Abstract
Patterns of synaptic connections in the visual system are remarkably precise. These connections dictate the receptive field properties of individual visual neurons and ultimately determine the quality of visual perception. Spontaneous neural activity is necessary for the development of various receptive field properties and visual feature maps. In recent years, attention has shifted to understanding the mechanisms by which spontaneous activity in the developing retina, lateral geniculate nucleus, and visual cortex instruct the axonal and dendritic refinements that give rise to orderly connections in the visual system. Axon guidance cues and a growing list of other molecules, including immune system factors, have also recently been implicated in visual circuit wiring. A major goal now is to determine how these molecules cooperate with spontaneous and visually evoked activity to give rise to the circuits underlying precise receptive field tuning and orderly visual maps.
Keywords: synaptogenesis, activity-dependent, visual plasticity, axonal refinement, retinal waves, axon guidance
INTRODUCTION
The wiring diagram of the vertebrate visual system is remarkably precise. Throughout the visual pathway, neurons are tuned to respond to specific features of the visual scene by virtue of the types and patterns of synaptic connections they receive. Moreover, visual connections are arranged into regular feature maps such as retinotopic maps, eye-specific layers, ocular dominance columns, orientation maps, and direction preference maps. Visual receptive fields and feature maps develop through a refinement process in which imprecise connections are weakened and eliminated and correctly targeted connections are strengthened and maintained (Katz & Shatz 1996). Much of this refinement is well underway or even completed before vision begins (Chapman et al. 1996, Chapman & Stryker 1993, Godement et al. 1984, Horton & Hocking 1996, Linden et al. 1981, McLaughlin & O’Leary 2005, Rakic 1976). Some visual circuit connections require vision to be maintained (White et al. 2001), whereas others are refractory to disruptions in visual experience (Wiesel & Hubel 1963a). Still others form only after eye-opening and require normal visual experience in order to develop in the first place (Li et al. 2006). Here, we review recent findings on mammalian visual circuit development. As a general framework for thinking about this process, we first review the sorts of activity-based and molecular-based cues that, in theory, are sufficient to instruct development of precise visual circuits. Second, we review what is currently known about the role of these cues in the development of particular types of receptive field properties and visual feature maps.
MECHANISMS TO INSTRUCT VISUAL CIRCUIT DEVELOPMENT
How do precise visual connections form? More than 40 years ago, Roger Sperry (1963) put forth the chemoaffinity hypothesis, the idea that molecular cues guide the formation of orderly connections between the eye and the brain (Sperry 1963). Around the same time, David Hubel and Torsten Wiesel discovered the importance of early visual experience for plasticity of visual circuits (Wiesel & Hubel 1963a, 1965a,1965b). Sperry, Hubel, and Wiesel all noted the remarkable degree of anatomical and functional precision that is present in the visual pathway before the onset of vision, and they concluded that the wiring of the visual system relies on “innate cues” (Sperry 1963, Wiesel & Hubel 1963b). Modern methods have further demonstrated the high degree of functional and anatomical precision that is attained in mammalian visual circuits, even before vision is possible. Retinotopic maps in the superior colliculus (SC) (Chalupa & Snider 1998, King et al. 1998, McLaughlin & O’Leary 2005), lateral geniculate nucleus (LGN) (Jeffery 1985, Pfeiffenberger et al. 2006), and primary visual cortex (V1) (Cang et al. 2005b), as well as eye-specific inputs to the LGN (Godement et al. 1984, Linden et al. 1981, Rakic 1976, Shatz 1983) and ocular dominance columns in V1 (Crowley & Katz 2002, Horton & Hocking 1996), all develop before photoreceptors can transduce light (Figure 1). Orientation-selective circuits in V1 (Chapman et al. 1996, Chapman & Stryker 1993, Issa et al. 1999, White et al. 2001) also begin to form before visual experience begins (Figure 1). The fact that such a high degree of wiring precision is achieved prior to the onset of visual experience implies that activity-independent cues such as axon guidance molecules establish the basic blueprint of visual circuit connections, and then visual experience subsequently modifies these connections during the “critical period” (reviewed in Hensch 2004). However, studies over the past two decades reveal that neurons in the developing visual system are spontaneously active long before the critical period and, indeed, even before photoreceptors become capable of responding to light (Galli & Maffei 1988, Maffei & Galli-Resta 1990). The spontaneous activity patterns present in the developing visual pathway are highly structured; retinotopic information, on-center vs. off-center receptive field information, and eye-specific information can all be found encoded in the patterns of spontaneous firing of retinal, LGN, and cortical neurons (Chiu &Weliky 2001, 2002; Feller 1999;Weliky & Katz 1999;Wong 1999). Once the visual system becomes capable of responding to light, sensory-evoked activity then stabilizes some of these nascent visual connections (Chapman et al. 1996, Chapman & Stryker 1993, Issa et al. 1999, White et al. 2001), refines them further (Tian & Copenhagen 2003), or induces additional circuit properties (Li et al. 2006). Some species also progress through an intermediate stage before eye-opening during which both spontaneous activity and vision through naturally closed eyelids coexist (Demas et al. 2003, Krug et al. 2001) and together drive refinement of visual connections (Akerman et al. 2002, Tian & Copenhagen 2003, White et al. 2001).
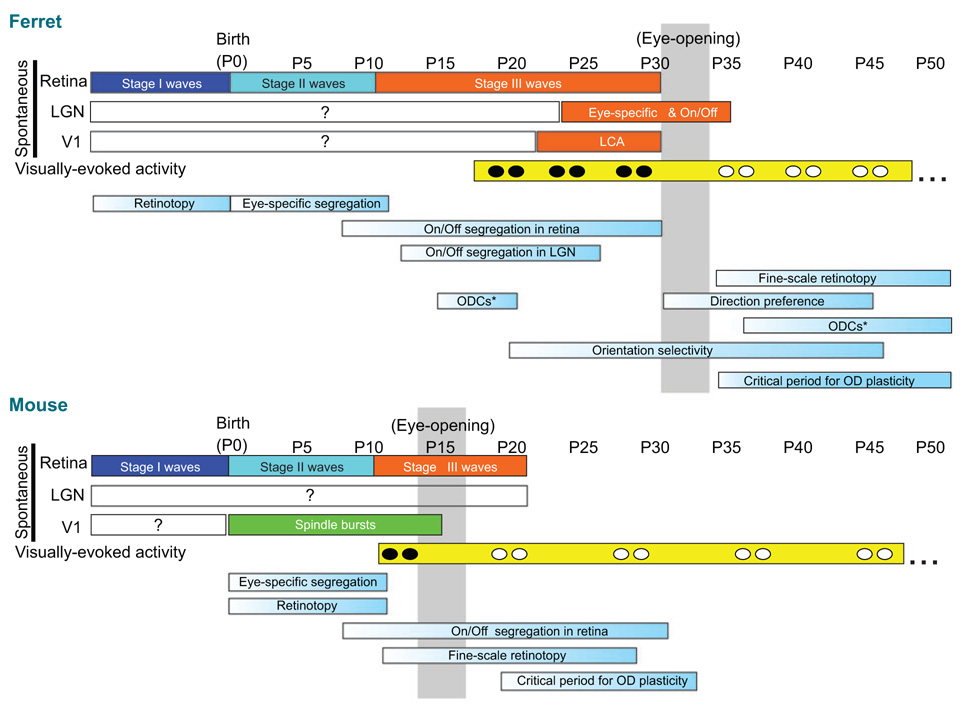
Figure 1.
Timing of the development of each of the major visual circuit properties in ferret and mouse (shown as bars with light blue gradients). Orange bars indicate ionotropic glutamate receptor-mediated retinal waves. Turquoise bars indicate nicotinic acetylcholine receptor–mediated retinal waves. Blue bars (stage I waves) indicate cholinergic and gap-junction-mediated activity. Gray column indicates the approximate age for eye opening in each species. Black icons within the “visually-evoked activity” yellow bar indicates the period in which vision occurs through naturally closed eye lids. White icons indicate vision through open eyelids. Asterisks in the two bars labeled “ODCs” indicate that two different time frames have been reported for ODC development in ferrets (early development of ODCs, Crowley & Katz 2000; later development of ODCs, Ruthazer et al. 1999) and which report is accurate remains controversial owing to technical limitations of the tracing techniques used in both studies (see main text). Note that ODCs, orientation maps, and direction maps are not listed for the mouse because mice lack these anatomical features, although single cells in mouse V1 do exhibit responses tuned according to these stimulus features. The timing of the emergence of these physiological features in the mouse has not yet been reported.
Here we review the types and patterns of spontaneous and visually evoked activity that can impact developing visual connections. We then discuss the role of molecules, such as axon guidance cues and cell adhesion molecules, in visual circuit development. For clarity, we present activity and molecular cues separately, but the reader should note that whether activity and guidance molecules are in fact independent of one another must be established on a case-by-case basis, especially in light of recent findings that guidance molecules can be modulated by altering patterns of spontaneous neural activity (Hanson & Landmesser 2004, Ming et al. 2001) and that guidance molecules can impact neural activity patterns as well (Bouzioukh et al. 2006, Sahay et al. 2005). In the second part of this review we consider how neural activity and axon guidance molecules contribute to the development of specific types of visual receptive fields and orderly feature maps.
Neural Activity
Spontaneous retinal waves
Prior to the onset of visual experience, the developing vertebrate retina spontaneously generates retinal waves. During a wave, retinal ganglion cells (RGCs) spontaneously fire correlated bursts of action potentials that propagate across the retina. Waves initiate from random locations in the retina and propagate over spatially restricted domains such that over time the whole retina is tiled by locally correlated activity (Feller et al. 1996, 1997). Retinal waves have been found in chickens, turtles, mice, ferrets, and monkeys(Warland et al. 2006,Wong 1999). In some species, such as primates, waves occur only prenatally, whereas in other species, such as mice and ferrets, they occur both prenatally and postnatally. In mammals, retinal waves begin prior to when photoreceptors are capable of transducing light, and they disappear around the time of eye-opening, regard-less of whether visual experience is prevented or not (Demas et al. 2003). The circuits that mediate retinal waves have been divided into three stages that are remarkably similar across species (Firth et al. 2005, Sernagor et al. 2001, Wong 1999). The waves that occur at each stage have somewhat different properties such as wave front velocities, domain sizes, and frequencies; thus the waves particular to each stage are positioned to instruct the development of particular types or aspects of visual circuit development.
Stage I waves emerge before birth (Bansal et al. 2000, Syed et al. 2004) (Figure 1). Gap junction blockers can inhibit stage I retinal waves in rabbit (Syed et al. 2004), whereas blocking nicotinic acetylcholine receptor (nAChR) inhibits some but not all of these early waves in mice (Bansal et al. 2000). Stage I waves are relatively infrequent, occurring every ~90–120 s.
Stage II waves emerge around the time of birth in mice and ferrets (Bansal et al. 2000, Syed et al. 2004). Stage II waves then last the first 1–2 postnatal weeks, coincident with retinotopic and eye-specific refinement (Figure 1). Stage II waves are driven by ACh released from starburst amacrine cells (Feller et al. 1996, Zheng et al. 2004). Acute application of nAChR antagonists blocks Stage II retinal waves (for reviews, see Firth et al. 2005, Zhou 1998). Stage II waves occur relatively infrequently (every 1–2 min) and propagate with an average wave-front velocity ranging from 100 to 300 microns/s, varying with species (Bansal et al. 2000, Feller et al. 1996). The propagating and restricted domain size of stage II waves allows them to relay information about the retinotopic relationship of RGCs to brain targets in an activity-dependent manner (Butts 2002). Also, because stage II waves are relatively infrequent, there is a low probability that RGCs residing at retinotopically similar positions in the two eyes will fire simultaneously. Thus, stage II waves also relay information identifying from which eye a given RGC axon originates to retinorecipient targets in the brain (Eglen 1999, Butts et al. 2007).
Mice lacking β2-subunits of neuronal nAChRs (β2nAChR−/−) do not exhibit stage II retinal waves (Bansal et al. 2000, Muir-Robinson et al. 2002) RGCs remain active in the β2nAChR−/− mouse, but rather than firing in correlated fashion, the RGCs in these mutants exhibit spontaneous spiking activity that is not correlated among neighboring RGCs and there is no propagating activity (Bansal et al. 2000, Muir-Robinson et al. 2002). Remarkably, waves at other developmental stages (I and III) are unaffected in the β2nAChR−/− mouse. The β2nAChR−/− mouse is thus an important animal model for studying the role of stage II waves in visual circuit development. Similarly, the cholinergic agonist epibatidine blocks stage II waves by desensitizing nAChRs (Penn et al. 1998). Chronic intraocular application of epibatidine to early postnatal mice or ferrets is a paradigm also commonly used to disrupt stage II and thereby study their role in visual circuit development.
Stage III waves begin at approximately P10–P12 in mice and ferrets and are mediated by glutamate released from retinal bipolar cells (Bansal et al. 2000, Wong et al. 2000, Zhou & Zhao 2000) (Figure 1). Stage III waves persist until around the time of eye-opening (Bansal et al. 2000, Demas et al. 2003, Syed et al. 2004,Wong et al. 1993). During stage III waves, activity still propagtes across the retina, but as it does so, it causes RGC firing to become more correlated among RGCs of the same sign (On-On or Off-Off) than it does between RGCs of the opposite sign (On vs. Off), in part because Off-RGCs manifest a higher frequency of burst firing than On-RGCs (Myhr et al. 2001, Wong & Oakley 1996). Thus, On vs. Off information is encoded in stage III waves and is relayed to central brain targets as differences in the temporal pattern of spiking and mean firing rate across these two RGC populations. Other types of functionally distinct RGCs, such as alpha, beta, and gamma RGCs, also exhibit differences in their spontaneous firing patterns during stage III waves (Liets et al. 2003), and these differences may contribute to the wiring of their distinct patterns of connectivity.
Spontaneous activity in the lateral geniculate nucleus
A few landmark studies used in vitro explants to confirm that the RGC spiking evoked during waves induces LGN neuron spiking (Mooney et al. 1996) and that such synaptic transmission can influence plasticity at developing retinogeniculate synapses (Mooney et al. 1996), two requisite features for wave-based activity-dependent models of visual circuit refinement (Butts 2002, Butts et al. 2007, Katz & Shatz 1996). Aside from these studies, however, virtually all our knowledge about spontaneous activity patterns in the developing LGN comes from experiments in which LGN activity was recorded with chronically implanted multi-electrodes in nonanesthetized ferret pups (Weliky & Katz 1999). These studies revealed that spontaneous activity is more correlated among neurons in same-eye (contralateral or ipsilateral) layers of the LGN than among neurons in opposite eye (contralateral vs. ipsilateral) layers. They also revealed higher correlations among neurons in same-sign (On or Off) sublaminae of the LGN than among neurons situated in opposite sign (On vs. Off) sublaminae. The youngest animals that Weliky and Katz were able to record from were ~P24, which is about one week before eye-opening in this species and coincides with stage III glutamate-mediated retinal waves (Figure 1). Indeed, spontaneous correlated neuron firing in the developing LGN was strongly enhanced by input from the contralateral retina and the ipsilateral visual cortex. Thus, the developing LGN is capable of intrinsically generating spontaneous correlated activity that is eye specific and On- vs. Off-center specific. Activity from the retina and the cortex further strengthens these eye-specific and On/Off correlations.
