Abstract
Background
The role of homocysteine in atherosclerosis is unclear. We examined the relationship between plasma homocysteine and infrarenal aortic calcification, the presence of homocysteine in human atheroma and the influence of homocysteine on osteogenic differentiation in vitro.
Methods and Results
In 194 patients with symptomatic peripheral artery disease or abdominal aortic aneurysm, fasting plasma total homocysteine was independently associated with the severity of infrarenal aortic calcification measured by Computer Tomography Angiography (odds ratio 1.91, 95% confidence interval 1.17–3.21 for calcification ≥ median). Homocysteine was identified in all 60 atheroma biopsies from 16 patients undergoing endarterectomy, and concentrations were significantly greater in the calcified biopsies (p=0.003). In vitro studies demonstrated that 100μmol/L homocysteine doubled the calcium deposition by mesenchymal stem cells during 16 days incubation in osteogenic medium (74±4 compared to 42±5 μg calcium/well without homocysteine, p<0.001). Homocysteine also stimulated monocytic THP1 cells to promote aortic smooth muscle cell calcification as evidenced by significant higher calcium deposition and alkaline phosphatase activity compared to incubation without homocysteine (p ≤ 0.05).
Conclusions
Homocysteine plays an important role in vascular calcification via multiple mechanisms. The presence of homocysteine in atheroma and its ability to enhance osteogenic cell differentiation may partly explain the association of homocysteine with atherosclerotic events.
Keywords: calcification, homocysteine, atheroma, osteogenic differentiation
1. Introduction
Vascular calcification is a significant risk factor for the occurrence and complications of atherosclerotic disease although its exact importance remains unclear 1, 2. Clinical investigations of vascular calcification have most frequently concentrated on the coronary circulation with relatively few studies investigating the abdominal aorta 1–4. Those investigators studying the abdominal aorta have commonly used subjective measures to quantify the severity of calcification, such as plain X-ray 4, or employed assessment techniques in which the measurement error is unknown or not stated 3. Recently, we have developed a reproducible technique to quantify infrarenal abdominal aortic calcification using Computer Tomography Angiography (CTA) 5. The technique allows the simultaneous assessment of both luminal narrowing and quantification of aortic calcification. Aortic calcification has been correlated with atherosclerotic disease in patients with abdominal aortic aneurysm 6 and with the prevalence of claudication and aortic aneurysm in hemodialysis patients 7. In addition to being a possible risk factor in arterial disease progression, calcification also has important consequences for the treatment options by preventing or complicating endovascular surgical repair 8, 9 In this study, aortic calcification is measured in patients with peripheral arterial disease and abdominal aortic aneurysm in search for possible risk factors.
The mechanisms and cellular control underlying vascular calcification are controversial 10, 11. The presence of bone remodelling cytokines and bone-like cells adjacent to areas of calcification within atherosclerosis suggests that arterial mineralisation is an active cell-mediated process similar to osteogenesis 12, 13. Cells controlling arterial mineralisation have been suggested to originate from two main sources 11. Most support has grown for the theory that aortic smooth muscle cells (AoSMC) resident in the atherosclerotic vascular wall are stimulated to adopt an osteogenic phenotype and become calcifying vascular cells 14, 15. An alternative theory suggests that circulating cells such as monocytic cells 16–18 or mesenchymal stem cells (MSC) 19–22 enter the atherosclerotic lesions and differentiate into osteogenic cells.
Another unsettled issue is the role of homocysteine (Hcy) in cardiovascular disease 23. Plasma total Hcy (tHcy) concentration has been repeatedly linked to the presence of clinical events associated with atherosclerosis 24, 25. A number of randomised trials however have failed to demonstrate a reduction in myocardial infarction events with vitamin therapy which has reduced blood tHcy concentration by approximately 20% 26, 27. However a 21–24% reduction in stroke events was observed after tHcy-lowering vitamin B therapy 27, 28. These data suggest that the effects of tHcy within atheroma may vary from one vascular bed to another and that improved understanding of the mechanisms linking atherosclerosis and tHcy is required in order to guide new therapy development. Circulating tHcy has previously been associated with coronary calcification in some 29, 30 but not 31, 32 all studies. Due to the paucity of studies accurately quantifying abdominal aortic calcification however, the importance of tHcy in calcification in peripheral arteries remains less clear 33, 34.
