promoting agent modafinil on sleep-promoting neurons from the ventrolateral preoptic nucleus:an in vitro pharmacologic study [see comment] Sleep. 2004;27:19–25. [PubMed] [Google Scholar]
- Garcia de Arriba S, Franke H, et al. Neuroprotection by ATP-dependent potassium channels in rat neocortical brain slices during hypoxia. Neurosci Lett. 1999;273:13–16. doi: 10.1016/s0304-3940(99)00603-5. [DOI] [PubMed] [Google Scholar]
- Gervasini G, Carrillo JA, et al. Potential role of cerebral cytochrome P450 in clinical pharmacokinetics. Clin Pharmacokinet. 2004;43:693–706. doi: 10.2165/00003088-200443110-00001. [DOI] [PubMed] [Google Scholar]
- Gervasini G, Martinez C, et al. Inhibition of cytochrome P450 2C9 activity in vitro by 5-hydroxytryptamine and adrenaline [article] Pharmacogenetics. 2001;11:29–37. doi: 10.1097/00008571-200102000-00004. [DOI] [PubMed] [Google Scholar]
- Gevins A, Smith ME, et al. High-resolution EEG mapping of cortical activation related to working memory: effects of task difficulty, type of processing, and practice. Cereb Cortex. 1997;7:374–85. doi: 10.1093/cercor/7.4.374. [DOI] [PubMed] [Google Scholar]
- Gevins A, Smith ME, et al. Monitoring working memory load during computer-based tasks with EEG pattern recognition methods. Hum Factors. 1998;40:79–91. doi: 10.1518/001872098779480578. [DOI] [PubMed] [Google Scholar]
- Gill M, Haerich P, et al. Cognitive performance following modafinil versus placebo in sleep-deprived emergency physicians: a double-blind randomized crossover study. Acad Emerg Med. 2006;13:158–65. doi: 10.1197/j.aem.2005.08.013. [DOI] [PubMed] [Google Scholar]
- Goel V, Dolan RJ. The functional anatomy of humor:segregating cognitive and affective components. Nat Neurosci. 2001;4:237–8. doi: 10.1038/85076. [DOI] [PubMed] [Google Scholar]
- Grabner RH, Stern E, et al. When intelligence loses its impact: neural efficiency during reasoning in a familiar area. Int J Psychophysiol. 2003;49:89–98. doi: 10.1016/s0167-8760(03)00095-3. [DOI] [PubMed] [Google Scholar]
- Granville DJ, Tashakkor B, et al. Reduction of ischemia and reperfusion-induced myocardial damage by cytochrome P450 inhibitors. Proc Natl Acad Sci USA. 2004;101:1321–6. doi: 10.1073/pnas.0308185100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart CL, Haney M, et al. Modafinil attenuates disruptions in cognitive performance during simulated night-shift work. Neuropsychopharmacology. 2005;31:1526–36. doi: 10.1038/sj.npp.1300991. [DOI] [PubMed] [Google Scholar]
- Heurteaux C, Lauritzen I, et al. Essential role of adenosine, adenosine A1 receptors, and ATP-sensitive K+ channels in cerebral ischemic preconditioning. Proc Natl Acad Sci USA. 1995;92:4666–70. doi: 10.1073/pnas.92.10.4666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou R, Freeman C, et al. Does modafinil activate the locus coeruleus in man? Comparison of modafinil and clonidine on arousal and autonomic functions in human volunteers. Psychopharmacology. 2005;181:537–49. doi: 10.1007/s00213-005-0013-8. [DOI] [PubMed] [Google Scholar]
- Huston JP, Haas HL, et al. Extracellular adenosine levels in neostriatum and hippocampus during rest and activity periods of rats. Neuroscience. 1996;73:99–107. doi: 10.1016/0306-4522(96)00021-8. [DOI] [PubMed] [Google Scholar]
- Ikeda M, Ikeda-Sagara M, et al. Brain oxidation is an initial process in sleep induction. Neuroscience. 2005;130:1029–40. doi: 10.1016/j.neuroscience.2004.09.057. [DOI] [PubMed] [Google Scholar]