Spontaneous activity in visual cortex
A few research groups have recorded spontaneous activity in the developing visual cortex of intact, nonanesthetized animals at stages prior to the onset of visual experience. Fiber optic recordings of calcium-sensitive dyes revealed the presence of propagating calcium waves in the cortex of newborn mice (Adelsberger et al. 2005). Other groups have recorded spindle burst oscillations in V1 of unanesthetized newborn rodents. From birth until ~P10, these spindle bursts are triggered by stage II retinal waves in the contralateral eye. After P10 they are triggered by light stimulation of the contralateral eye (Hanganu et al. 2006). The specific role of spindle bursts in visual circuit wiring remains unknown.
Does spontaneous activity in V1 contribute to visual circuit refinement? Multisite electrode recordings from the visual cortex of P22–P28 ferrets revealed patches ofV1neurons that spontaneously exhibit synchronous firing. These patches were separated by ~1 mm and resembled ferret ocular dominance columns (ODCs). This long-range correlated activity (LCA) (Chiu &Weliky 2001) is intrinsic to the cortex because it persists despite LGN activity blockade, but it is modulated by subcortical inputs. A subsequent study combined light stimulation of the retina and multisite recordings from V1 to show that LCA domains are associated with ODCs (Chiu &Weliky 2002). However, LCA was also observed in large monocular portions of V1 that do not have ODCs. Thus LCA in developing V1 is generated by circuits intrinsic to V1 and is modified by eye-specific connections from the retina via the LGN.
Collectively, the above-described studies demonstrate that patterns of spontaneous activity generated within the developing retina, LGN, and V1 are highly structured and that, by virtue of nascent connections within and between these visual areas, the overall strength of eye-specific and On/Off correlations is enhanced. This arrangement suggests a hierarchical organization whereby increasingly specific information is encoded in the patterns of spontaneous activity as the visual pathway matures and visual experience begins.
Early patterns of visually driven activity
Vision begins at birth in species born with their eyes open, and it begins several days to weeks after birth in species born with their eyes closed such as mice, ferrets, and cats. It is tempting to assume that the statistical properties of the visual scene reliably predict the activity patterns in the developing visual pathway from the onset of vision, and that those activity patterns contribute to visual receptive field development. However, very few studies have examined what sorts of visually driven activity patterns are actually present in the visual pathway at the earliest stages of vision. Moreover, in mice and ferrets, visual experience begins before eye-opening through naturally closed eye lids (Krug et al. 2001) at a time when stage III spontaneous retinal waves and correlated LGN and V1 activity are also occuring (Figure 1). Although dark-rearing before eye-opening disrupts some aspects of visual circuit refinement (Akerman et al. 2002, White et al. 2001), determining what activity patterns are present in the visual pathway at these stages requires that the investigators record activity from awake unanaesthetized animals (so as not to eliminate spontaneous activity) while the animals view natural scenes through closed eyelids (to recapitulate normal experience). LGN recordings under these conditions have recently been carried out in postnatal ferrets (Ohshiro & Weliky 2006). These experiments revealed that the correlations among like sign (On- or Off-center) LGN neurons generated by spontaneous activity closely match the correlations generated by the animal viewing natural scenes. Thus, spontaneous and visually generated neural activity patterns could sharpen or induce receptive fields in the visual pathway, even before eye-opening.
Molecular Guidance Cues
Molecules such as axon guidance cues (Goodman & Shatz 1993) and cell adhesion molecules (Sanes & Yamagata 1999, Yamagata et al. 2002) that are traditionally thought of as activity independent can induce a considerable degree of wiring precision in the developing visual pathway. Indeed, the development of virtually all the visual circuit properties we discuss below could, in theory, involve molecular guidance cues. Although we do not focus extensively on the mechanisms by which these guidance cues function, several excellent reviews on this topic are cited as further reading. We now consider the types of visual circuit wiring that molecular guidance cues can induce.
Graded expression of axon guidance cues
Sperry (1963) first hypothesized that graded expression of molecules in inputs and targets regulates visual map development. Considerable evidence now demonstrates that gradients of the ephrin/Eph family of axon guidance cues are key to the development of topographic maps not only in the visual system, but also in many other sensory systems (reviewed in Flanagan 2006, McLaughlin & O’Leary 2005) (Figure 2a). Quantitative models demonstrate how graded expression of Ephs in the retina and ephrin ligands in the target instructs mapping of the cardinal retinal axes [(nasal-temporal (N-T), dorsal-ventral (D-V)], although, as we discuss below, such models also need to incorporate activity-dependent synaptic refinements to fully explain map development as it occurs in vivo.
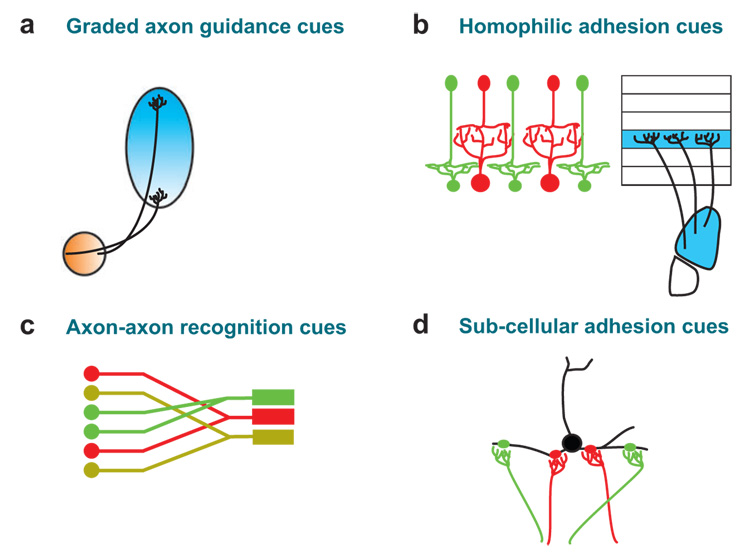
Figure 2.
Schematic diagrams of the types of molecular cues that direct wiring specificity in developing visual circuits. (a) Graded expression of guidance cues in axons and in their targets can guide specific patterns of visual connections according to matching of ligand and receptor levels (see reviews by McLaughlin & O’Leary 2005, Flanagan 2006) and see Figure 3. (b) Homophilic adhesion cues expressed in the axons and dendrites of pre- and postsynaptic neurons can lead to highly specific patterns of connectivity (red cells connect to red cells, green cells to green cells, etc.) (e.g., Yamagata et al. 2002). Similarly, the expression of adhesion molecules in neurons within one structure (shown in blue; schematic of the mouse LGN) and within the specific layer of their targets to which they project (also shown in blue; schematic of cortex) can induce highly specific patterns of connectivity (e.g., Poskanzer et al. 2003). (c) Adhesive cues expressed among axons arising from a common cell type (schematized here as red, green or yellow) can segregate these axons into distinct fiber tracts and/or portions of fiber tracts, which can then lead to segregation of their axons within the final target (reviewed in Chen & Flanagan 2006, Mombaerts 2006). (d ) Different adhesion cues expressed at different sites along the dendritic arbor of an individual postsynaptic neuron can segregate synaptic inputs arising from different cells/sources at the subcellular level (e.g., Ango et al. 2004, Di Cristo et al. 2004) and thereby impact the receptive field properties of the postsynaptic neuron (e.g., Sherman 2004).
Graded expression of ephrins can also induce binary axon guidance decisions (Dufour et al. 2003, Huberman et al. 2005b). This sort of pathfinding is well-suited to segregate many of the highly specialized functionally visual processing streams (motion, form, color, etc.) from one another along fiber pathways and within target structures (Callaway 2005).
Layer specific adhesion molecules
One hallmark feature of the visual system is layer-specific connectivity. In the retina, SC, LGN, and visual cortex, functionally distinct visual pathways arise through layer-specific axonal and dendritic connections. A few molecules have been identified that regulate layer-specific connectivity in the retina, such as sidekicks and DSCAMS (Yamagata et al. 2002, Yamagata & Sanes 2008) (Figure 2b). Also N-cadherin, in collaboration with neural activity, regulates layer specificity of geniculo-cortical projections (Poskanzer et al. 2003, Uesaka et al. 2007, Yamamoto et al. 2000) (Figure 2b). No one has yet reported regarding the molecules that mediate layer-specific targeting of functionally defined visual processing streams, although such cues have been proposed to exist (Meissirel et al. 1997; Kawasaki et al. 2004).
Axon-axon recognition molecules
One way to ensure selective wiring of axons or dendrites arising from one functional subclass of visual neurons (say, for example, On-center or motion-sensitive RGCs) is to have those cells express adhesion molecules that keep them bound together along the fiber tracts and/or within their targets (Figure 2c). Indeed, axons arising from RGCs in retinotopically similar locations in the retina establish retinotopic order within the optic tract prior to target innervation (reviewed in Plas et al. 2005), and the axons of functionally distinct RGCs segregate from one another along fiber pathways (Reese 1987, Reese & Cowey 1988) and segregate into different layers within their targets (Sur & Sherman 1982). The molecules that regulate axon-axon recognition and segregation in the mammalian visual system have not been identified, but one such molecule—heparin sulfate—can control axon-axon segregation of retinotopically distinct RGCs in the optic tract of zebrafish (Lee et al. 2004). Neural activity may also regulate axon-axon recognition and targeting, but purely activity-dependent models cannot explain why, for instance eye-specific layers or On-center versus Off-center sublaminae always form in the same stereotyped locations in their target (Stryker & Zahs 1983). Therefore, a molecular or other activity-independent mechanism, such as variation in ingrowth timing (Walsh & Guillery 1985), must ensure this feature. In this context, axon sorting on the basis of molecular guidance cues is a well-established phenomenon in developing olfactory circuits, but neural activity also contributes to axon sorting in that system (Chen & Flanagan 2006, Mombaerts 2006, Yu et al. 2004).
Subcellular recognition molecules
The specific receptive field properties of a given visual neuron arise by virtue of the type, number, strength, and location of synaptic inputs onto that cell (Chapman et al. 1991, Nelson et al. 1978, Reid & Alonso 1995, Sherman 2004, Tavazoie & Reid 2000). In recent years, studies have begun to explore the factors that regulate where particular inputs form synapses on a post-synaptic cell. Huang and coworkers have shown that the perisomatic innervation of pyramidal neurons by GABAergic parvalbumin neurons in the visual cortex occurs even in the complete absence of sensory experience (Di Cristo et al. 2004). The same group has also shown, however, that GABAergic signaling plays an important role in the emergence of perisomatic inhibition (Chattopadhyaya et al. 2007). Thus both restricted expression of cell adhesion molecules (Ango et al. 2004) (Figure 2d ) and neural activity likely control the targeting of highly specific patterns of subcellular connectivity observed in the mature visual system. We now consider the role of neural activity and molecular cues in the development of connectivity underlying specific types of receptive field properties and visual feature maps.
DEVELOPMENT OF VISUAL MAPS AND RECEPTIVE FIELD PROPERTIES
Retinotopic Mapping
Visual connections are organized to convey information about the position of stimuli in the visual field. Neighboring RGCs in the retina project to neighboring cells in the brain, thereby forming retinotopic maps of the visual field in the LGN and SC. Retinotopic mapping is also present in the pattern of projections from the LGN to V1. Numerous studies have shown that retinotopic maps develop through a process of refinement; when RGC axons initially innervate the SC they overshoot their correct termination zone. A fine precision retinotopic map gradually emerges as RGCs arborize interstitial branches in the appropriate target location and the overshooting portion of the axon degenerates (Figure 3a and b). Retinotopic refinement is completed prior to eye-opening and the onset of vision (Figure 1).Two major forces instruct refinement of retinotopic maps: gradients of molecular cues and correlated neural activity (reviewed in McLaughlin & O’Leary 2005).
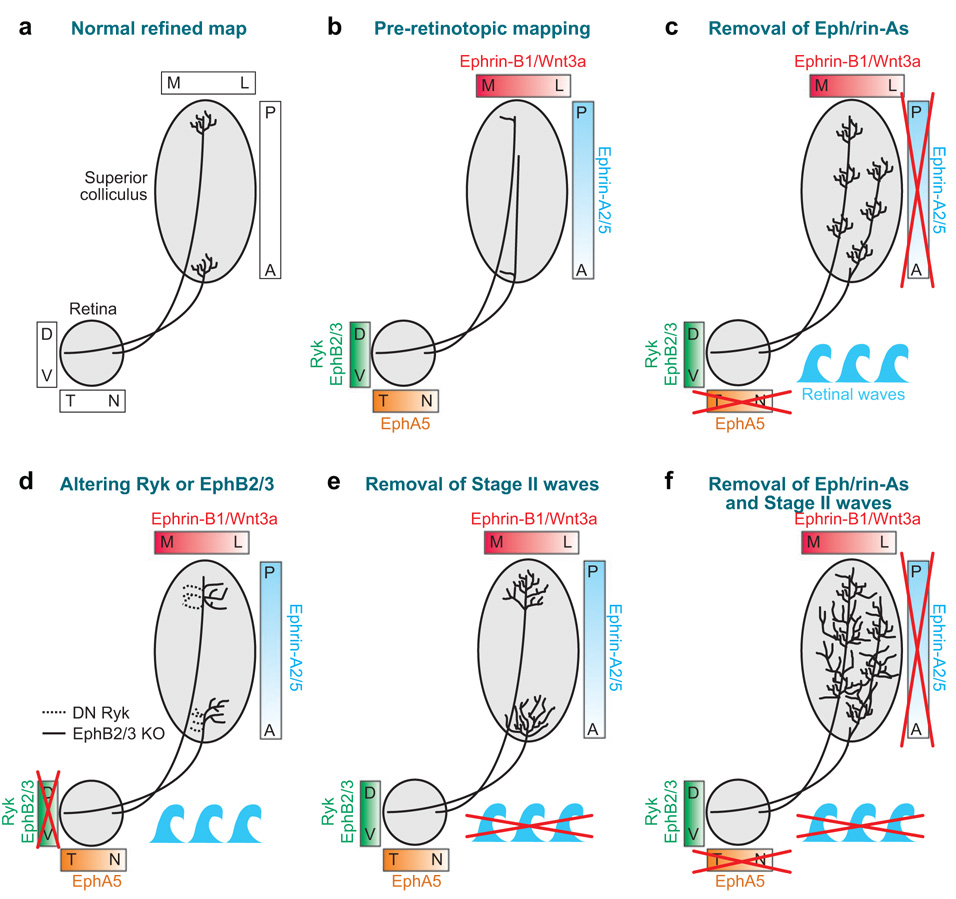
Figure 3.