In the present study, we examined the relationship between plasma tHcy and abdominal aortic calcification and investigated the presence of tHcy in human atheroma biopsies in relation to calcification. We also conducted a series of in vitro mechanistic studies assessing the influence of Hcy on the osteogenic differentiation of MSC, monocytic cells and AoSMC. We postulate that Hcy may contribute to vascular calcification by enhancing the osteogenic potential of these cells.
2. Methods
Study design
In this study we investigated the following hypotheses:
Plasma Hcy is independently associated with the severity of infrarenal abdominal aortic calcification.
Hcy is present in atheroma and correlates to the severity of calcification.
Hcy stimulates in vitro calcification by enhancing osteogenic differentiation of cells involved in atheroma development.
Patients
Ethical approval for the study was granted by the local Institutional Ethics Committee and written informed consent was obtained from all participants. We prospectively entered consecutive patients undergoing CTA referred to our vascular service for investigation of symptomatic peripheral artery disease (PAD) or abdominal aortic aneurysm (AAA) between May 2004 and June 2007. For ethical reasons to be included in the study the patient had to require a CTA for assessment of their PAD according to the treating consultant vascular surgeon. The primary local indications for CTA include assessment of the suitability of PAD for angioplasty or stenting in patients with intermittent claudication and anatomical assessment of AAA. Patients with critical lower limb ischaemia are usually investigated by conventional angiography and therefore were excluded. Other exclusion criteria included: Previous open or endovascular aortic surgery, contrast allergy and renal impairment evidenced by serum creatinine ≥ 150μmol/L.
Patient assessment and definitions
On entry to the study patients were interviewed and examined by a consultant vascular surgeon who recorded: Age, sex, presenting complaint, past history of coronary heart disease (CHD), diabetes, hypertension, smoking, current medications and physical examination 35. Intermittent claudication was defined by an appropriate history obtained by a vascular specialist, a positive Edinburgh claudication questionnaire and confirmation of significant stenosis (>50%) or occlusion of lower limb arteries on CTA. AAA was defined by a maximum aortic diameter of ≥ 30mm on CTA. Hypertension was defined by a history of high blood pressure or receiving treatment to reduce blood pressure. Diabetes was defined by a fasting blood glucose ≥ 7.0mmol/L, or history of, or treatment for hyperglycaemia. Smoking status was classified into current smokers (smoked within the last month), ex-smokers (given up for more than one month) and never smokers 36.
Blood assessment
Fasting venous blood samples were collected from patients on the morning of the CTA. Hemoglobin, white blood count, serum creatinine, glucose, cholesterol, triglyceride, high density lipoprotein (HDL), low density lipoprotein (LDL), C-reactive protein (CRP) and plasma tHcy (sum of Hcy, homocystine Hcy-Hcy, mixed disulfides Hcy-Cys and protein-bound forms of Hcy) were measured by automated assays, as previously described 35–37.
Quantification of infrarenal aortic calcification volume
CTA images were obtained using a 16-slice multiscanner (Philips). The imaging and workstation protocols utilised to measure infrarenal abdominal aortic calcification have previously been validated in detail 5. Briefly selected images were reconstructed on a workstation utilising defined thresholds (Center Hounsfield Unit (CH) level 1400 and Window width Hounsfield Unit (WH) 2000), automated function setting and image magnification. Intra- and inter-observer coefficients of variation were approximately 1%.
Assessment of human atheroma biopsies
During the course of the study 16 patients underwent open surgical treatment of their PAD by carotid (n=14) or femoral (n=2) endarterectomy. These samples were utilised to investigate the presence of Hcy within atherosclerosis and its relationship to calcification. 5mm2 biopsies were cut from the site of main macroscopic disease from each endarterectomy sample. Most samples gave rise to multiple biopsies (n=1–9) with half of the patient samples providing 4 biopsies. Biopsies were defined as calcified when appearing hard or bone-like on handling and cutting. Biopsies were stored at −80°C for later analysis. Subsequently proteins were extracted from the biopsies using cracking buffer (30mmol/L Tris–HCl, 3.2% sodium dodecyl sulphate, 32% glycerol, 0.2mol/L dithiothreitol). The presence of protein-bound Hcy within atheroma protein samples was assessed by dot blot method applying a 1/10000 dilution of rabbit polyclonal anti-Hcy antibody (ab15154, Abcam). In addition, total Hcy (tHcy) within atheroma biopsies was quantified using a fluorescence polarisation immunoassay (AxSYM System, Abbott) according to the instructions of the AxSYM® Homocysteine Assay (sensitivity ≤ 0.8μmol/L, coefficient of variation ≤ 5.1%). Using biopsies from one patient in which a large femoral atheroma was removed from both groins, calcification was also estimated by measuring calcium (Ca) concentrations within biopsies (Cobas® Integra Ca-assay kit and Cobas Integra 700 Spectrophotometer, Roche). Both tHcy and Ca concentrations were normalised against total protein content measured with the Bradford technique (Biorad) 38, 39.