- Ishizuka T, Sakamoto Y, et al. Modafinil increases histamine release in the anterior hypothalamus of rats. Neurosci Lett. 2003;339:143–6. doi: 10.1016/s0304-3940(03)00006-5. [DOI] [PubMed] [Google Scholar]
- Jausovec N. Differences in EEG alpha activity related to giftedness. Intelligence. 1996;23:159–73. [Google Scholar]
- Jausovec N. Differences in cognitive processes between gifted, intelligent, creative, and average individuals while solving complex problems: an EEG study. Intelligence. 2000;28:213–37. [Google Scholar]
- Jenner P, Zeng BY, et al. Antiparkinsonian and neuroprotective effects of modafinil in the mptp-treated common marmoset. Exp Brain Res. 2000;133:178–88. doi: 10.1007/s002210000370. [DOI] [PubMed] [Google Scholar]
- Jones BE. From waking to sleeping: neuronal and chemical substrates. Trends Pharmacol Sci. 2005;26:578–86. doi: 10.1016/j.tips.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Killgore WDS, McBride SA, et al. The effects of caffeine, dextroamphetamine, and modafinil on humor appreciation during sleep deprivation. Sleep. 2006;29:841–7. doi: 10.1093/sleep/29.6.841. [DOI] [PubMed] [Google Scholar]
- Klimesch W. EEG alpha and theta oscillations reflect cognitive and memory performance: a review and analysis. Brain Res Brain Res Rev. 1999;29:169–95. doi: 10.1016/s0165-0173(98)00056-3. [DOI] [PubMed] [Google Scholar]
- Klose TS, Blaisdell JA, et al. Gene structure of CYP2C8 and extra-hepatic distribution of the human CYP2Cs. J Biochem Mol Toxicol. 1999;13:289–95. doi: 10.1002/(sici)1099-0461(1999)13:6<289::aid-jbt1>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Liley DTJ, Alexander DM, et al. Alpha rhythm emerges from large-scale netowrks of realistically coupled multicompartmental model of cortical neurons. Network: Computation in Neural Systems. 1999;10:79–92. [PubMed] [Google Scholar]
- Lin JS, Hou Y, et al. Potential brain neuronal targets for amphetamine-, methylphenidate-, and modafinil-induced wakefulness, evidenced by c-fos immunocytochemistry in the cat. Proc Natl Acad Sci USA. 1996;93:14128–33. doi: 10.1073/pnas.93.24.14128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llerena A, Berecz R, et al. CYP2C9 gene and susceptibility to major depressive disorder. Pharmacogenomics J. 2003;3:300–2. doi: 10.1038/sj.tpj.6500197. [DOI] [PubMed] [Google Scholar]
- Madras BK, Xie Z, et al. Modafinil occupies dopamine and norepinephrine transporters in vivo and modulates the transporters and trace amine activity in vitro. J Pharmacol Exp Ther. 2006;319:561–9. doi: 10.1124/jpet.106.106583. [DOI] [PubMed] [Google Scholar]
- Markov D, Goldman M. Normal sleep and circadian rhythms: neurobiologic mechanisms underlying sleep and wakefulness. Psychiatr Clin North Am. 2006;29:841–53. doi: 10.1016/j.psc.2006.09.008. [DOI] [PubMed] [Google Scholar]
- McFadyen MCE, Melvin WT, et al. Regional distribution of individual forms of cytochrome P450 mRNA in normal adult human brain. Biochem Pharmacol. 1998;55:825–30. doi: 10.1016/s0006-2952(97)00516-9. [DOI] [PubMed] [Google Scholar]
- McMorris T, Harris RC, et al. Effect of creatine supplementation and sleep deprivation, with mild exercise, on cognitive and psychomotor performance, mood state, and plasma concentrations of catecholamines and cortisol. Psychopharmacology. 2006;185:93–103. doi: 10.1007/s00213-005-0269-z. [DOI] [PubMed] [Google Scholar]