Schematic diagrams of (a) the mature retinotopic map in the SC, (b) the immature unrefined retinotopic map in the SC (and the expression of molecular cues that guide formation of retinotopic maps) (see McLaughlin & O’Leary 2005). (c) Disruptions in retinotopic mapping in ephrin-A2/5−/− or EphA5−/− mice. Multiple dense termination zones are observed along the N-T axis of the target (Feldheim et al. 2000, 2004). Waves denote that retinal waves are intact in these animals. (d ) Disruptions in retinotopic mapping in EphB2/3−/− mice or in response to disrupting Wnt/ryk signaling; RGC terminals shift more medially (ryk disruption; dashed lines in target) (Schmitt et al. 2006) or shift more laterally (EphB2/3 knockout; solid lines) (Hindges et al. 2002). Similar results are observed after disruption of BMPs (Chandrasekaran et al. 2005). (e) The overall retinotopic map forms when stage II retinal waves are eliminated (because ephrin-A2/5 signaling is still intact), but RGC axons fail to form dense terminal arbors in their correct topopographic locations and are abnormally broad (McLaughlin et al. 2003, Grubb et al. 2003). (f )When stage II waves are prevented in ephrin-A2/5−/− mice, N-T mapping of RGC projections is abolished (Pfeiffenberger et al. 2006). For all the manipulations shown here, the retino-SC projection is depicted. The same general defect pattern is observed in the LGN and V1, where ephrin-As and Bs and retinal waves regulate topographic map formation (see main text for details).
The ephrin family of axon guidance molecules organizes the overall layout of retinotopic maps. Gradients of ephrins and their receptors, Ephs, are expressed in the retina and its central visual targets (Flanagan 2006, McLaughlin & O’Leary 2005). In mammals, EphA5 is expressed in a low nasal to high temporal gradient in RGCs, and the repellant ligands, ephrin-A2/A5, are expressed in low anterior to high posterior gradients in the SC (Feldheim et al. 1998, McLaughlin & O’Leary 2005). Mutant mice lacking ephrin-A2/5 (ephrin-A2/5−/−) or EphA5 (EphA5−/−) show aberrant retinotopic mapping of the N-T retinal axis (visual field azimuth representation) in the SC and in the LGN(Feldheim et al. 2000, 2004). If an anterograde tracer is focally injected at one location in the retina of a wildtype (wt) mouse, a single dense focus of RGC terminal arbors is seen in the retinotopically appropriate location in the SC and in the LGN. When such injections are made in mice lacking ephrin-A2 and ephrin-A5, however, multiple topographically incorrect RGC termination zones are observed, consistent with the loss of the repellent cues in the targets (Feldheim et al. 1998, 2000; Frisen et al. 1998; Pfeiffenberger et al. 2006) (Figure 3c). Similarly, ectopic expression studies indicate that a gradient of EphA5 expression in the LGN and ephrin-A5 in the visual cortex controls retinotopic mapping of the N-T retina axis (visual field azimuth) in the geniculocortical projection (Cang et al. 2005a).
Retinotopic targeting of RGCs along the D-V axis of the retina (corresponding to visual field elevation) relies on ephrin-/EphBs and wnt/ryk signaling. Wnt3 and EphrinB ligands are expressed in high-to-low gradients along the medial-lateral axis of the SC/tectum, and their receptors, ryk and EphBs respectively, are expressed in high-to-low gradients along the ventral-to-dorsal axis in the retina (Hindges et al. 2002) and optic tectum (Braisted et al. 1997) (Figure 3d ). EphB2−/− and EphB3−/− or kinase-inactive EphB2 mice exhibit D-V mapping errors in the SC (Hindges et al. 2002), and disruption of Wnt signaling by dominant-negative ryk expression in the retina also alters D-V mapping but in the opposite direction of EphB2/3−/− mice (Schmitt et al. 2006) (Figure 3d ). Thus the balance between Ephrin-B and Wnt/ryk signaling guides RGC axons to their correct medial-lateral termination zone in the SC/tectum (see review by Salinas & Zou 2008, in this volume).
Computational models suggest that molecular cues alone cannot specify the precision of retinotopic mapping observed in vivo (McLaughlin & O’Leary 2005, O’Leary & McLaughlin 2005, Yates et al. 2004) and that the correlated firing of neighboring RGCs is necessary to enhance the precision of retinotopic mapping through Hebbian “fire together wire together” type mechanisms (Butts et al. 2007, Yates et al. 2004). Such models predict that, in the absence of correlated RGC firing, ephrins will guide RGC axons to the correct locations in their targets, but the axons will fail to form dense terminations in those locations, effectively blurring the local visual field representation. Experimental evidence now supports this idea. Retinotopic refinement of RGC and LGN projections occurs during the same developmental period as stage II retinal waves (Figure 1). In the β2nAChR−/− mouse or in mice that receive intraocular injections of epibatidine, stage II waves are abolished, andRGC axons project to the correct retinotopic location in the SC and LGN but therein form abnormally large, diffuse axonal arborizations (Grubb et al. 2003, McLaughlin et al. 2003, Pfeiffenberger et al. 2006) (Figure 3e). As a result of these diffuse arbors, SC and LGN neurons exhibit larger-than-normal receptive fields in these animals (Chandrasekaran et al. 2005, Grubb et al. 2003, Mrsic-Flogel et al. 2005). Likewise, epibatidine-treated and β2nAChR−/− mice also exhibit altered retinotopy in the geniculocortical pathway (Cang et al. 2005b). Poorly refined retinotopic maps are also observed if the readout of correlated activity is prevented in target neurons by N-methyl-D-aspartate(NMDA)receptor blockers (Huang & Pallas 2001, Simon et al. 1992). These findings strongly reinforce Hebbian activity–dependent models of retinotopic map refinement (Butts 2002).
Rather than regulate each other, activity and ephrin-As act in parallel to control mapping of the nasal temporal retinal axis in visual targets. When β2nAChR−/− mice are crossed to ephrin-A2/5−/− mice, mapping of the N-T axis is abolished (Cang et al. 2008, Pfeiffenberger et al. 2006) (Figure 3f ). Correlated activity refines the retinotopic map predominantly along the N-T axis; mapping of the D-V axis is unaffected by loss of waves (Grubb et al. 2003; Pfeiffenberger et al. 2006; Cang et al. 2008). Why waves affect only N-T mapping remains unknown. Waves are not thought to exhibit locational differences along the N-T or D-V axes or according to eccentricity (distance from the center of the retina), but it should be noted that regional differences in wave properties have never been examined systematically. Mapping along the D-V axis may be refractory to altering stage II waves because D-V mapping is established before target innervation within the optic tract (Plas et al. 2005). D-V mapping in the optic tract may be controlled by as-yet-undiscovered axon-axon recognition cues (Figure 1c) and/or by stage I waves.
Fine-Scale Retinotopic Refinement
After RGC axons map to their appropriate retinotopic locations in their targets and form dense axonal arborizations at those locations, they continue to undergo fine-scale retinotopic refinement. In vitro experiments that combine optic tract stimulation with whole-cell recordings from LGN neurons indicate that on P10, approximately 12–30 RGCs provide weak synaptic input onto each LGN neuron. Significant elimination of retinogeniculate synapses occurs within the following week so that by P16 each LGN neuron receives strong input from only 1–3 RGCs (Chen & Regehr 2000, Hooks & Chen 2006, Jaubert-Miazza et al. 2005) (reviewed in Huberman 2007). This fine-scale retinotopic refinement has direct implications for sharpening receptive fields in the LGN. Indeed, in ferrets around the time of eye-opening, LGN receptive fields are significantly larger and more elongated than they are three weeks later when the loss of all but one to two RGC inputs onto each LGN neuron has occurred (Tavazoie & Reid 2000). Stage III retinal waves coincide with fine-scale retinotopic refinement (Figure 1) and drive this process. Hooks & Chen (2006) used chronic intravitreal application of tetrodotoxin (TTX) from P11 to P15 to block stage III wave-induced RGC spiking. TTX prevented both the normal elimination of weak RGC inputs onto single LGN neurons and the strengthening of any of the supernumerary weak inputs. Vision in mice begins at ~P11, which is about four days before eye-opening (Figure 1). Hooks & Chen (2006) therefore tested the role of visual experience in fine-scale retinotopic refinement. Visual deprivation from birth until P28 had no impact on this process, but surprisingly, dark-rearing from P25 to P30 caused the retinogeniculate projections to revert to a multiply innervated state of ~10–12 inputs. These findings indicate that spontaneous activity is critical for fine-scale retinotopic refinement in the LGN and suggest that light-induced maturation of inhibitory circuits and/or cortical feedback may be involved in maintenance of newly refined circuits. TTX likely eliminates all RGC spiking induced by stage III waves. Thus the issue of whether the specific patterns of RGC spiking generated during stage III waves are instructive for fine-scale refinement still needs to be determined using manipulations that alter the structure of RGC activity during stage III waves but do not eliminate RGC spiking altogether. Tools to accomplish this have not yet been identified.
Some of the molecular cues that act down-stream of activity to mediate fine-scale retinotopic refinement have been identified. Components of the classical complement cascade of immune proteins are expressed at developing retinogeniculate synapses, and in their absence, much of the normal synaptic elimination that occurs during fine-scale retinotopic refinement is prevented (Stevens et al. 2007). The expression of other immune molecules, such as MHCI (Corriveau et al. 1998, Huh et al. 2000) and neuronal pentraxins (NPs) (Bjartmar et al. 2006), is regulated by activity. MHC I is essential for plasticity events leading to synaptic weakening (Huh et al. 2000), and NPs are critical for glutamate receptor insertion at developing retinogeniculate synapses (S. Koch and E. Ullian submitted). Proteins in the complement pathway as well as MHC I and NPs are all also required for anatomical eye-specific refinement (Bjartmar et al. 2006, Huh et al. 2000, S. Koch and E. Ullian submitted) (Figure 4t). Immune genes are thus emerging as important mediators of activity-dependent plasticity in visual circuit refinement.
Figure 4.
Schematic representations of the eye-specific projection patterns to the LGN of the ferret (panels a– j ) and the mouse (panels k–t) during normal development and the results of experiments examining the role of spontaneous retinal activity, ephrin-As, and immune genes in eye-specific retino-LGN segregation. (b, l ) The early prerefined pattern of RGC inputs to the LGN in the newborn ferret (b) and mouse (l ). Red areas of the LGN correspond to territory occupied by RGC axons arising from the right (red ) eye, and green areas correspond the territory occupied by RGC axons from the left (green) eye. Yellow corresponds to the LGN territory where red and green axons from the two eyes overlap. The ages depicted are shown in parentheses. The manipulations leading to each phenotype are described in each box, as well as in the main text. The asterisk above *NP1/2 and * P25 in panel t refers to the fact that the lack of eye-specific segregation observed in the P10 NP1/2−/− mouse changes to a pattern similar to panel p by P25. By contrast, C1q−/− mice and MHCI−/− mice exhibit defects in eye-specific segregation until at least P25.
Eye-Specific Segregation in the LGN
In mammals, RGC axons from the two eyes project to both sides of the brain and are segregated into eye-specific domains within their targets, including the LGN, the SC, vLGN, pretectum, and suprachiasmatic nucleus (Muscat et al. 2003). Whether eye-specific segregation is important for visual processing remains unknown. Nevertheless, the development of segregated eye-specific projections is a prominent model system for exploring how precise patterns of synaptic connections emerge during development and, in particular, for exploring the role of neural activity in axonal refinement. The development of eye-specific segregation has been studied mostly in the LGN because the patterns of eye-specific projections are so stereotyped in this target.Within a given species, axons from the contralateral and ipsilateral eye terminate in domains of stereotyped shape, position, and size (Figure 4). In carnivores and primates, where much of the work on the development of eye-specific retinogeniculate projections has been carried out, eye-specific inputs are also mirrored eye-specific cellular layers in the LGN (Linden et al. 1981, Rakic 1976, Shatz 1983). This feature allows one to study the relationship between axon targeting and cytoarchitectural development as well (Casagrande & Condo 1988, Huberman et al. 2002).
Development of eye-specific connections occurs before the onset of visual experience (Figure 1) through a refinement process whereby RGC axons from the two eyes initially overlap (Figure 4a,b). In macaques and humans, eye-specific retinogeniculate segregation occurs in the second trimester (Hevner 2000, Huberman et al. 2005a, Rakic 1976). In cats this process occurs shortly before birth (E49–60) (Shatz 1983). In mice and ferrets, eyespecific retinogeniculate segregation occurs during the first ten days of postnatal life (P1–10) (Godement et al. 1984, Jaubert-Miazza et al. 2005) (Figure 1). Individual-axon labeling studies indicate that eye-specific segregation reflects two general processes: (a) synapse and axon arbor elaboration and (b) synapse and axon arbor elimination (Campbell & Shatz 1992; Sretavan & Shatz 1984, 1987). Electrophysiological recordings indicate that before segregation, RGC axons from both eyes can drive activity in individual LGN neurons. Then as segregation proceeds, inputs from one eye are weakened and removed and inputs from the remaining eye are strengthened and maintained (Jaubert-Miazza et al. 2005, Shatz & Kirkwood 1984).
Competitive interactions between RGC axons from the two eyes are critical for eye-specific segregation because if one eye is removed at the overlap stage, RGC axons from the intact eye maintain arbors throughout the LGN (Morgan & Thompson 1993, Sretavan & Shatz 1986). Early studies used intracranial infusions of TTX to demonstrate that spiking of LGN neurons is necessary for eye-specific segregation (Sretavan et al. 1988).
Is spontaneous retinal activity necessary for eye-specific segregation? Stage II retinal waves coincide with the period of eye-specific segregation in ferrets (Penn et al. 1998) and in mice (Bansal et al. 2000) (Figure 1). As described above, stage II waves encode eye-specific information. In a landmark study, Penn et al. (1998) tested whether stage II waves are necessary for eye-specific retinogeniculate segregation. To block stage II waves, they applied epibatidine at high concentrations to one or both retinas of ferrets from P1 to P10 (the normal period of eye-specific segregation in this species). In P10 ferrets that had waves blocked in both eyes, eye-specific segregation was completely prevented; RGC axons from the two eyes remained intermingled to the same extent observed in normal P1 ferrets (before segregation) (Figure 4c). When wave activity was blocked in just one eye, however, retinogeniculate projections still segregated: The territory arising from the activity-blocked eye shrank dramatically at the expense of the normally active eye, which expanded its axonal territory (Figure 4d ). Penn et al. (1998) was thus the first to demonstrate that spontaneous retinal activity mediates the binocular competition underlying eye-specific segregation.
Subsequent studies used intraocular injections of cAMP analogs to increase the size and frequency of retinal waves in one or both eyes of ferrets (Stellwagen et al. 1999, Stellwagen & Shatz 2002). Results of these studies indicated that the more active eye always wins more territory in the LGN (Figure 4e). Thus the relative levels of spontaneous activity in the two retinas, rather than a requirement for some threshold level of retinal activity, dictate how much LGN territory axons from a given retina will occupy.
Do the specific patterns of RGC spiking induced by retinal waves instruct eye-specific segregation? Answering this question requires that one abolish the correlated firing pattern of neighboring RGCs without eliminating RGC spiking or changing overall retinal activity levels. The first study to accomplish this task used immunotoxin ablation of starburst amacrine cells in newborn ferrets to significantly reduce the correlated firing of neighboring RGCs. Eye-specific retinogeniculate segregation was normal in these animals (Huberman et al. 2003) (Figure 4f ). It was therefore concluded that spontaneous retinal activity is permissive than instructive for eye-specific segregation. By contrast, in the β2nAChR−/− mouse, which has altered stage-II waves, eye-specific segregation fails to occur in the normal time frame (Muir-Robinson et al. 2002, Rossi et al. 2001) (Figure 4n).
