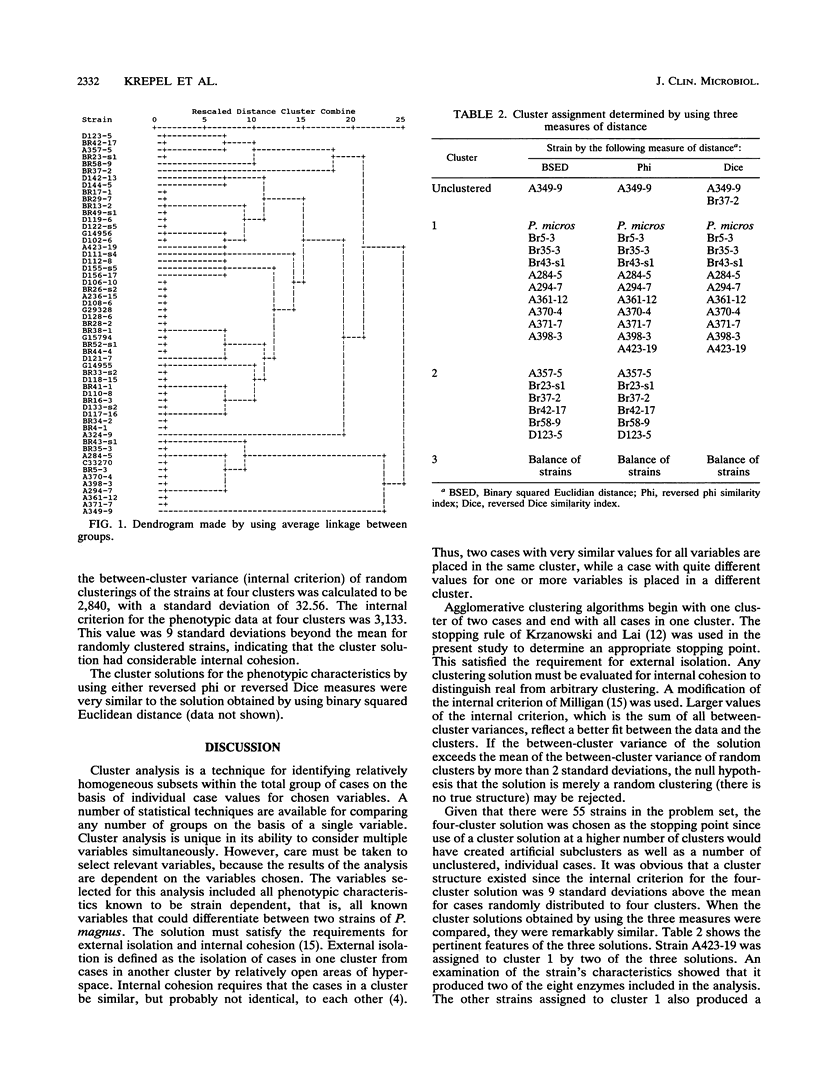
Abstract
Fifty-four strains of Peptostreptococcus magnus (11 were recovered from abdominal infections, 18 were from nonpuerperal breast abscesses, and 21 were from diabetic foot infections; the type strain and three other strains were from the American Type Culture Collection, Rockville, Md.) and the type strain of Peptostreptococcus micros were tested for their ability to produce various enzymes, including catalase, hippurate hydrolase, serine dehydratase, threonine dehydratase, collagenase, gelatinase, alkaline phosphatase, and esterase C4. The data were analyzed by cluster analysis. The results showed that all but one strain could be assigned to either of two distinct, valid clusters. The first cluster of 11 strains was composed of strains that were relatively inactive, having produced one or two of the eight strain-dependent enzymes. The second was a large cluster of strains (n = 43) that were considerably more active, all having produced at least three enzymes; the vast majority of strains (89%) produced four or more enzymes. The unclustered strain produced one enzyme that was different from that produced by the strains in the first cluster. The chi 2 test of homogeneity applied to the clustering solution indicated that greater enzyme activity was significantly associated with the site of infection (P less than 0.001). The more enzymatically active P. magnus strains were recovered significantly more often from nonpuerperal breast abscesses and diabetic foot infections than they were from abdominal infections. These results may provide insight into the nature of certain polymicrobial soft tissue infections and suggest that (i) P. magnus may participate more in nonpuerperal breast and diabetic foot infections than in abdominal infections and that (ii) peptostreptococcal production of proteolytic enzymes may have an important adjunctive effect on the pathogenesis of certain soft tissue infections.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bourgault A. M., Rosenblatt J. E., Fitzgerald R. H. Peptococcus magnus: a significant human pathogen. Ann Intern Med. 1980 Aug;93(2):244–248. doi: 10.7326/0003-4819-93-2-244. [DOI] [PubMed] [Google Scholar]
- Edmiston C. E., Jr, Walker A. P., Krepel C. J., Gohr C. The nonpuerperal breast infection: aerobic and anaerobic microbial recovery from acute and chronic disease. J Infect Dis. 1990 Sep;162(3):695–699. doi: 10.1093/infdis/162.3.695. [DOI] [PubMed] [Google Scholar]
- Habif D. V., Perzin K. H., Lipton R., Lattes R. Subareolar abscess associated with squamous metaplasia of lactiferous ducts. Am J Surg. 1970 May;119(5):523–526. doi: 10.1016/0002-9610(70)90167-4. [DOI] [PubMed] [Google Scholar]
- Kaufman E. J., Mashimo P. A., Hausmann E., Hanks C. T., Ellison S. A. Fusobacterial infection: enhancement by cell free extracts of Bacteroides melaninogenicus possessing collagenolytic activity. Arch Oral Biol. 1972 Mar;17(3):577–580. doi: 10.1016/0003-9969(72)90073-8. [DOI] [PubMed] [Google Scholar]
- Krepel C. J., Gohr C. M., Edmiston C. E., Jr, Farmer S. G. Anaerobic pathogenesis: collagenase production by Peptostreptococcus magnus and its relationship to site of infection. J Infect Dis. 1991 May;163(5):1148–1150. doi: 10.1093/infdis/163.5.1148. [DOI] [PubMed] [Google Scholar]
- Leach R. D., Eykyn S. J., Phillips I. Vaginal manipulation and anaerobic breast abscesses. Br Med J (Clin Res Ed) 1981 Feb 21;282(6264):610–611. doi: 10.1136/bmj.282.6264.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louie T. J., Bartlett J. G., Tally F. P., Gorbach S. L. Aerobic and anaerobic bacteria in diabetic foot ulcers. Ann Intern Med. 1976 Oct;85(4):461–463. doi: 10.7326/0003-4819-85-4-461. [DOI] [PubMed] [Google Scholar]
- Notari M. A., Mittler B. E. An unusual mixed infection in a diabetic patient: Citrobacter freundii and Peptococcus magnus. J Foot Surg. 1985 Jul-Aug;24(4):251–254. [PubMed] [Google Scholar]
- Sanderson P. J. Infection of the foot with Peptococcus magnus. J Clin Pathol. 1977 Mar;30(3):266–268. doi: 10.1136/jcp.30.3.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffen E. K., Hentges D. J. Hydrolytic enzymes of anaerobic bacteria isolated from human infections. J Clin Microbiol. 1981 Aug;14(2):153–156. doi: 10.1128/jcm.14.2.153-156.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheat L. J., Allen S. D., Henry M., Kernek C. B., Siders J. A., Kuebler T., Fineberg N., Norton J. Diabetic foot infections. Bacteriologic analysis. Arch Intern Med. 1986 Oct;146(10):1935–1940. [PubMed] [Google Scholar]