- Mignot E, Nishino S, et al. Modafinil binds to the dopamine uptake carrier site with low affinity. Sleep. 1994;17:436–7. doi: 10.1093/sleep/17.5.436. [DOI] [PubMed] [Google Scholar]
- Mignot E, Sharhad T, et al. Sleeping with the hypothalamus: emerging therapeutic targets for sleep disorders. Nat Neurosci. 2002;5:1071–5. doi: 10.1038/nn944. [DOI] [PubMed] [Google Scholar]
- Mobbs D, Greicius MD, et al. Humor modulates the mesolimbic reward centers. Neuron. 2003;40:1041–8. doi: 10.1016/s0896-6273(03)00751-7. [DOI] [PubMed] [Google Scholar]
- Moran JM, Wig GS, et al. Neural correlates of humor detection and appreciation. Neuroimage. 2004;21:1055–60. doi: 10.1016/j.neuroimage.2003.10.017. [DOI] [PubMed] [Google Scholar]
- Muller U, Steffenhagen N, et al. Effects of modafinil on working memory processes in humans. Psychopharmacology. 2004;177:161–9. doi: 10.1007/s00213-004-1926-3. [DOI] [PubMed] [Google Scholar]
- Neubauer AC, Fink A, et al. Intelligence and neural efficiency: the influence of task content and sex on the brain-IQ relationship. Intelligence. 2002;30:515–36. doi: 10.1016/j.cogbrainres.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Nunez P. Toward a quantitative description of large-scale neocortical dynamic function and EEG. Behav Brain Sci. 2000;23:371–437. doi: 10.1017/s0140525x00003253. [DOI] [PubMed] [Google Scholar]
- Nunez P, Wingeier BM, et al. Spatial-temporal sturctures of human alpha rhythms:teory, microcurrent sources, multiscale measurements, and global binding of local networks. Hum Brain Mapp. 2001;13:125–64. doi: 10.1002/hbm.1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace-Schott E, Hobson JA. The neurobiology of sleep:genetics, cellular physiology and subcortical networks. Nat Rev Neurosci. 2002;3:591–605. doi: 10.1038/nrn895. [DOI] [PubMed] [Google Scholar]
- Paus T, Breve S, et al. Time-related changes in neural systems underlying attention and arousal during the performance of an auditory vigilance task. J Cogn Neurosci. 1997;9:392–408. doi: 10.1162/jocn.1997.9.3.392. [DOI] [PubMed] [Google Scholar]
- Perez de la Mora M, Aguilar-Garcia A, et al. Effects of the vigilance promoting drug modafinil on the synthesis of GABA and glutamate in slices of rat hypothalamus. Neurosci Lett. 1999;259:181–5. doi: 10.1016/s0304-3940(98)00905-7. [DOI] [PubMed] [Google Scholar]
- Pierard C, Satabin P, et al. Effects of a vigilance-enhancing drug, modafinil, on rat brain metabolism:2D COSY 1H-NMR study. Brain Res. 1995;693:251–6. doi: 10.1016/0006-8993(95)00711-x. [DOI] [PubMed] [Google Scholar]
- Porkka-Heiskanen T, Strecker RE, et al. Adenosine: a mediator of the sleep-inducing effects of prolonged wakefulness. Science. 1997;276:1265–8. doi: 10.1126/science.276.5316.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porkka-Heiskanen T, Strecker RE, et al. Brain site-specificity of extracellular adenosine concentration changes during sleep deprivation and spontaneous sleep: and in vivo microdialysis study. Neuroscience. 2000;99:507–17. doi: 10.1016/s0306-4522(00)00220-7. [DOI] [PubMed] [Google Scholar]
- Randall DC, Cafferty FH, et al. Chronin treatment with modafinil may not be beneficial in patients with chronic fatigue syndrome. Psychopharmacology (Berl) 2005a;19:647–60. doi: 10.1177/0269881105056531. [DOI] [PubMed] [Google Scholar]
- Randall DC, Fleck NL, et al. The cognitive-enhancing properties of modafinil are limited in non-sleep-deprived middle-aged volunteers. Pharmacol Biochem Behav. 2004;77:547–55. doi: 10.1016/j.pbb.2003.12.016. [DOI] [PubMed] [Google Scholar]