Pharmacologic studies confirm that the lack of segregation observed in the β2nAChR−/− mouse is not caused by a lack of β2nAChRs in the LGN or other brain regions but rather is due to defects in stage II spontaneous retinal activity (Cang et al. 2005b, Chandrasekaran et al. 2005, Rossi et al. 2001) (Figure 4m). Therefore, how can researchers explain the discrepancy between the results seen in the β2nAChR−/− mouse and immunotoxin-treated ferrets? Several possibilities exist. First, ferrets have eye-specific cellular layers in the LGN, whereas mice do not. Cues besides patterned activity—such as graded (Huberman et al. 2005b) or layer-specific adhesion molecules (Figure 2)—might cooperate with activity to induce eye-specific segregation in species that have eye-specific cellular laminae in the LGN. Second, eye-specific segregation may fail to occur in β2nAChR−/− mice, not because correlated RGC firing is abolished in this animal but because a fraction of RGCs are rendered silent in these animals (McLaughlin et al. 2003). However, some RGCs are rendered silent in immunotoxin treated retinas too, yet this does not impact eye-specific segregation (Huberman et al. 2002, 2003). Third, in the immunotoxin studies, early calcium waves were spared, and whether RGCs were correlated over a larger distance in the retina was not investigated (Huberman et al. 2003) and might explain why eye-specific segregation persisted in these animals.
To define which spatiotemporal features of retinal activity are critical for eye-specific targeting, a recent study examined a transgenic mouse that lacks the gap junction protein, Cx36 (Cx36−/−). Cx36−/− mice continue to exhibit retinal waves but show significant increases in the number of asynchronous RGC action potentials, which significantly reduces the nearest neighbor correlation observed in normal mice (Torborg et al. 2005). In contrast with β2nAChR−/− mice, Cx36−/− mice exhibit normal eye-specific segregation in the LGN (Figure 4o). By comparing the firing patterns of normal mice and Cx36−/− mice to β2nAChR−/−, investigators concluded that high-frequency bursts synchronized across nearby RGCs are correlated with eye-specific segregation, whereas additional asynchronous spikes do not inhibit segregation (Torborg et al. 2005).
The debate about whether waves are instructive or permissive for eye-specific segregation remains unresolved. Binocular TTX injections from birth to P10 delay but do not prevent eye-specific segregation (Cook et al. 1999). But whether TTX blocked all RGC spiking in those animals remains unclear. A recent study concluded that waves are permissive for the proper readout of ephrin-induced repulsion during RGC axonal targeting (Nicol et al. 2007), but these results have not yet been confirmed in vivo. Experiments that alter or, ideally, control RGC spiking patterns in reliable ways are needed to resolve the controversy about whether correlated RGC firing induced by waves is permissive or instructive for eye-specific segregation.
In the β2nAChR−/− mouse and in mice or ferrets treated with epibatidine in both eyes from P1 to P10, eye-specific segregation fails to occur (Penn et al. 1998, Rossi et al. 2001) (Figure 4c,m,n). But if these animals are allowed to survive until P25, stage III waves return and rescue eye-specific segregation (Huberman et al. 2002, Muir-Robinson et al. 2002). The overall organization of eye-specific zones that form, however, is highly perturbed; whereas normally eye-specific territories are highly stereotyped, stage II wave blockade followed by a recovery period induces a patchy, random pattern of eye-specific zones in the LGN, and the layout of these zones is highly variable from one animal to the next (Huberman et al. 2002, Muir-Robinson et al. 2002) (Figure 4g,p). These findings indicate that retinal activity is necessary for eye-specific segregation but that factors other than activity that are available only during the normal period of eye-specific segregation (P1–P10 in the ferrets and mice) control the location in the target in which the projections from one or the other eye arborize.
Ephrin-As control the spatial patterning of eye-specific layers in the LGN. This is perhaps not surprising given that “eye-specific” projections arise from nasal-retina (contralateral) versus temporal-retina (ipsilateral) RGC axons, and EphA5 is differentially expressed across theN-Tretinal axis. In ephrin-A2/3/5−/− mice (ephrin-A2/3/5 are the ephrin ligands normally expressed in the LGN during eye-specific segregation), eye-specific segregation occurs but in a patchy, random pattern rather than in stereotyped contralateral and ipsilateral eye territories (Pfeiffenberger et al. 2005) (Figure 4q). Likewise, EphAs and ephrin-As are expressed in the ferret retina and LGN during eye-specific segregation in a pattern suggestive of an eye-specific pathfinding role. If EphA3 or EphA5 are ectopically expressed in RGCs of newborn ferrets, eye-specific patterning and segregation are perturbed (Huberman et al. 2005b) (Figure 4i). Together these findings indicate that ephrin-A/EphA interactions are critical for controlling the position, shape, and size of eye-specific territories in the LGN.
Both the timing and pattern of ephrin-A expression in the LGN are critical for patterning of eye-specific territories. Ephrin-As are downregulated in the LGN after the normal period of eye-specific segregation. Misexpression of EphA receptors in RGCs after the normal period of eye-specific segregation has no impact on patterning of RGC projections to the LGN (Huberman et al. 2005b). Thus, timed ephrin-A expression in theLGNregulates the early critical period for eye-specificLGNlayer formation observed in other studies (Muir-Robinson et al. 2002, Huberman et al. 2002). Also, in experiments where RGC axons are experimentally rewired to novel, non-visual targets, ipsilateral RGC axons avoid regions of high ephrin-A expression and segregate from contralateral eye RGC axons in those novel targets (Ellsworth et al. 2005), consistent with the idea that the expression pattern of ephrin-As unique to each subcortical RGC target dictates the characteristic spatial organization of eye-specific territories that will form in that target.
Collectively, the above-described studies indicate that spontaneous RGC spiking that occurs during stage II waves is critical for eyespecific segregation and that ephrin-As dictate where eye-specific domains form within the LGN. Indeed, if ephrin-A2/3/5−/− mice receive binocular injections of epibatidine from P1 to P10, to eliminate stage II waves, RGC axons from the two eyes remain permanently intermingled in a salt and pepper fashion throughout the entire LGN (Pfeiffenberger et al. 2005; D. Feldheim, personal communication) (Figure 4r). As with retinotopic mapping, ephrin-As and activity act in parallel to induce eye-specific pathfinding; removal or overexpression of Eph/ephrin-As does not effect neural activity patterns, nor does altering activity impact ephrin expression in the retina or LGN (Huberman et al. 2005b, Pfeiffenberger et al. 2005). Whether specific patterns of RGC spiking are critical to instruct segregation remains controversial and demands further investigation.
Maintenance of Eye-Specific Projections
After eye-specific segregation is complete, retinal waves help maintain segregation of RGC axons from the two eyes. In young ferrets (P10–P30), the drug APB completely blocks stage III waves (Chapman 2000). If binocular injections of APB are applied to both eyes of ferrets from P10 to P25, axons from the two eyes revert to an overlapping state in the LGN (Chapman 2000). The pattern of overlap caused by a stage III wave blockade noticeably differs from that observed by stage II wave blockade. Whereas blocking stage II waves causes contralateral and ipsilateral eye axons to overlap throughout most of theLGN(Figure 4c,m,n), blocking stage III waves causes RGC axons from the ipsilateral eye to collapse from layer A1 into the inner segment of theLGN(termed layer A in ferrets and cats) where they overlap with axons from the contralateral eye, leaving the territory normally occupied by axons from the ipsilateral eye (A1) is left devoid of retinal input (Chapman 2000) (Figure 4h). These findings suggest that stage III retinal waves maintain eye-specific segregation by preventing collapse of ipsilateral RGC axons into the more attractive (or less repulsive) contralateral eye territory. Interestingly, expression of the axon-repellant ligands ephrin-A2/5 is lower in contralateral eye territory (layer A) than it is in ipsilateral eye (A1) territory (Huberman et al. 2005b).
Do the specific activity patterns that occur during stage III waves instruct maintenance of eye-specific projections, or is RGC spiking simply permissive to maintain segregation? Demas et al. (2006) discovered that in nob mice (no b-wave mice) stage II waves and eye-specific segregation are normal, but stage III retinal waves are excessively frequent and persist abnormally past eye-opening. These aberrantly patterned and persistent stage III waves cause desegregation of eye-specific retinogeniculate inputs, indicating that normally stage III spontaneous retinal activity patterns instruct stabilization of newly re- fined eye-specific circuits (Demas et al. 2006) (reviewed in Huberman 2006) (Figure 4s). As in the earlier APB studies (Chapman 2000), Demas et al. (2006) found that ipsilateral eye axons were preferentially disrupted in nob mice. Why might ipsilateral axons be affected differently by activity manipulations than would other RGC axons? The ipsilateral visual pathway is less efficient in driving postsynaptic activity than the contralateral pathway early in development (Crair et al. 1998, 2001). Also, the RGCs that project ipsilaterally are molecularly distinct in terms of their expression of certain EphAs (Huberman et al. 2005b) and EphBs (Williams et al. 2003, 2006) and zinc transporters (Land & Shamalla-Hannah 2001). Indeed, even in animals that lack an optic chiasm and in which all RGC axons project to the same side of the brain, axons from RGCs in the temporal retina (that normally do not cross at the chiasm) segregate from the axons arising from other RGCs in the retina (that normally cross at the chiasm) in the LGN(Williams et al. 1994) (Figure 4j ), indicating that these two axon populations are molecularly distinct. Microarray gene profiling of contralaterally vs. ipsilaterally projecting RGCs would be useful for discovering the genes that regulate the differential targeting of these two cell populations.
Ocular Dominance Column Development
In many carnivore and primate species, including humans, segregation of eye-specific pathways is evident not only in the pattern of eye-specific inputs to the LGN, but also in the pattern of LGN projections to V1 (Hubel & Wiesel 1962). In these species, the axons of LGN neurons located in one or the other eye-specific layer project to V1 in a series of nonoverlapping, alternating stripes called ocular dominance columns (ODCs) (Wiesel et al. 1974). ODCs are a classic model system for exploring the role of early visual experience in the plasticity of cortical circuits (Katz & Crowley 2002). Patterns of visually evoked activity during the critical period instruct the layout of ODCs through binocular competition and Hebbian-type plasticity (reviewed in Hensch 2004). ODCs emerge before the critical period, however, and do not require vision to form (Crair et al. 1998, Horton & Hocking 1996, Wiesel & Hubel 1974).
The anatomical events that underlie ODC development still remain unclear. Early studies that used monocular injection of transneuronal tracers (retino>LGN>V1) to label geniculocortical axons found that in young animals the pattern of proline label in V1 was continuous, whereas in more mature animals, an alternating pattern of proline label (ODCs) was seen (LeVay et al. 1978, Rakic 1976, Ruthazer et al. 1999). Thus investigators concluded that LGN axons representing the two eyes intermingle extensively before segregating into ODCs. One potential problem with this conclusion, however, is that in young animals transneuronal tracers spill over into neighboring eye-specific layers in the LGN (LeVay et al. 1978, Ruthazer et al. 1999, Stryker & Harris 1986), which can lead to incorrect conclusions regarding the absence of ODCs in V1. Indeed, subsequent optical imaging studies (Crair et al. 1998, 2001) and anatomical studies that used focal injections of anterograde tracers into the LGN (Crowley & Katz 2000, 2002) concluded that ODCs are present much earlier than can be observed by transneuronal labeling from the eye (LeVay et al. 1978, Ruthazer et al. 1999, Stryker & Harris 1986). Neither set of studies resolved, however, whether ODCs form precisely from the outset or through refinement. Researchers in this field eagerly await the discovery of anterograde trans-synaptic tracers that are not prone to spillover to firmly resolve this controversial issue.
Stage II and III retinal waves and spontaneous LGN activity are present throughout the ODC development period (Wong et al. 1993, Wong & Oakley 1996) (Figure 1), and as discussed above, all these sources of activity are structured to relay eye-specific information to V1. Classic experiments concluded that blocking retinal activity with TTX disrupts the formation of ODCs (Stryker & Harris 1986) and that stimulating the two optic nerves simultaneously prevents ODC segregation (Stryker & Strickland, 1984). However, optical imaging studies (Crair et al. 1998, 2001) later showed that these experiments were carried out after ODCs had already formed and thus could implicate activity only in the maintenance of ODCs, not in their initial formation.
The idea that activity-independent cues drive the initial ODC segregation was actually first proposed by Hubel & Wiesel (1963). Crowley & Katz (1999) tested this idea by removing one or both eyes from newborn ferrets then allowed the animals to mature into adulthood, when they labeled thalamocortical axons with anterograde tracers injected directly into the inner or outer half of the LGN. When the eyes are removed early in development, eyespecific cellular layers fail to form in the LGN (Guillery et al. 1985, Morgan & Thompson 1993)]. In the ferrets lacking eyes from birth, patches of label with a periodicity similar to that of ODCs were observed in V1 (Crowley & Katz 1999). Although these eye-removal experiments did not completely rule out a role for neural activity in ODC formation, they did challenge the traditional model in which retinal activity patterns control the process (reviewed in Crowley & Katz 2002). Indeed, prior studies from the same lab showed that eye removal significantly reduces (but does not eliminate) the strength of eye-specific correlations in the developing LGN, at least for ages P24 and older (Weliky & Katz 1999) (Figure 1). Crowley & Katz (1999), therefore, concluded that ODCs form according to eye-specific molecular cues in the LGN and/or V1 and not on the basis of Hebbian-type plasticity. Subsequent studies showed that ODCs appeared earlier in development than previously thought and were well segregated from the outset without undergoing significant refinement (Crowley & Katz 2000). These findings reinforced the hypothesis that innate (molecular) cues induce ODC development, though others have argued that the patches of label observed in V1 after focal LGN injections are not actually ODCs, but instead reflect some other patchy organization of the thalamocortical pathway. Given the potential implications of these findings for models of ODC development, and thalamocortical patterning in general, this issue is crucial to resolve. One way to determine if the patches observed in V1 after focal injections into theLGN are indeed ODCs would be to focally inject an anterograde tracer into the monocular portion of the LGN. This region of the LGN is innervated by the nasal portion of the contralateral eye and never projects to V1 in ODCs. Thus, such an injection should lead to a uniform label in V1, not patches. Molecularly distinct portions of theLGNthat corresponded to functionally specific, but not eye-specific, visual pathways have been identified (Kawasaki et al. 2004). Still, molecules that mediate ODC formation may exist. Considering the established role of ephrin-As in eye-specific pathfinding in the LGN (Huberman et al. 2005b, Pfeiffenberger et al. 2005), it would be interesting to examine ephrin-A expression in V1 of species such as ferrets, cats, or macaques, which have ODCs. Other cues that might regulate ODC formation are the molecular cues that are differentially expressed in RGCs that take different courses at the optic chiasm, such as zinc transporters (Land & Shamalla-Hannah 2001), EphBs, and zic transcription factors (see review by Petros et al. 2008, in this volume). The expression of these molecules in V1 of species that have ODCs has not been reported.