Cell culture studies
To examine potential cellular targets for Hcy, we carried out a range of in vitro studies exposing human MSC, human monocytes (both peripheral isolated and THP-1 cell line) and AoSMC to osteogenic medium and assessing the effect of Hcy on calcification. Human MSC (PT2501) and AoSMC (CC2571) were purchased from Lonza and maintained in MSC growth medium (PT3001, Lonza) and AoSMC growth medium (CC3182, Lonza) respectively. The monocyte THP-1 cell line was kindly provided by Dr. Rajiv Khanna (Queensland Institute of Medical Research) and maintained in RPMI, supplemented with 25mmol/L HEPES (JRH Biosciences), penicillin (100U/mL), streptomycin (100μg/mL) and 10% fetal bovine serum (Invitrogen). Human peripheral blood mononuclear cells (PBMNC) were isolated from heparinised blood obtained from healthy adults by density gradient centrifugation through Histopaque-1077 (Sigma). For all experiments, cells between passage 3 and 6 were cultured in T75 flasks (Sarsted) in a humidified 5% CO2 atmosphere at 37°C. Medium was refreshed every 2–3 days and at confluence, cells were replated in osteogenic medium (containing β-glycerophosphate, PT3002, Lonza) for use in the following experiments.
The effect of Hcy on circulating cells
MSC (3 × 104/well), freshly isolated PBMNC’s (6 × 105/well) and THP-1 cells (6 × 105/well) were replated in 6 well plates (Nunc) and cultured in osteogenic medium in the absence or presence of 100μmol/L DL-Hcy (Sigma). For comparison, in an additional 6 well plate, cells were maintained in growth medium. Medium was refreshed every 2–3 days and at day 16, osteogenic differentiation was evaluated as explained below.
The effect of Hcy on AoSMC
To study the ability of Hcy to induce osteogenic differentiation, AoSMC were cultured in T75 flasks (7.5 × 105/mL) in growth medium with 0, 10 and 100 μmol/L DL-Hcy. After 14 days, RNA was extracted from cells using the RNeasy Mini kit (Qiagen) and analysed on the HP 2100 Bioanalyzer (Agilent Technologies) for integrity. RNA hybridisation was performed, and gene expression profiles determined, using the CodeLink™ Human Whole Human Genome Bioarray (GE Healthcare Life Sciences). Four biological replicates were prepared for each treatment condition. Processed chips were scanned using an Axon GenePix 4000B scanner at 635 nm and images analyzed with CodeLink™ Expression Analysis software (GE Healthcare Life Sciences). Background-adjusted spot intensity values were imported into the web-based microarray analysis system, GeneSifter™ (http://www.genesifter.net/web/) for gene expression data comparison and analysis. In a separate experiment, AoSMC were replated in 6 well plates until confluent and switched to osteogenic medium in the absence or presence of 100μmol/L DL-Hcy. For comparison, in an additional 6 well plate, cells were maintained in growth medium. Medium was refreshed every 2–3 days and at day 16, osteogenic differentiation was evaluated as explained below. In a further experiment AoSMC were pre-incubated with osteogenic medium for 12 days. The pre-incubated cells (5 × 104/well) were cultured an additional 12 days in osteogenic medium with or without 100μmol/L DL-Hcy and osteogenic differentiation was evaluated.
The effect of Hcy on the interaction between monocytic cells and AoSMC
We investigated if the ability of monocytic cells to alter osteogenic differentiation of AoSMC was influenced by the presence of Hcy. Non-activated and activated (+160nmol/L phorbol myristate acetate (PMA), Sigma) THP-1 cells were cultured in osteogenic medium in the absence or presence of 100μmol/L Hcy for 2 days followed by 24h incubation with fresh osteogenic medium without Hcy or PMA. The conditioned media from the later 24h period (i.e. not containing any Hcy or PMA) was collected by centrifugation and applied to AoSMC which had been pre-incubated in osteogenic medium for 12 days (5 × 104/well). The THP-conditioned medium was refreshed every 3 days and after an additional 12 days, the osteogenic differentiation of the AoSMC was evaluated.