- Randall DC, Shneerson JM, et al. Cognitive effects of modafinil in student volunteers may depend on IQ. Pharmacol Biochem Behav. 2005b;82:133–9. doi: 10.1016/j.pbb.2005.07.019. [DOI] [PubMed] [Google Scholar]
- Randall DC, Shneerson JM, et al. Modafinil affects mood, but not cognitive function, in healthy young volunteers. Human Psychopharmacol. 2003;18:163–73. doi: 10.1002/hup.456. [DOI] [PubMed] [Google Scholar]
- Robertson P, Decory HH, et al. In vitro inhibition and induction of human hepatic cytochrome P450 enzymes by modafinil. Drug Metab Dispos. 2000;28:644–71. [PubMed] [Google Scholar]
- Sadato N, Nakamura S, et al. Neural networks for generation and suppression of alpha rhythm: a PET study. Neuroreport. 1998;9:893–7. doi: 10.1097/00001756-199803300-00024. [DOI] [PubMed] [Google Scholar]
- Saletu M, Anderer P, et al. EEG-tomographic studies with LORETA on vigilance differences between narcolepsy patients and controls and subsequent double-blind, placebo-controlled studies with modafinil. J Neurol. 2004;251:1354–63. doi: 10.1007/s00415-004-0543-8. [DOI] [PubMed] [Google Scholar]
- Saletu MT, Anderer P, et al. EEG-mapping differences between narcolepsy patients and controls and subsequent double-blind, placebo-controlled studies with modafinil. Eur Arch Psychiatry Clin Neurosc. 2005;255:20–32. doi: 10.1007/s00406-004-0530-1. [DOI] [PubMed] [Google Scholar]
- Saper CB, Chou TC, et al. The sleep switch: hypothalamic control of sleep and wakefulness. Trends Neurosci. 2001;24:726–31. doi: 10.1016/s0166-2236(00)02002-6. [DOI] [PubMed] [Google Scholar]
- Saper CB, Scammell TE. Modafinil: a drug in search of a mechanism. Sleep. 2004;27:19–25. [PubMed] [Google Scholar]
- Scammell TE, Estabrooke IV, et al. Hypothalamic arousal regions are activated during modafinil-induced wakefulness. J Neurosci. 2000;20:8620–8. doi: 10.1523/JNEUROSCI.20-22-08620.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebban C, Tesolin-Decros B, et al. Contrasting EEG profiles elicited by antipsychotic agents in the prefrontal cortex of the conscious rat: antagonism of the effects of clozapine by modafinil. Br J Pharmacol. 1999a;128:1055–63. doi: 10.1038/sj.bjp.0702893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebban C, Zhang XQ, et al. Changes in EEG spectral power in the prefrontal cortex of conscious rats elicited by drugs interacting with dopaminergic and noradrenergic transmission. Br J Pharmacol. 1999b;128:1045–54. doi: 10.1038/sj.bjp.0702894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevy S, Rosenthal MH, et al. Double-blind, placebo-controlled, study of modafinil for fatigue and cognition in schizophrenia patients treated with psychotropic medications. J Clin Psychiatry. 2005;66:839–43. doi: 10.4088/jcp.v66n0705. [DOI] [PubMed] [Google Scholar]
- Shammi P, Stuss DT. Humour appreciation: a role of the right frontal lobe. Brain. 1999;122:657–66. doi: 10.1093/brain/122.4.657. [DOI] [PubMed] [Google Scholar]
- Simon P, Hemet C, et al. Non-amphetaminic mechanism of stimulant locomotor effect of modafinil in mice. Eur Neuropsychopharmacol. 1995;5:509–14. doi: 10.1016/0924-977x(95)00041-m. [DOI] [PubMed] [Google Scholar]
- Skulachev VP. Cytochrome C in the apoptotic and antioxidant cascades. FEBS Lett. 1998;423:275–80. doi: 10.1016/s0014-5793(98)00061-1. [DOI] [PubMed] [Google Scholar]