Given the controversy over whether spontaneous retinal activity mediates ODC development, a recent study used binocular epibatidine injections to prevent stage II retinal waves in ferrets and then transneuronally labeled thalamo-cortical projections in those animals at adulthood, when spillover is not an issue. A key feature of this study’s design is that epibatidine treatment was carried out before ODCs normally form. Results showed that preventing stage II waves severely disrupts ODC segregation and patterning (Huberman et al. 2006). Moreover, eliminating stage II waves caused the size of binocular receptive fields in V1 to increase ~30-fold, whereas monocular receptive fields were unaffected. These findings indicate that stage II waves mediate ODC formation and binocular competition leading to binocular receptive field patterning in V1 and generally support the activity-dependent model of ODC formation.
Epibatidine treatment only alters spontaneous retinal activity patterns in ferrets from P1 until ~P10–P11 (Huberman et al. 2002, Penn et al. 1998). At those ages, LGN axons reside in the subplate (Herrmann et al. 1994), a transient structure that sends massive synaptic input to the developing cortex and is critical for LGN axon pathfinding to V1 (Ghosh et al. 1990, Ghosh & Shatz 1992b), as well as for ODC and ODC plasticity formation (Kanold et al. 2003) and maintenance (Ghosh & Shatz 1992a) during the critical period (Kanold & Shatz 2006). Thus stage II waves may cause LGN axons to segregate into ODCs while they still reside in the subplate, which might explain why Crowley & Katz (2000) observed that ODCs are segregated as they grow out of the subplate and into cortical layer four. Given its importance in so many aspects of thalamo-cortical development, the subplate is a terrific source for candidate cues (activity-dependent or otherwise) that regulate ODC development and plasticity. Microarray gene profiling of cells in the developing subplate under normal conditions and under conditions of binocular epibatidineinduced wave blockade (which prevents ODC segregation) ought to be very informative.
Development of Orientation Selectivity
Neurons in the primary visual cortex of adult mammals are tuned for the orientation of visual stimuli. In carnivores and primates, orientation selectivity is organized in a columnar fashion (Hubel &Wiesel 1962, Thompson et al. 1983) and mapped smoothly across cortex (Blasdel & Salama 1986, Grinvald et al. 1986). The development of orientation selectivity was first studied in traditional animal models of the visual system, monkeys and cats. In rhesus monkeys, at least some cells demonstrate orientation-specific responses at birth (Wiesel & Hubel 1974). In kittens studied at the end of the first postnatal week, before natural eye opening, the degree of orientation tuning reported in the literature varied widely, from 0% of cells exhibiting orientation selectivity (Barlow & Pettigrew 1971, Pettigrew 1974), to 25%–30% (Blakemore & Van Sluyters 1975, Buisseret & Imbert 1976, Fregnac & Imbert 1978), to 100% (Hubel & Wiesel 1963). These discrepancies may be due to the difficulty of performing electrophysiological recording in young kittens where cells respond sluggishly and habituate rapidly (Hubel & Wiesel 1963), and where small changes in blood pressure or expired carbon dioxide levels can change an orientation-selective cell into a nonselective or unresponsive cell (Blakemore & Van Sluyters 1975). For this reason, more recent studies of orientation selectivity development have used the ferret as an animal model. The ferret has a visual system quite similar to that of the cat (Law et al. 1988) but is born about three weeks earlier in development (Linden et al. 1981). The ferret therefore provides a terrific model system for exploring the mechanisms underlying development of orientation selectivity (Chapman & Stryker 1993, Krug et al. 2001).
Studies of ferret primary visual cortex have shown that some degree of orientation tuning is present as early as visual responses can be elicited, two weeks before the time of natural eye opening, but that adult-like tuning levels are not reached until about a week after eye opening (Chapman & Stryker 1993, Krug et al. 2001) (Figure 1). Studies of the development of orientation maps in V1 have shown that orientation maps are observed prior to eye opening and that the overall map layout is very stable throughout development (Chapman et al. 1996).
The role of visual experience in the development of orientation selectivity has been widely studied. The fact that orientation-selective responses are already present at or before the time of eye opening suggested that vision is not necessary for the initial emergence of cortical cell orientation tuning. However, cortical neurons can be driven in an orientation-selective manner, even before natural eye-opening, through closed eyelids (Eysel 1979, Krug et al. 2001, Spear et al. 1978), so dark-rearing beginning at stages prior to natural eye-opening were carried out. Experiments comparing the orientation maps in normal and dark-reared animals showed that orientation selectivity can in fact develop in the absence of visual experience but that normal mature levels of selectivity are never achieved (White et al. 2001). In contrast, binocular deprivation by lid suture after the normal period of eye-opening produced much more devastating effects on orientation selectivity, allowing little orientation selectivity maturation beyond that seen in very young normal ferrets (Chapman & Stryker 1993, White et al. 2001). These results suggest that visual experience is not necessary for the initial phase of orientation development but that vision is needed for its maturation. The impact of early dark-rearing furthermore shows that abnormal visual experience through closed eyelids disrupts the initial emergence of circuits underlying orientation selectivity. A somewhat different picture emerges from studies of orientation selectivity development in the cat visual cortex: In these studies, binocular visual deprivation did not prevent the initial development of orientation selectivity, although it did prevent map maintenance (Crair et al. 1998). It is not clear whether this difference is due to a difference in the amount of vision possible through a closed eyelid in the two species (i.e., that a lid-sutured cat may experience very little vision and therefore respond like a dark-reared ferret) or whether it is due to a real difference in the role of visual experience in the initial development of orientation selectivity between the two species.
Although visual experience is not necessary for the initial development of orientation selectivity, spontaneous neuronal activity is necessary. Silencing all spontaneous action potential activity in the visual cortex with TTX completely abolishes the maturation of orientation selectivity (Chapman & Stryker 1993). Several experiments have addressed whether activity is playing an instructive role in orientation selectivity development, that is, whether altering the spatio-temporal properties of neuronal activity produces an effect. Computational models have suggested that the patterns of spontaneous activity in On- and Off-center RGCs may instruct the development of orientation tuning in cortical cells. Correlations in neighboring RGC firing of like center type (On or Off) and anticorrelations in the firing of cells with opposite center type result in the development of oriented cortical cell receptive fields in these models (Miller 1992, 1994). Pharmacalogically silencing On-RGC activity does indeed prevent the maturation of orientation selectivity, supporting this class of computational model and suggesting that activity is, in fact, playing an instructive role (Chapman & Godecke 2000). An alternative method of altering activity patterns during development, artificially increasing correlations of all RGCs by electrically stimulating the optic nerve, likewise weakened orientation selectivity maturation (Weliky & Katz 1997). Thus correlation among On- versus Off-center LGN inputs to V1 appears to instruct development of orientation tuning. The molecular basis for detecting the correlational structure of On- and Off-center inputs to cortical cells is likely to be NMDA receptor activation; blocking NMDA receptor function prevents orientation selectivity maturation (Ramoa et al. 2001).
The exact mechanisms underlying the development of cortical cell orientation selectivity remain to be elucidated. Patterns of spontaneous activity before eye-opening are clearly involved in the initial development of orientation tuning, and visual experience is involved in later maturation and maintenance of the circuits underlying this receptive field feature. A role for molecular cues in orientation selectivity development has not been tested. However, the positional stability of the overall layout of orientation maps as they emerge (Chapman et al. 1996) suggests that factors other than activity are involved.
Development of Direction Preference
Neurons at various stages of the visual pathway are tuned to the movement direction of stimuli in the visual field. In some species, such as rabbits and mice, RGCs exhibit direction selectivity, whereas in other species such as ferrets, cats, and monkeys, this property emerges first in V1. The circuit that underlies retinal direction selectivity has been extensively studied (Demb 2007, Vaney & Taylor 2002), but the mechanisms that control retinal direction selectivity development remain a mystery.
Some neurons in the SC exhibit directionselective responses (Hoffmann & Sherman 1974). Manipulations that alter early activity patterns, such as strobe rearing, disrupt direction-selective cell tuning in the SC (Chalupa & Rhoades 1978, Flandrin & Jeannerod 1975); however, dark-rearing animals during the same period does not disrupt direction tuning of SC cells (Chalupa & Rhoades 1978), which indicates that direction-selective circuits in the SC do not require vision to form.
In some species, neurons in V1 exhibit direction preference, and optical imaging experiments have shown that V1 cells are organized into regular maps for preferred direction (Weliky et al. 1996). Direction selectivity in the cortex is absent at eye opening in ferrets and then develops during the subsequent two weeks from P30 to P45, making direction one of the last basic visual circuit properties to form. Patterned visual experience is the cue that instructs development of cortical direction selectivity. If ferrets are dark reared after eye-opening, direction maps fail to form and single V1 neurons lack direction tuning (Li et al. 2006). However, a brief exposure to moving visual stimuli in a single direction will allow the development of cells tuned to that direction, whereas other cells remain untuned for direction. This finding indicates that visual experience is instructive, not merely permissive, for the emergence of cortical direction selectivity.
Allowing vision to return at P45 rescues many response properties of V1 neurons such as contrast sensitivity and orientation, but direction preference is permanently abolished. This result indicates that, even after eye opening, the initial development—not just the plasticity—of direction-selective circuits in V1 encounters an early critical period. The critical period for ocular dominance plasticity begins in the ferret at ~P35 and peaks at P42 (Issa et al. 1999) (Figure 1). Thus even though the cortical circuits underlying eye specificity form before eye-opening and then are subject to plastic changes at this time, some circuit properties such as direction selectivity are still in the process of developing. Why direction-selective circuits in retina and SC do not require vision to form, whereas direction-selective circuits in V1 do, remains unclear. A better understanding of the neural circuits underlying direction selectivity in each visual area (retina, SC, V1) will shed light on this issue.
SYNTHESIS, CONCLUSIONS, FUTURE DIRECTIONS
Spontaneous and early visually evoked neural activity is necessary for anatomical and functional refinement of developing visual circuits. In the past decade, investigators have debated whether specific activity patterns matter for this refinement or whether activity is permissive for readout of guidance cues. The emerging model posits that activity patterns do matter, but which specific patterns matter and which plasticity rules they engage at nascent synapses to refine connections and induce each circuit property remain a mystery. With the discovery of molecules that translate activity into structural changes in the developing visual system (Huh et al. 2000, Bjartmar et al. 2006, Stevens et al. 2007), it will be interesting to see if activity-dependent synaptic refinement employs general or specialized mechanisms depending on the circuit property in question. At the same time, growing evidence indicates that activity-independent factors are responsible for inducing the highly stereotyped features of visual circuity such as layer position and overall map layout. Again, the question arises as to whether general rules and principles will emerge for this category of cues and if they are truly activity independent (as appears to be the case for ephrin- As in retinotopic and eye-specific map formation). Much remains to be learned about the development of visual maps and receptive field properties, both for the sake of understanding visual system development and for the sake of understanding the mechanisms of neural circuit refinement throughout the central nervous system.
ACKNOWLEDGMENTS
Work in the laboratory of M.B. Feller related to the topic of this review was supported by Klingenstein Foundation, Whitehall Foundation, March of Dimes, McKnight Scholars Fund and NIH RO1 EY13528. Work in the laboratory of B. Chapman related to the topic of this review was supported by NIH EY11369. A. D. Huberman was supported by a Helen Hay Whitney Postdoctoral Fellowship. We are grateful to David Feldheim, Michael Susman, and Jocelyn Krey for critical reading and helpful comments.