Osteogenic differentiation
The influence of Hcy on calcification was assessed by measuring the calcium (Ca) content of the cultured cell layer, performing Von Kossa staining for mineralised deposits and quantifying alkaline phosphatase (ALP) activity as a measure of osteogenic differentiation.
Ca quantification
Appropriate culture wells were rinsed twice with phosphate-buffered saline and the contents of the well scraped into 0.5N HCl. Ca was extracted by overnight shaking at 4°C followed by 5 min centrifugation at 1000g. The Ca content in the supernatant was determined colorimetrically by measuring the complexed product with phenolsulponephthalein at 612 nm (Sunrise™ Microplate Reader, Tecan) according to instructions of the Quantichrom™ Calcium Assay Kit (DICA-500, BioAssay Systems). Ca deposition was expressed as μg Ca/well or normalised for total protein content as determined by the Bradford method (Bio-Rad Protein Assay) 38, 39.
Von Kossa staining
Mineralisation of the cell layer was investigated using Von Kossa staining as previously described 40. Briefly, fixed cell layers were incubated in 5% silver nitrate (Pro Sci Tech) solution for 30 min in the dark, washed with distilled water and exposed to bright light for 45 min. Staining was evaluated microscopically.
ALP activity
ALP activity of the cell layer was quantified colorimetrically according to the instructions of the Sensolyte™ ALP Assay kit (AnaApec). Enzyme activity was calculated measuring the yellow p-nitrophenol product formed at 405 nm (Sunrise™ Microplate Reader, Tecan) and expressed as ng ALP normalised for total protein content of the cell layer as determined by the Bradford method (Biorad) 38, 39.
Statistical Analysis
Infrarenal abdominal aortic calcification values were not normally distributed even after log transformation. The relationship between plasma tHcy and infrarenal aortic calcification was therefore examined with non-parametric statistics, including Spearman’s correlation and binary logistic regression analysis. For multiple regression analysis infrarenal aortic calcification volumes were defined as < or ≥ median values. We examined the association of plasma tHcy (per 5μM increase) with upper calcification volume median adjusting for age (per 5 years), sex, diabetes, CHD, hypertension, smoking history, medications, serum creatinine (per 100μM) and maximum aortic diameter (per 5mm). The amount of tHcy that could be extracted from atheroma biopsies was reported as pmol per mg of total protein and compared between calcified and non-calcified biopsies using box plots and Mann-Whitney test. The correlation between extracted tHcy and Ca was assessed with Spearman’s test. Cell culture results were expressed as mean±sem and compared by Mann-Whitney test. All microarray data were log transformed. Genes were considered to be differentially expressed based on a 1.5 fold change in response to Hcy and a p value <0.05 by ANOVA with Benjamini and Hochberg correction. The data from the individual bioarrays have been deposited in NCBIs Gene Expression Omnibus (GEO; http://www.ncbi.nlm.nih.gov/geo/) and are accessible through GEO accession number GSE9490.
3. Results
Plasma tHcy is associated with infrarenal aortic calcification volume
A total of 194 patients were recruited into the study over a 3 year period. The characteristics of the patients are shown in Table 1. The severity of infrarenal abdominal aortic calcification was correlated with plasma tHcy concentration (Spearman’s correlation coefficient, r=0.44, p<0.0001, Figure 1) and patients’ age (Spearman’s correlation coefficient, r=0.37, p<0.0001). Plasma tHcy was independently associated with aortic calcification volume ≥ median after adjusting for other risk factors and medication (odds ratio 1.91, 95% confidence interval 1.17–3.12, Table 2).
Table 1.
Characteristics of the patients.
| Characteristics | Characteristics | ||
|---|---|---|---|
| Number | 194 | Hemoglobin (g/L) | 142 (130–150) |
| Male | 140 (72%) | White blood cell (109/L) | 7.8 (6.3–9.5) |
| Age (years) | 69.0 (63.0–74.0) | Glucose (mM) | 5.5 (5.0–6.5) |
| Intermittent claudication | 130 (67%) | CRP (mg/L) | 4.0 (2.0–7.0) |
| AAA | 82 (42%)* | Plasma tHcy (μM) | 12.0 (10.0–15.0) |
| Diabetes | 54 (28%) | Cholesterol (mM) | 4.55 (4.00–5.60) |
| Hypertension | 130 (67%) | Triglyceride (mM) | 1.70 (1.20–2.40) |
| Current smoking | 63 (32%) | LDL (mM) | 2.50 (1.90–3.28) |
| Ex smoker | 75 (39%) | HDL (mM) | 1.20 (1.01–1.50) |
| Never smoker | 56 (29%) | Calcium channel blockers | 59 (30%) |
| Aspirin | 125 (64%) | Angiotensin II receptor blocker | 27 (14%) |
| Statin | 100 (52%) | Beta blockers | 55 (28%) |
| ACE inhibitor | 50 (26%) | Infrarenal aortic calcification volume (mm3) | 1224 (500–2662) |
Data are numbers and percentages for nominal variables and median (IQR) for continuous variables.