- Smit AS, Eling PATM, et al. Mental effort affects vigilance enduringly: after-effects in EEG and behavior. Int J Psychophysiol. 2004;53:239–43. doi: 10.1016/j.ijpsycho.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Spence SA, Green RD, et al. Modafinil modulates anterior cingulate function in chronic schizophrenia. Br J Psychiatry. 2005;187:55–61. doi: 10.1192/bjp.187.1.55. [DOI] [PubMed] [Google Scholar]
- Stone EA, Cotecchia S, et al. Role of brain alpha 1B-adrenoceptors in modafinil-induced behavioral activity. Synapse. 2002;46:269–70. doi: 10.1002/syn.10127. [DOI] [PubMed] [Google Scholar]
- Stone EA, Lin Y, et al. Stress-induced subsensitivity to modafinil and its prevention by corticosteroids. Pharmacol Biochem Behav. 2002;73:971–8. doi: 10.1016/s0091-3057(02)00962-0. [DOI] [PubMed] [Google Scholar]
- Strecker RE, Morairty S, et al. Adenosinergic modulation of basal forebrain and preoptic/anterior hypothalamic neuronal activity in the control of behavioral state. Behav Brain Res. 2000;115:183. doi: 10.1016/s0166-4328(00)00258-8. [DOI] [PubMed] [Google Scholar]
- Tanganelli S, de la Mora MP, et al. Modafinil and cortical [gamma]-aminobutyric acid outflow. Modulation by 5-hydroxytryptamine neurotoxins. Eur J Pharmacol. 1995;273:63–71. doi: 10.1016/0014-2999(94)00675-w. [DOI] [PubMed] [Google Scholar]
- Turner D, Robbins T, et al. Cognitive enhancing effects of modafinil in healthy volunteers. Psychopharmacology. 2003;165:260–9. doi: 10.1007/s00213-002-1250-8. [DOI] [PubMed] [Google Scholar]
- Turner DC, Clark L, et al. Modafinil improves cognition and response inhibition in adult attention-deficit/hyperactivity disorder. Biol Psychiatry. 2004a;55:1031–40. doi: 10.1016/j.biopsych.2004.02.008. [DOI] [PubMed] [Google Scholar]
- Turner DC, Clark, et al. Modafinil improves cognition and attentional set shifting in patients with chronic schizophrenia. Neuropsychopharmacology. 2004b;29:1363–73. doi: 10.1038/sj.npp.1300457. [DOI] [PubMed] [Google Scholar]
- Uftring SJ, Wachtel SR, et al. An fMRI study of the effect of amphetamine on brain activity. Neuropsychopharmacology. 2001;25:925–35. doi: 10.1016/S0893-133X(01)00311-6. [DOI] [PubMed] [Google Scholar]
- Voirol P, Jonzier-Perey M, et al. Cytochrome P-450 activities in human and rat brain microsomes. Brain Res. 2000;855:235–43. doi: 10.1016/s0006-8993(99)02354-9. [DOI] [PubMed] [Google Scholar]
- Walsh JK, Randazzo AC, et al. Modafinil improves alertness, vigilance, and executive function during simulated night shifts. Sleep. 2004;27:434–9. doi: 10.1093/sleep/27.3.434. [DOI] [PubMed] [Google Scholar]
- Willie JT, Renthal W, et al. Modafinil more effectively induces wakefulness in orexin-null mice than in wild-type littermates. Neuroscience. 2005;130:983–95. doi: 10.1016/j.neuroscience.2004.10.005. [DOI] [PubMed] [Google Scholar]
- Wisor JP, Eriksson KS. Dopaminergic-adrenergic interactions in the wake promoting mechanism of modafinil. Neuroscience. 2005;132:1027–34. doi: 10.1016/j.neuroscience.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Wisor JP, Nishino S, et al. Dopaminergic role in stimulant-induced wakefulness. J Neurosci. 2001;21:1787–94. doi: 10.1523/JNEUROSCI.21-05-01787.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Y-L, Fu J-M, et al. Neuroprotective mechanism of modafinil on Parkinson disease induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Acta Pharmacologica Sinica. 2004;25:301–5. [PubMed] [Google Scholar]