Glossary
- Superior colliculus (SC)
a major subcortical target of RGC axons important for visual orienting. The mammalian homolog to the optic tectum
- Lateral geniculate nucleus (LGN)
also often referred to as the dorsal LGN. Receives direct input from RGCs and relays visual information to the visual cortex
- V1
primary visual cortex
- On-center cells (also called “On” cells)
neurons in the retina and LGN that respond to increments in light presented to the center of their receptive field
- Off-center cells (also called “Off” cells)
neurons in the retina and LGN that respond to decrements in light presented to the center of their receptive field
- Retinal wave
spontaneous activity in the retina that correlates the depolarizations of neighboring retinal cells, including RGCs
- Retinal ganglion cells (RGCs)
the neurons that project out of the eye to subcortical visual areas in the brain
- Ferret
a carnivore species with a visual system similar to that of the cat, but it is born earlier, when visual circuits are still very immature
- Epibatidine
a nicotinic cholinergic agonist that can block retinal waves
- Long-range correlated activity (LCA)
A pattern of spontaneous activity whereby groups of neurons that are separated from each other by ~1 mm fire synchronously
- N-T
nasal-temporal
- D-V
dorsal-ventral
- Immunotoxin
a conjugate of an antibody that recognizes a cellular protein and a toxin, which kills the cell after the antibody binds to the protein target
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any biases that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- Adelsberger H, Garaschuk O, Konnerth A. Cortical calcium waves in resting newborn mice. Nat. Neurosci. 2005;8:988–990. doi: 10.1038/nn1502. [DOI] [PubMed] [Google Scholar]
- Akerman CJ, Smyth D, Thompson ID. Visual experience before eye-opening and the development of the retinogeniculate pathway. Neuron. 2002;36:869–879. doi: 10.1016/s0896-6273(02)01010-3. [DOI] [PubMed] [Google Scholar]
- Ango F, Di Cristo G, Higashiyama H, Bennett V, Wu P, Huang ZJ. Ankyrin-based subcellular gradient of neurofascin, an immunoglobulin family protein, directs GABAergic innervation at Purkinje axon initial segment. Cell. 2004;119:257–272. doi: 10.1016/j.cell.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Bansal A, Singer JH, Hwang BJ, Xu W, Beaudet A, Feller MB. Mice lacking specific nicotinic acetylcholine receptor subunits exhibit dramatically altered spontaneous activity patterns and reveal a limited role for retinal waves in forming ON and OFF circuits in the inner retina. J. Neurosci. 2000;20:7672–7681. doi: 10.1523/JNEUROSCI.20-20-07672.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow HB, Pettigrew JD. Lack of specificity of neurones in the visual cortex of young kittens. J. Physiol. 1971;218:P98–P100. [PubMed] [Google Scholar]
- Bjartmar L, Huberman AD, Ullian EM, Renteria RC, Liu X, et al. Neuronal pentraxins mediate synaptic refinement in the developing visual system. J. Neurosci. 2006;26:6269–6281. doi: 10.1523/JNEUROSCI.4212-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore C, Van Sluyters RC. Innate and environmental factors in the development of the kitten’s visual cortex. J. Physiol. 1975;248:663–716. doi: 10.1113/jphysiol.1975.sp010995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasdel GG, Salama G. Voltage-sensitive dyes reveal a modular organization in monkey striate cortex. Nature. 1986;321:579–585. doi: 10.1038/321579a0. [DOI] [PubMed] [Google Scholar]
- Bouzioukh F, Daoudal G, Falk J, Debanne D, Rougon G, Castellani V. Semaphorin3A regulates synaptic function of differentiated hippocampal neurons. Eur. J. Neurosci. 2006;23:2247–2254. doi: 10.1111/j.1460-9568.2006.04783.x. [DOI] [PubMed] [Google Scholar]
- Braisted JE, McLaughlin T, Wang HU, Friedman GC, Anderson DJ, O’Leary DD. Graded and lamina-specific distributions of ligands of EphB receptor tyrosine kinases in the developing retinotectal system. Dev. Biol. 1997;191:14–28. doi: 10.1006/dbio.1997.8706. [DOI] [PubMed] [Google Scholar]
- Buisseret P, Imbert M. Visual cortical cells: their developmental properties in normal and dark reared kittens. J. Physiol. 1976;255:511–525. doi: 10.1113/jphysiol.1976.sp011293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butts DA. Retinal waves: implications for synaptic learning rules during development. Neuroscientist. 2002;8:243–253. doi: 10.1177/1073858402008003010. [DOI] [PubMed] [Google Scholar]
- Butts DA, Kanold PO, Shatz CJ. A burst-based “Hebbian” learning rule at retinogeniculate synapses links retinal waves to activity-dependent refinement. PLoS Biol. 2007;5:e61. doi: 10.1371/journal.pbio.0050061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaway EM. Structure and function of parallel pathways in the primate early visual system. J. Physiol. 2005;566:13–19. doi: 10.1113/jphysiol.2005.088047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell G, Shatz CJ. Synapses formed by identified retinogeniculate axons during the segregation of eye input. J. Neurosci. 1992;12:1847–1858. doi: 10.1523/JNEUROSCI.12-05-01847.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cang J, Kaneko M, Yamada J, Woods G, Stryker MP, Feldheim DA. Ephrin-as guide the formation of functional maps in the visual cortex. Neuron. 2005a;48:577–589. doi: 10.1016/j.neuron.2005.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cang J, Renteria RC, Kaneko M, Liu X, Copenhagen DR, Stryker MP. Development of precise maps in visual cortex requires patterned spontaneous activity in the retina. Neuron. 2005b;48:797–809. doi: 10.1016/j.neuron.2005.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cang J, Niell CM, Liu X, Pfeiffenberger C, Feldheim DA, Stryker MP. Selective disruption of one cartesian axis of cortical maps and receptive fields by deficiency in ephrin-As and structured activity. Neuron. 2008;57:511–523. doi: 10.1016/j.neuron.2007.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casagrande VA, Condo GJ. The effect of altered neuronal activity on the development of layers in the lateral geniculate nucleus. J. Neurosci. 1988;8:395–416. doi: 10.1523/JNEUROSCI.08-02-00395.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalupa LM, Rhoades RW. Directional selectivity in hamster superior colliculus is modified by strobe-rearing but not by dark-rearing. Science. 1978;199:998–1001. doi: 10.1126/science.622583. [DOI] [PubMed] [Google Scholar]
- Chalupa LM, Snider CJ. Topographic specificity in the retinocollicular projection of the developing ferret: an anterograde tracing study. J. Comp. Neurol. 1998;392:35–47. doi: 10.1002/(sici)1096-9861(19980302)392:1<35::aid-cne3>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Chandrasekaran AR, Plas DT, Gonzalez E, Crair MC. Evidence for an instructive role of retinal activity in retinotopic map refinement in the superior colliculus of the mouse. J. Neurosci. 2005;25:6929–6938. doi: 10.1523/JNEUROSCI.1470-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman B. Necessity for afferent activity to maintain eye-specific segregation in ferret lateral geniculate nucleus. Science. 2000;287:2479–2482. doi: 10.1126/science.287.5462.2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman B, Godecke I. Cortical cell orientation selectivity fails to develop in the absence of ON-center retinal ganglion cell activity. J. Neurosci. 2000;20:1922–1930. doi: 10.1523/JNEUROSCI.20-05-01922.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman B, Stryker MP. Development of orientation selectivity in ferret visual cortex and effects of deprivation. J. Neurosci. 1993;13:5251–5262. doi: 10.1523/JNEUROSCI.13-12-05251.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman B, Stryker MP, Bonhoeffer T. Development of orientation preference maps in ferret primary visual cortex. J. Neurosci. 1996;16:6443–6453. doi: 10.1523/JNEUROSCI.16-20-06443.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman B, Zahs KR, Stryker MP. Relation of cortical cell orientation selectivity to alignment of receptive fields of the geniculocortical afferents that arborize within a single orientation column in ferret visual cortex. J. Neurosci. 1991;11:1347–1358. doi: 10.1523/JNEUROSCI.11-05-01347.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyaya B, Di Cristo G, Wu CZ, Knott G, Kuhlman S, et al. GAD67-mediated GABA synthesis and signaling regulate inhibitory synaptic innervation in the visual cortex. Neuron. 2007;54:889–903. doi: 10.1016/j.neuron.2007.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Regehr WG. Developmental remodeling of the retinogeniculate synapse. Neuron. 2000;28:955–966. doi: 10.1016/s0896-6273(00)00166-5. [DOI] [PubMed] [Google Scholar]
- Chen Y, Flanagan JG. Follow your nose: axon pathfinding in olfactory map formation. Cell. 2006;127:881–884. doi: 10.1016/j.cell.2006.11.017. [DOI] [PubMed] [Google Scholar]
- Chiu C, Weliky M. Spontaneous activity in developing ferret visual cortex in vivo. J. Neurosci. 2001;21:8906–8914. doi: 10.1523/JNEUROSCI.21-22-08906.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu C, Weliky M. Relationship of correlated spontaneous activity to functional ocular dominance columns in the developing visual cortex. Neuron. 2002;35:1123–1134. doi: 10.1016/s0896-6273(02)00867-x. [DOI] [PubMed] [Google Scholar]
- Cook PM, Prusky G, Ramoa AS. The role of spontaneous retinal activity before eye opening in the maturation of form and function in the retinogeniculate pathway of the ferret. Vis. Neurosci. 1999;16:491–501. doi: 10.1017/s0952523899163107. [DOI] [PubMed] [Google Scholar]
- Corriveau RA, Huh GS, Shatz CJ. Regulation of class I MHC gene expression in the developing and mature CNS by neural activity. Neuron. 1998;21:505–520. doi: 10.1016/s0896-6273(00)80562-0. [DOI] [PubMed] [Google Scholar]
- Crair MC, Gillespie DC, Stryker MP. The role of visual experience in the development of columns in cat visual cortex. Science. 1998;279:566–570. doi: 10.1126/science.279.5350.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crair MC, Horton JC, Antonini A, Stryker MP. Emergence of ocular dominance columns in cat visual cortex by 2 weeks of age. J. Comp. Neurol. 2001;430:235–249. doi: 10.1002/1096-9861(20010205)430:2<235::aid-cne1028>3.0.co;2-p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley JC, Katz LC. Development of ocular dominance columns in the absence of retinal input. Nat. Neurosci. 1999;2:1125–1130. doi: 10.1038/16051. [DOI] [PubMed] [Google Scholar]
- Crowley JC, Katz LC. Early development of ocular dominance columns. Science. 2000;290:1321–1324. doi: 10.1126/science.290.5495.1321. [DOI] [PubMed] [Google Scholar]
- Crowley JC, Katz LC. Ocular dominance development revisited. Curr. Opin. Neurobiol. 2002;12:104–109. doi: 10.1016/s0959-4388(02)00297-0. [DOI] [PubMed] [Google Scholar]
- Demas J, Eglen SJ, Wong RO. Developmental loss of synchronous spontaneous activity in the mouse retina is independent of visual experience. J. Neurosci. 2003;23:2851–2860. doi: 10.1523/JNEUROSCI.23-07-02851.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demas J, Sagdullaev BT, Green E, Jaubert-Miazza L, McCall MA, et al. Failure to maintain eye-specific segregation in nob, a mutant with abnormally patterned retinal activity. Neuron. 2006;50:247–259. doi: 10.1016/j.neuron.2006.03.033. [DOI] [PubMed] [Google Scholar]
- Demb JB. Cellular mechanisms for direction selectivity in the retina. Neuron. 2007;55:179–186. doi: 10.1016/j.neuron.2007.07.001. [DOI] [PubMed] [Google Scholar]
- Di Cristo G, Wu C, Chattopadhyaya B, Ango F, Knott G, et al. Subcellular domain-restricted GABAergic innervation in primary visual cortex in the absence of sensory and thalamic inputs. Nat. Neurosci. 2004;7:1184–1186. doi: 10.1038/nn1334. [DOI] [PubMed] [Google Scholar]
- Dufour A, Seibt J, Passante L, Depaepe V, Ciossek T, et al. Area specificity and topography of thalamocortical projections are controlled by ephrin/Eph genes. Neuron. 2003;39:453–465. doi: 10.1016/s0896-6273(03)00440-9. [DOI] [PubMed] [Google Scholar]
- Eglen SJ. The role of retinal waves and synaptic normalization in retinogeniculate development. Philos. Trans. R. Soc. London Ser. B. 1999;354:497–506. doi: 10.1098/rstb.1999.0400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellsworth CA, Lyckman AW, Feldheim DA, Flanagan JG, Sur M. Ephrin-A2 and -A5 influence patterning of normal and novel retinal projections to the thalamus: conserved mapping mechanisms in visual and auditory thalamic targets. J. Comp. Neurol. 2005;488:140–151. doi: 10.1002/cne.20602. [DOI] [PubMed] [Google Scholar]
- Eysel UT. Maintained activity, excitation and inhibition of lateral geniculate neurons after monocular deafferentation in the adult cat. Brain Res. 1979;166:259–271. doi: 10.1016/0006-8993(79)90212-9. [DOI] [PubMed] [Google Scholar]
- Feldheim DA, Kim YI, Bergemann AD, Frisen J, Barbacid M, Flanagan JG. Genetic analysis of ephrin-A2 and ephrin-A5 shows their requirement in multiple aspects of retinocollicular mapping. Neuron. 2000;25:563–574. doi: 10.1016/s0896-6273(00)81060-0. [DOI] [PubMed] [Google Scholar]
- Feldheim DA, Nakamoto M, Osterfield M, Gale NW, DeChiara TM, et al. Loss-of-function analysis of EphA receptors in retinotectal mapping. J. Neurosci. 2004;24:2542–2550. doi: 10.1523/JNEUROSCI.0239-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldheim DA, Vanderhaeghen P, Hansen MJ, Frisen J, Lu Q, et al. Topographic guidance labels in a sensory projection to the forebrain. Neuron. 1998;21:1303–1313. doi: 10.1016/s0896-6273(00)80650-9. [DOI] [PubMed] [Google Scholar]
- Feller MB. Spontaneous correlated activity in developing neural circuits. Neuron. 1999;22:653–656. doi: 10.1016/s0896-6273(00)80724-2. [DOI] [PubMed] [Google Scholar]
- Feller MB, Butts DA, Aaron HL, Rokhsar DS, Shatz CJ. Dynamic processes shape spatiotemporal properties of retinal waves. Neuron. 1997;19:293–306. doi: 10.1016/s0896-6273(00)80940-x. [DOI] [PubMed] [Google Scholar]
- Feller MB, Wellis DP, Stellwagen D, Werblin FS, Shatz CJ. Requirement for cholinergic synaptic transmission in the propagation of spontaneous retinal waves. Science. 1996;272:1182–1187. doi: 10.1126/science.272.5265.1182. [DOI] [PubMed] [Google Scholar]
- Firth SI, Wang CT, Feller MB. Retinal waves: mechanisms and function in visual system development. Cell Calcium. 2005;37:425–432. doi: 10.1016/j.ceca.2005.01.010. [DOI] [PubMed] [Google Scholar]
- Flanagan JG. Neural map specification by gradients. Curr. Opin. Neurobiol. 2006;16:59–66. doi: 10.1016/j.conb.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Flandrin JM, Jeannerod M. Superior colliculus: environmental influences on the development of directional responses in the kitten. Brain Res. 1975;89:348–352. doi: 10.1016/0006-8993(75)90726-x. [DOI] [PubMed] [Google Scholar]
- Fregnac Y, Imbert M. Early development of visual cortical cells in normal and dark-reared kittens: relationship between orientation selectivity and ocular dominance. J. Physiol. 1978;278:27–44. doi: 10.1113/jphysiol.1978.sp012290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisen J, Yates PA, McLaughlin T, Friedman GC, O’Leary DD, Barbacid M. Ephrin-A5 (AL-1/RAGS) is essential for proper retinal axon guidance and topographic mapping in the mammalian visual system. Neuron. 1998;20:235–243. doi: 10.1016/s0896-6273(00)80452-3. [DOI] [PubMed] [Google Scholar]