18(9%) patients presenting with intermittent claudication also had an AAA identified. AAA= Abdominal aortic aneurysm; ACE= Angiotensin converting enzyme; CRP= C-reactive protein; LDL= Low density lipoprotein; HDL= High density lipoprotein.
Figure 1.
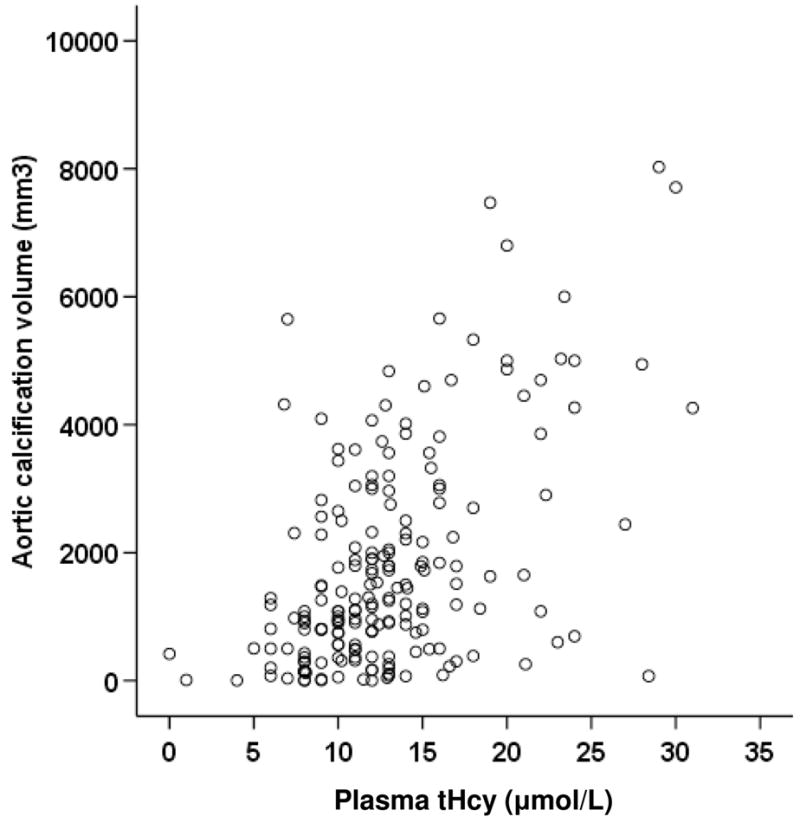
Correlation between plasma tHcy concentration and infrarenal aortic calcification volume (r=0.44).
Table 2.
Variables associated with infrarenal abdominal aortic calcification
| Variable | OR (95% CI) | Variable | OR (95% CI) |
|---|---|---|---|
| Age (per 5 years) | 1.73 (1.29–2.31) | Plasma tHcy (per 5mM) | 1.91 (1.17–3.12) |
| Male sex | 1.03 (0.42–2.62) | Creatinine (per 100μM) | 0.27 (0.07–1.14) |
| Hypertension | 1.56 (0.56–4.37) | Beta blocker | 0.86 (0.35–2.12) |
| Current smoking | 0.98 (0.28–1.35) | ACE inhibitor | 1.02 (0.41–2.56) |
| Diabetes | 1.03 (0.41–2.63) | Angiotensin II inhibitor | 0.73 (0.21–2.43) |
| CHD | 1.16 (0.53–2.54) | Statin | 1.40 (0.62–3.52) |
| LDL (per 1mM) | 1.0 (0.73–1.36) | Aspirin | 0.70 (0.29–1.67) |
| HDL (per 1mM) | 0.72 (0.22–2.43) | Maximum infrarenal aortic | 0.88 (0.75–1.02) |
| CRP (per 1mg/L) | 1.01 (0.99–1.03) | diameter (per 5mm) |
Relationship between 17 vasriables and infrarenal aortic calcification volume medians by logistic regression. R2=0.3 χ2=39.4, p<0.001. Odds ratios (OR) for nominal variables refer to the effect of having the risk factor compared to not. Odds ratios for continuous variables refer to the effect of an approximate one standard deviation increase in the variable. CI= confidence intervals; CHD= coronary heart disease LDL= low density lipoprotein; HDL= high density lipoprotein; CRP= C-reactive protein; ACE= angiotensin converting enzyme.