- Galli L, Maffei L. Spontaneous impulse activity of rat retinal ganglion cells in prenatal life. Science. 1988;242:90–91. doi: 10.1126/science.3175637. [DOI] [PubMed] [Google Scholar]
- Ghosh A, Antonini A, McConnell SK, Shatz CJ. Requirement for subplate neurons in the formation of thalamocortical connections. Nature. 1990;347:179–181. doi: 10.1038/347179a0. [DOI] [PubMed] [Google Scholar]
- Ghosh A, Shatz CJ. Involvement of subplate neurons in the formation of ocular dominance columns. Science. 1992a;255:1441–1443. doi: 10.1126/science.1542795. [DOI] [PubMed] [Google Scholar]
- Ghosh A, Shatz CJ. Pathfinding and target selection by developing geniculocortical axons. J. Neurosci. 1992b;12:39–55. doi: 10.1523/JNEUROSCI.12-01-00039.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godement P, Salaun J, Imbert M. Prenatal and postnatal development of retinogeniculate and retinocollicular projections in the mouse. J. Comp. Neurol. 1984;230:552–575. doi: 10.1002/cne.902300406. [DOI] [PubMed] [Google Scholar]
- Goodman CS, Shatz CJ. Developmental mechanisms that generate precise patterns of neuronal connectivity. Cell. 1993;72 Suppl.:77–98. doi: 10.1016/s0092-8674(05)80030-3. [DOI] [PubMed] [Google Scholar]
- Grinvald A, Lieke E, Frostig RD, Gilbert CD, Wiesel TN. Functional architecture of cortex revealed by optical imaging of intrinsic signals. Nature. 1986;324:361–364. doi: 10.1038/324361a0. [DOI] [PubMed] [Google Scholar]
- Grubb MS, Rossi FM, Changeux JP, Thompson ID. Abnormal functional organization in the dorsal lateral geniculate nucleus of mice lacking the β2 subunit of the nicotinic acetylcholine receptor. Neuron. 2003;40:1161–1172. doi: 10.1016/s0896-6273(03)00789-x. [DOI] [PubMed] [Google Scholar]
- Guillery RW, LaMantia AS, Robson JA, Huang K. The influence of retinal afferents upon the development of layers in the dorsal lateral geniculate nucleus of mustelids. J. Neurosci. 1985;5:1370–1379. doi: 10.1523/JNEUROSCI.05-05-01370.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanganu IL, Ben-Ari Y, Khazipov R. Retinal waves trigger spindle bursts in the neonatal rat visual cortex. J. Neurosci. 2006;26:6728–6736. doi: 10.1523/JNEUROSCI.0752-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson MG, Landmesser LT. Normal patterns of spontaneous activity are required for correct motor axon guidance and the expression of specific guidance molecules. Neuron. 2004;43:687–701. doi: 10.1016/j.neuron.2004.08.018. [DOI] [PubMed] [Google Scholar]
- Hensch TK. Critical period regulation. Annu. Rev. Neurosci. 2004;27:549–579. doi: 10.1146/annurev.neuro.27.070203.144327. [DOI] [PubMed] [Google Scholar]
- Herrmann K, Antonini A, Shatz CJ. Ultrastructural evidence for synaptic interactions between thalamocortical axons and subplate neurons. Eur. J. Neurosci. 1994;6:1729–1742. doi: 10.1111/j.1460-9568.1994.tb00565.x. [DOI] [PubMed] [Google Scholar]
- Hevner RF. Development of connections in the human visual system during fetal midgestation: a DiI-tracing study. J. Neuropathol. Exp. Neurol. 2000;59:385–392. doi: 10.1093/jnen/59.5.385. [DOI] [PubMed] [Google Scholar]
- Hindges R, McLaughlin T, Genoud N, Henkemeyer M, O’Leary DD. EphB forward signaling controls directional branch extension and arborization required for dorsal-ventral retinotopic mapping. Neuron. 2002;35:475–487. doi: 10.1016/s0896-6273(02)00799-7. [DOI] [PubMed] [Google Scholar]
- Hoffmann KP, Sherman SM. Effects of early monocular deprivation on visual input to cat superior colliculus. J. Neurophysiol. 1974;37:1276–1286. doi: 10.1152/jn.1974.37.6.1276. [DOI] [PubMed] [Google Scholar]
- Hooks BM, Chen C. Distinct roles for spontaneous and visual activity in remodeling of the retinogeniculate synapse. Neuron. 2006;52:281–291. doi: 10.1016/j.neuron.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Horton JC, Hocking DR. An adult-like pattern of ocular dominance columns in striate cortex of newborn monkeys prior to visual experience. J. Neurosci. 1996;16:1791–1807. doi: 10.1523/JNEUROSCI.16-05-01791.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L, Pallas SL. NMDA antagonists in the superior colliculus prevent developmental plasticity but not visual transmission or map compression. J. Neurophysiol. 2001;86:1179–1194. doi: 10.1152/jn.2001.86.3.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. Receptive fields, binocular interaction and functional architecture in the cat’s visual cortex. J. Physiol. 1962;160:106–154. doi: 10.1113/jphysiol.1962.sp006837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. Receptive fields of cells in striate cortex of very young, visually inexperienced kittens. J. Neurophysiol. 1963;26:994–1002. doi: 10.1152/jn.1963.26.6.994. [DOI] [PubMed] [Google Scholar]
- Huberman AD. Nob mice wave goodbye to eye-specific segregation. Neuron. 2006;50:175–177. doi: 10.1016/j.neuron.2006.04.006. [DOI] [PubMed] [Google Scholar]
- Huberman AD. Mechanisms of eye-specific visual circuit development. Curr. Opin. Neurobiol. 2007;17:73–80. doi: 10.1016/j.conb.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Huberman AD, Dehay C, Berland M, Chalupa LM, Kennedy H. Early and rapid targeting of eye-specific axonal projections to the dorsal lateral geniculate nucleus in the fetal macaque. J. Neurosci. 2005a;25:4014–4023. doi: 10.1523/JNEUROSCI.4292-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huberman AD, Murray KD, Warland DK, Feldheim DA, Chapman B. Ephrin-As mediate targeting of eye-specific projections to the lateral geniculate nucleus. Nat. Neurosci. 2005b;8:1013–1021. doi: 10.1038/nn1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huberman AD, Speer CM, Chapman B. Spontaneous retinal activity mediates development of ocular dominance columns and binocular receptive fields in v1. Neuron. 2006;52:247–254. doi: 10.1016/j.neuron.2006.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huberman AD, Stellwagen D, Chapman B. Decoupling eye-specific segregation from lamination in the lateral geniculate nucleus. J. Neurosci. 2002;22:9419–9429. doi: 10.1523/JNEUROSCI.22-21-09419.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huberman AD, Wang GY, Liets LC, Collins OA, Chapman B, Chalupa LM. Eye-specific retinogeniculate segregation independent of normal neuronal activity. Science. 2003;300:994–998. doi: 10.1126/science.1080694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh GS, Boulanger LM, Du H, Riquelme PA, Brotz TM, Shatz CJ. Functional requirement for class I MHC in CNS development and plasticity. Science. 2000;290:2155–2159. doi: 10.1126/science.290.5499.2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issa NP, Trachtenberg JT, Chapman B, Zahs KR, Stryker MP. The critical period for ocular dominance plasticity in the ferret’s visual cortex. J. Neurosci. 1999;19:6965–6978. doi: 10.1523/JNEUROSCI.19-16-06965.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaubert-Miazza L, Green E, Lo FS, Bui K, Mills J, Guido W. Structural and functional composition of the developing retinogeniculate pathway in the mouse. Vis. Neurosci. 2005;22:661–676. doi: 10.1017/S0952523805225154. [DOI] [PubMed] [Google Scholar]
- Jeffery G. Retinotopic order appears before ocular separation in developing visual pathways. Nature. 1985;313:575–576. doi: 10.1038/313575a0. [DOI] [PubMed] [Google Scholar]
- Kanold PO, Kara P, Reid RC, Shatz CJ. Role of subplate neurons in functional maturation of visual cortical columns. Science. 2003;301:521–525. doi: 10.1126/science.1084152. [DOI] [PubMed] [Google Scholar]
- Kanold PO, Shatz CJ. Subplate neurons regulate maturation of cortical inhibition and outcome of ocular dominance plasticity. Neuron. 2006;51:627–638. doi: 10.1016/j.neuron.2006.07.008. [DOI] [PubMed] [Google Scholar]
- Katz LC, Crowley JC. Development of cortical circuits: lessons from ocular dominance columns. Nat. Rev. Neurosci. 2002;3:34–42. doi: 10.1038/nrn703. [DOI] [PubMed] [Google Scholar]
- Katz LC, Shatz CJ. Synaptic activity and the construction of corical circuits. Science. 1996;274:1133–1138. doi: 10.1126/science.274.5290.1133. [DOI] [PubMed] [Google Scholar]
- Kawasaki H, Crowley JC, Livesey FJ, Katz LC. Molecular organization of the ferret visual thalamus. J. Neurosci. 2004;24:9962–9970. doi: 10.1523/JNEUROSCI.2165-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AJ, Schnupp JW, Thompson ID. Signals from the superficial layers of the superior colliculus enable the development of the auditory space map in the deeper layers. J. Neurosci. 1998;18:9394–9408. doi: 10.1523/JNEUROSCI.18-22-09394.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krug K, Akerman CJ, Thompson ID. Responses of neurons in neonatal cortex and thalamus to patterned visual stimulation through the naturally closed lids. J. Neurophysiol. 2001;85:1436–1443. doi: 10.1152/jn.2001.85.4.1436. [DOI] [PubMed] [Google Scholar]
- Land PW, Shamalla-Hannah L. Transient expression of synaptic zinc during development of uncrossed retinogeniculate projections. J. Comp. Neurol. 2001;433:515–525. doi: 10.1002/cne.1157. [DOI] [PubMed] [Google Scholar]
- Law MI, Zahs KR, Stryker MP. Organization of primary visual cortex (area 17) in the ferret. J. Comp. Neurol. 1988;278:157–180. doi: 10.1002/cne.902780202. [DOI] [PubMed] [Google Scholar]
- Lee JS, von der Hardt S, Rusch MA, Stringer SE, Stickney HL, et al. Axon sorting in the optic tract requires HSPG synthesis by ext2 (dackel) and extl3 (boxer) Neuron. 2004;44:947–960. doi: 10.1016/j.neuron.2004.11.029. [DOI] [PubMed] [Google Scholar]
- LeVay S, Stryker MP, Shatz CJ. Ocular dominance columns and their development in layer IV of the cat’s visual cortex: a quantitative study. J. Comp. Neurol. 1978;179:223–244. doi: 10.1002/cne.901790113. [DOI] [PubMed] [Google Scholar]
- Li Y, Fitzpatrick D, White LE. The development of direction selectivity in ferret visual cortex requires early visual experience. Nat. Neurosci. 2006;9:676–681. doi: 10.1038/nn1684. [DOI] [PubMed] [Google Scholar]
- Liets LC, Olshausen BA, Wang GY, Chalupa LM. Spontaneous activity of morphologically identified ganglion cells in the developing ferret retina. J. Neurosci. 2003;23:7343–7350. doi: 10.1523/JNEUROSCI.23-19-07343.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden DC, Guillery RW, Cucchiaro J. The dorsal lateral geniculate nucleus of the normal ferret and its postnatal development. J. Comp. Neurol. 1981;203:189–211. doi: 10.1002/cne.902030204. [DOI] [PubMed] [Google Scholar]
- Maffei L, Galli-Resta L. Correlation in the discharges of neighboring rat retinal ganglion cells during prenatal life. Proc. Natl. Acad. Sci. USA. 1990;87:2861–2864. doi: 10.1073/pnas.87.7.2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin T, O’Leary DD. Molecular gradients and development of retinotopic maps. Annu. Rev. Neurosci. 2005;28:327–355. doi: 10.1146/annurev.neuro.28.061604.135714. [DOI] [PubMed] [Google Scholar]
- McLaughlin T, Torborg CL, Feller MB, O’Leary DD. Retinotopic map refinement requires spontaneous retinal waves during a brief critical period of development. Neuron. 2003;40:1147–1160. doi: 10.1016/s0896-6273(03)00790-6. [DOI] [PubMed] [Google Scholar]
- Meissirel C, Wikler KC, Chalupa LM, Rakic P. Early divergence of magnocellular and parvocellular functional subsystems in the embryonic primate visual system. Proc. Natl. Acad. Sci. USA. 1997;94:5900–5905. doi: 10.1073/pnas.94.11.5900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller KD. Development of orientation columns via competition between ON- and OFF-center inputs. NeuroReport. 1992;3:73–76. doi: 10.1097/00001756-199201000-00019. [DOI] [PubMed] [Google Scholar]
- Miller KD. Models of activity-dependent neural development. Prog. Brain Res. 1994;102:303–318. doi: 10.1016/S0079-6123(08)60548-8. [DOI] [PubMed] [Google Scholar]
- Ming G, Henley J, Tessier-Lavigne M, Song H, Poo M. Electrical activity modulates growth cone guidance by diffusible factors. Neuron. 2001;29:441–452. doi: 10.1016/s0896-6273(01)00217-3. [DOI] [PubMed] [Google Scholar]
- Mombaerts P. Axonal wiring in the mouse olfactory system. Annu. Rev. Cell Dev. Biol. 2006;22:713–737. doi: 10.1146/annurev.cellbio.21.012804.093915. [DOI] [PubMed] [Google Scholar]
- Mooney R, Penn AA, Gallego R, Shatz CJ. Thalamic relay of spontaneous retinal activity prior to vision. Neuron. 1996;17:863–874. doi: 10.1016/s0896-6273(00)80218-4. [DOI] [PubMed] [Google Scholar]
- Morgan J, Thompson ID. The segregation of ON- and OFF-center responses in the lateral geniculate nucleus of normal and monocularly enucleated ferrets. Vis. Neurosci. 1993;10:303–311. doi: 10.1017/s0952523800003709. [DOI] [PubMed] [Google Scholar]
- Mrsic-Flogel TD, Hofer SB, Creutzfeldt C, Cloëz-Tayarani I, Changeux JP, et al. Altered map of visual space in the superior colliculus of mice lacking early retinal waves. J. Neurosci. 2005;25:6921–6928. doi: 10.1523/JNEUROSCI.1555-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muir-Robinson G, Hwang BJ, Feller MB. Retinogeniculate axons undergo eye-specific segregation in the absence of eye-specific layers. J. Neurosci. 2002;22:5259–5264. doi: 10.1523/JNEUROSCI.22-13-05259.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muscat L, Huberman AD, Jordan CL, Morin LP. Crossed and uncrossed retinal projections to the hamster circadian system. J. Comp. Neurol. 2003;466:513–524. doi: 10.1002/cne.10894. [DOI] [PubMed] [Google Scholar]
- Myhr KL, Lukasiewicz PD, Wong RO. Mechanisms underlying developmental changes in the firing patterns of ON and OFF retinal ganglion cells during refinement of their central projections. J. Neurosci. 2001;21:8664–8671. doi: 10.1523/JNEUROSCI.21-21-08664.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson R, Famiglietti EV, Jr, Kolb H. Intracellular staining reveals different levels of stratification for on- and off-center ganglion cells in cat retina. J. Neurophysiol. 1978;41:472–483. doi: 10.1152/jn.1978.41.2.472. [DOI] [PubMed] [Google Scholar]
- Nicol X, Voyatzis S, Muzerelle A, Narboux-Nême N, Südhof TC, et al. cAMP oscillations and retinal activity are permissive for ephrin signaling during the establishment of the retinotopic map. Nat. Neurosci. 2007;10:340–347. doi: 10.1038/nn1842. [DOI] [PubMed] [Google Scholar]
- Ohshiro T, Weliky M. Simple fall-off pattern of correlated neural activity in the developing lateral geniculate nucleus. Nat. Neurosci. 2006;9:1541–1548. doi: 10.1038/nn1799. [DOI] [PubMed] [Google Scholar]
- O’Leary DD, McLaughlin T. Mechanisms of retinotopic map development: Ephs, ephrins, and spontaneous correlated retinal activity. Prog. Brain Res. 2005;147:43–65. doi: 10.1016/S0079-6123(04)47005-8. [DOI] [PubMed] [Google Scholar]
- Penn AA, Riquelme PA, Feller MB, Shatz CJ. Competition in retinogeniculate patterning driven by spontaneous activity. Science. 1998;279:2108–2112. doi: 10.1126/science.279.5359.2108. [DOI] [PubMed] [Google Scholar]
- Petros TJ, Rebsam A, Mason C. Retinal axon growth at the optic chasm: to cross or not to cross. Annu. Rev. Neurosci. 2008;31:295–315. doi: 10.1146/annurev.neuro.31.060407.125609. [DOI] [PubMed] [Google Scholar]
- Pettigrew JD. The effect of visual experience on the development of stimulus specificity by kitten cortical neurones. J. Physiol. 1974;237:49–74. doi: 10.1113/jphysiol.1974.sp010469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffenberger C, Cutforth T, Woods G, Yamada J, Renteria RC, et al. Ephrin-As and neural activity are required for eye-specific patterning during retinogeniculate mapping. Nat. Neurosci. 2005;8:1022–1027. doi: 10.1038/nn1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffenberger C, Yamada J, Feldheim DA. Ephrin-As and patterned retinal activity act together in the development of topographic maps in the primary visual system. J. Neurosci. 2006;26:12873–12884. doi: 10.1523/JNEUROSCI.3595-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plas DT, Lopez JE, Crair MC. Pretarget sorting of retincollicular axons in the mouse. J. Comp. Neurol. 2005;491:305–319. doi: 10.1002/cne.20694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poskanzer K, Needleman LA, Bozdagi O, Huntley GW. N-cadherin regulates ingrowth and laminar targeting of thalamocortical axons. J. Neurosci. 2003;23:2294–2305. doi: 10.1523/JNEUROSCI.23-06-02294.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakic P. Prenatal genesis of connections subserving ocular dominance in the rhesus monkey. Nature. 1976;261:467–471. doi: 10.1038/261467a0. [DOI] [PubMed] [Google Scholar]
- Ramoa AS, Mower AF, Liao D, Jafri SI. Suppression of cortical NMDA receptor function prevents development of orientation selectivity in the primary visual cortex. J. Neurosci. 2001;21:4299–4309. doi: 10.1523/JNEUROSCI.21-12-04299.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reese BE. The position of the crossed and uncrossed optic axons, and the nonoptic axons, in the optic tract of the rat. Neuroscience. 1987;22:1025–1039. doi: 10.1016/0306-4522(87)92978-2. [DOI] [PubMed] [Google Scholar]
- Reese BE, Cowey A. Segregation of functionally distinct axons in the monkey’s optic tract. Nature. 1988;331:350–351. doi: 10.1038/331350a0. [DOI] [PubMed] [Google Scholar]
- Reid RC, Alonso JM. Specificity of monosynaptic connections from thalamus to visual cortex. Nature. 1995;378:281–284. doi: 10.1038/378281a0. [DOI] [PubMed] [Google Scholar]
- Rossi FM, Pizzorusso T, Porciatti V, Marubio LM, Maffei L, Changeux JP. Requirement of the nicotinic acetylcholine receptor beta 2 subunit for the anatomical and functional development of the visual system. Proc. Natl. Acad. Sci. USA. 2001;98:6453–6458. doi: 10.1073/pnas.101120998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruthazer ES, Baker GE, Stryker MP. Development and organization of ocular dominance bands in primary visual cortex of the sable ferret. J. Comp. Neurol. 1999;407:151–165. [PMC free article] [PubMed] [Google Scholar]
- Sahay A, Kim CH, Sepkuty JP, Cho E, Huganir RL, et al. Secreted semaphorins modulate synaptic transmission in the adult hippocampus. J. Neurosci. 2005;25:3613–3620. doi: 10.1523/JNEUROSCI.5255-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salinas PC, Zou Y. Wnt signaling and neuronal circuit assembly. Annu. Rev. Neurosci. 2008;31:339–358. doi: 10.1146/annurev.neuro.31.060407.125649. [DOI] [PubMed] [Google Scholar]
- Sanes JR, Yamagata M. Formation of lamina-specific synaptic connections. Curr. Opin. Neurobiol. 1999;9:79–87. doi: 10.1016/s0959-4388(99)80010-5. [DOI] [PubMed] [Google Scholar]
- Schmitt AM, Shi J, Wolf AM, Lu CC, King LA, Zou Y. Wnt-Ryk signalling mediates medial-lateral retinotectal topographic mapping. Nature. 2006;439:31–37. doi: 10.1038/nature04334. [DOI] [PubMed] [Google Scholar]
- Sernagor E, Eglen SJ, Wong RO. Development of retinal ganglion cell structure and function. Prog. Retin. Eye Res. 2001;20:139–174. doi: 10.1016/s1350-9462(00)00024-0. [DOI] [PubMed] [Google Scholar]
- Shatz CJ. The prenatal development of the cat’s retinogeniculate pathway. J. Neurosci. 1983;3:482–499. doi: 10.1523/JNEUROSCI.03-03-00482.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shatz CJ, Kirkwood PA. Prenatal development of functional connections in the cat’s retinogeniculate pathway. J. Neurosci. 1984;4:1378–1397. doi: 10.1523/JNEUROSCI.04-05-01378.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman SM. Interneurons and triadic circuitry of the thalamus. Trends Neurosci. 2004;27:670–675. doi: 10.1016/j.tins.2004.08.003. [DOI] [PubMed] [Google Scholar]
- Simon DK, Prusky GT, O’Leary DD, Constantine-Paton M. N-methyl-d-aspartate receptor antagonists disrupt the formation of a mammalian neural map. Proc. Natl. Acad. Sci. USA. 1992;89:10593–10597. doi: 10.1073/pnas.89.22.10593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear PD, Tong L, Langsetmo A. Striate cortex neurons of binocularly deprived kittens respond to visual stimuli through the closed eyelids. Brain Res. 1978;155:141–146. doi: 10.1016/0006-8993(78)90315-3. [DOI] [PubMed] [Google Scholar]
- Sperry RW. Chemoaffinity in the orderly growth of nerve fiber patterns and connections. Proc. Natl. Acad. Sci. USA. 1963;50:703–710. doi: 10.1073/pnas.50.4.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sretavan D, Shatz CJ. Prenatal development of individual retinogeniculate axons during the period of segregation. Nature. 1974;308:845–848. doi: 10.1038/308845a0. [DOI] [PubMed] [Google Scholar]
- Sretavan DW, Shatz CJ. Prenatal development of cat retinogeniculate axon arbors in the absence of binocular interactions. J. Neurosci. 1986;6:990–1003. doi: 10.1523/JNEUROSCI.06-04-00990.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sretavan DW, Shatz CJ. Axon trajectories and pattern of terminal arborization during the prenatal development of the cat’s retinogeniculate pathway. J. Comp. Neurol. 1987;255:386–400. doi: 10.1002/cne.902550306. [DOI] [PubMed] [Google Scholar]
- Sretavan DW, Shatz CJ, Stryker MP. Modification of retinal ganglion cell axon morphology by prenatal infusion of tetrodotoxin. Nature. 1988;336:468–471. doi: 10.1038/336468a0. [DOI] [PubMed] [Google Scholar]
- Stellwagen D, Shatz CJ. An instructive role for retinal waves in the development of retinogeniculate connectivity. Neuron. 2002;33:357–367. doi: 10.1016/s0896-6273(02)00577-9. [DOI] [PubMed] [Google Scholar]
- Stellwagen D, Shatz CJ, Feller MB. Dynamics of retinal waves are controlled by cyclic AMP. Neuron. 1999;24:673–685. doi: 10.1016/s0896-6273(00)81121-6. [DOI] [PubMed] [Google Scholar]
- Stevens B, Allen NJ, Vasquez LE, Christopherson KS, Nouri N, et al. The classical complement cascade mediates developmental synapse elimination. Cell. 2007;131:1164–1178. doi: 10.1016/j.cell.2007.10.036. [DOI] [PubMed] [Google Scholar]
- Stryker MP, Harris WA. Binocular impulse blockade prevents the formation of ocular dominance columns in cat visual cortex. J. Neurosci. 1986;6:2117–2133. doi: 10.1523/JNEUROSCI.06-08-02117.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stryker MP, Strickland S. Physiological segregation of oculr dominance columns depends on the pattern of afferent electrical activity. Invest. Opthal. Vis. Sci. (Suppl) 1984;25:278. [Google Scholar]
- Stryker MP, Zahs KR. On and off sublaminae in the lateral geniculate nucleus of the ferret. J. Neurosci. 1983;3:1943–1951. doi: 10.1523/JNEUROSCI.03-10-01943.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sur M, Sherman SM. Retinogeniculate terminations in cats: morphological differences between X and Y cell axons. Science. 1982;218:389. doi: 10.1126/science.7123239. [DOI] [PubMed] [Google Scholar]
- Syed MM, Lee S, Zheng J, Zhou ZJ. Stage-dependent dynamics and modulation of spontaneous waves in the developing rabbit retina. J. Physiol. 2004;560:533–549. doi: 10.1113/jphysiol.2004.066597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavazoie SF, Reid RC. Diverse receptive fields in the lateral geniculate nucleus during thalamocortical development. Nat. Neurosci. 2000;3:608–616. doi: 10.1038/75786. [DOI] [PubMed] [Google Scholar]
- Thompson ID, Kossut M, Blakemore C. Development of orientation columns in cat striate cortex revealed by 2-deoxyglucose autoradiography. Nature. 1983;301:712–715. doi: 10.1038/301712a0. [DOI] [PubMed] [Google Scholar]
- Tian N, Copenhagen DR. Visual stimulation is required for refinement of ON and OFF pathways in postnatal retina. Neuron. 2003;39:85–96. doi: 10.1016/s0896-6273(03)00389-1. [DOI] [PubMed] [Google Scholar]
- Torborg CL, Hansen KA, Feller MB. High frequency, synchronized bursting drives eye-specific segregation of retinogeniculate projections. Nat. Neurosci. 2005;8:72–78. doi: 10.1038/nn1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uesaka N, Hayano Y, Yamada A, Yamamoto N. Interplay between laminar specificity and activity-dependent mechanisms of thalamocortical axon branching. J. Neurosci. 2007;27:5215–5223. doi: 10.1523/JNEUROSCI.4685-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaney DI, Taylor WR. Direction selectivity in the retina. Curr. Opin. Neurobiol. 2002;12:405–410. doi: 10.1016/s0959-4388(02)00337-9. [DOI] [PubMed] [Google Scholar]
- Walsh C, Guillery RW. Age-related fiber order in the optic tract of the ferret. J. Neurosci. 1985;5:3061–3069. doi: 10.1523/JNEUROSCI.05-11-03061.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warland DK, Huberman AD, Chalupa LM. Dynamics of spontaneous activity in the fetal macaque retina during development of retinogeniculate pathways. J. Neurosci. 2006;26:5190–5197. doi: 10.1523/JNEUROSCI.0328-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weliky M, Bosking WH, Fitzpatrick D. A systematic map of direction preference in primary visual cortex. Nature. 1996;379:725–728. doi: 10.1038/379725a0. [DOI] [PubMed] [Google Scholar]
- Weliky M, Katz LC. Disruption of orientation tuning in visual cortex by artificially correlated neuronal activity. Nature. 1997;386:680–685. doi: 10.1038/386680a0. [DOI] [PubMed] [Google Scholar]
- Weliky M, Katz LC. Correlational structure of spontaneous neuronal activity in the developing lateral geniculate nucleus in vivo. Science. 1999;285:599–604. doi: 10.1126/science.285.5427.599. [DOI] [PubMed] [Google Scholar]
- White LE, Coppola DM, Fitzpatrick D. The contribution of sensory experience to the maturation of orientation selectivity in ferret visual cortex. Nature. 2001;411:1049–1052. doi: 10.1038/35082568. [DOI] [PubMed] [Google Scholar]
- Wiesel TN, Hubel DH. Effects of visual deprivation on morphology and physiology of cells in the cats lateral geniculate body. J. Neurophysiol. 1963a;26:978–993. doi: 10.1152/jn.1963.26.6.978. [DOI] [PubMed] [Google Scholar]
- Wiesel TN, Hubel DH. Single-cell responses in striate cortex of kittens deprived of vision in one eye. J. Neurophysiol. 1963b;26:1003–1017. doi: 10.1152/jn.1963.26.6.1003. [DOI] [PubMed] [Google Scholar]
- Wiesel TN, Hubel DH. Comparison of the effects of unilateral and bilateral eye closure on cortical unit responses in kittens. J. Neurophysiol. 1965a;28:1029–1040. doi: 10.1152/jn.1965.28.6.1029. [DOI] [PubMed] [Google Scholar]
- Wiesel TN, Hubel DH. Extent of recovery from the effects of visual deprivation in kittens. J. Neurophysiol. 1965b;28:1060–1072. doi: 10.1152/jn.1965.28.6.1060. [DOI] [PubMed] [Google Scholar]
- Wiesel TN, Hubel DH. Ordered arrangement of orientation columns in monkeys lacking visual experience. J. Comp. Neurol. 1974;158:307–318. doi: 10.1002/cne.901580306. [DOI] [PubMed] [Google Scholar]
- Wiesel TN, Hubel DH, Lam DM. Autoradiographic demonstration of ocular-dominance columns in the monkey striate cortex by means of transneuronal transport. Brain Res. 1974;79:273–279. doi: 10.1016/0006-8993(74)90416-8. [DOI] [PubMed] [Google Scholar]
- Williams RW, Hogan D, Garraghty PE. Target recognition and visual maps in the thalamus of achiasmatic dogs. Nature. 1994;367:637–639. doi: 10.1038/367637a0. [DOI] [PubMed] [Google Scholar]
- Williams SE, Grumet M, Colman DR, Henkemeyer M, Mason CA, Sakurai T. A role for Nr-CAM in the patterning of binocular visual pathways. Neuron. 2006;50:535–547. doi: 10.1016/j.neuron.2006.03.037. [DOI] [PubMed] [Google Scholar]
- Williams SE, Mann F, Erskine L, Sakurai T, Wei S, et al. Ephrin-B2 and EphB1 mediate retinal axon divergence at the optic chiasm. Neuron. 2003;39:919–935. doi: 10.1016/j.neuron.2003.08.017. [DOI] [PubMed] [Google Scholar]
- Wong RO. Retinal waves and visual system development. Annu. Rev. Neurosci. 1999;22:29–47. doi: 10.1146/annurev.neuro.22.1.29. [DOI] [PubMed] [Google Scholar]
- Wong RO, Meister M, Shatz CJ. Transient period of correlated bursting activity during development of the mammalian retina. Neuron. 1993;11:923–938. doi: 10.1016/0896-6273(93)90122-8. [DOI] [PubMed] [Google Scholar]
- Wong RO, Oakley DM. Changing patterns of spontaneous bursting activity of on and off retinal ganglion cells during development. Neuron. 1996;16:1087–1095. doi: 10.1016/s0896-6273(00)80135-x. [DOI] [PubMed] [Google Scholar]
- Wong WT, Myhr KL, Miller ED, Wong RO. Developmental changes in the neurotransmitter regulation of correlated spontaneous retinal activity. J. Neurosci. 2000;20:351–360. doi: 10.1523/JNEUROSCI.20-01-00351.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagata M, Sanes JR. Dscam and Sidekick proteins direct lamina-specific synaptic connections in vertebrate retina. Nature. 2008;451:465–469. doi: 10.1038/nature06469. [DOI] [PubMed] [Google Scholar]
- Yamagata M, Weiner JA, Sanes JR. Sidekicks: synaptic adhesion molecules that promote lamina-specific connectivity in the retina. Cell. 2002;110:649–660. doi: 10.1016/s0092-8674(02)00910-8. [DOI] [PubMed] [Google Scholar]
- Yamamoto N, Matsuyama Y, Harada A, Inui K, Murakami F, Hanamura K. Characterization of factors regulating lamina-specific growth of thalamocortical axons. J. Neurobiol. 2000;42:56–68. doi: 10.1002/(sici)1097-4695(200001)42:1<56::aid-neu6>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Yates PA, Holub AD, McLaughlin T, Sejnowski TJ, O’Leary DD. Computational modeling of retinotopic map development to define contributions of EphA-ephrinA gradients, axon-axon interactions, and patterned activity. J. Neurobiol. 2004;59:95–113. doi: 10.1002/neu.10341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu CR, Power J, Barnea G, O’Donnell S, Brown HE, et al. Spontaneous neural activity is required for the establishment and maintenance of the olfactory sensory map. Neuron. 2004;42:553–566. doi: 10.1016/s0896-6273(04)00224-7. [DOI] [PubMed] [Google Scholar]
- Zheng JJ, Lee S, Zhou ZJ. A developmental switch in the excitability and function of the starburst network in the mammalian retina. Neuron. 2004;44:851–864. doi: 10.1016/j.neuron.2004.11.015. [DOI] [PubMed] [Google Scholar]
- Zhou ZJ. Direct participation of starburst amacrine cells in spontaneous rhythmic activities in the developing mammalian retina. J. Neurosci. 1998;18:4155–4165. doi: 10.1523/JNEUROSCI.18-11-04155.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou ZJ, Zhao D. Coordinated transitions in neurotransmitter systems for the initiation and propagation of spontaneous retinal waves. J. Neurosci. 2000;20:6570–6577. doi: 10.1523/JNEUROSCI.20-17-06570.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]