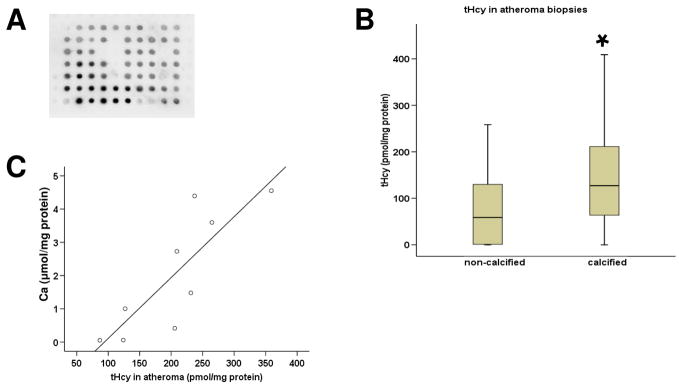
Identification of Hcy in human atheroma
A total of 60 atheroma biopsies from 16 patients were examined. Protein-bound Hcy was identified in the proteins extracted from all atheroma samples by dot blot (Figure 2A). tHcy in atheroma was quantified by AxSYM® Homocysteine immunoassay and found to be higher in the calcified atheroma than in the non-calcified biopsies (p=0.003, Figure 2B). In one patient who had a large area of intimal atheroma removed from both femoral arteries it was possible to obtain nine biopsies. This patient had a plasma tHcy of 30μmol/L. The amount of atheroma tHcy and Ca extracted from these biopsies were closely correlated (Spearman’s correlation coefficient, r=0.95, p<.0001, Figure 2C).
Figure 2.
Hcy in human atheroma. (A) The presence of protein-bound Hcy within the atheroma biopsies was identified using dot blot. (B) tHcy was higher in the calcified versus the non calcified biopsies using immunoassay quantification.* p=0.003 using Mann-Whitney comparison to non-calcified biopsies. (C) Multiple biopsies (n=9) from one patient showed close correlation between extracted tHcy and Ca concentrations (r=0.95).
Hcy promotes calcification in vitro
The effect of Hcy on the in vitro osteogenic differentiation of MSC, monocytic cells and AoSMC was investigated.
The effect of Hcy on MSC and monocytic cells
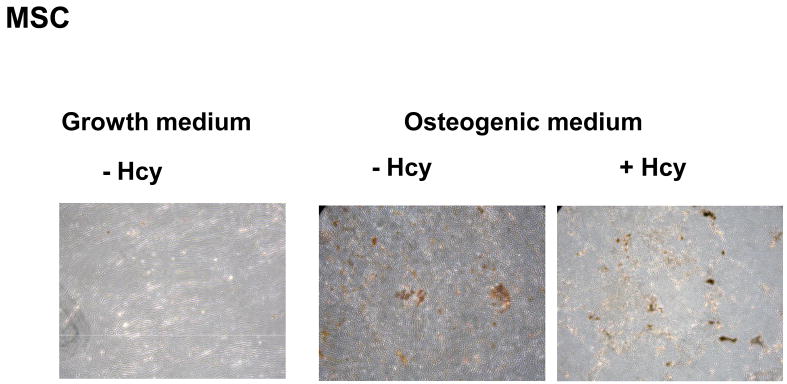
As a model of circulating progenitor cells, MSC were cultured in osteogenic medium in the absence and presence of Hcy for 16 days. Hcy stimulated osteogenic differentiation resulting in a significant two-fold higher Ca deposition (74±4 compared to 42±5 ug Ca/well in the absence of Hcy, p<0.001). These results were supported by Von kossa staining showing increased mineral deposition in osteogenic medium, especially in the presence of Hcy (Figure 3A). In our experiments, both PBMNCs and the monocytic THP-1 cell line failed to carry out osteogenic differentiation when cultured for 16 days in osteogenic medium.
Figure 3.
Hcy stimulates calcium deposition by MSC. Microscopic images (10X) were taken of Von Kossa stained MSC cultured in growth medium and osteogenic medium with or without 100μmol/L Hcy.
The effect of Hcy on human AoSMC
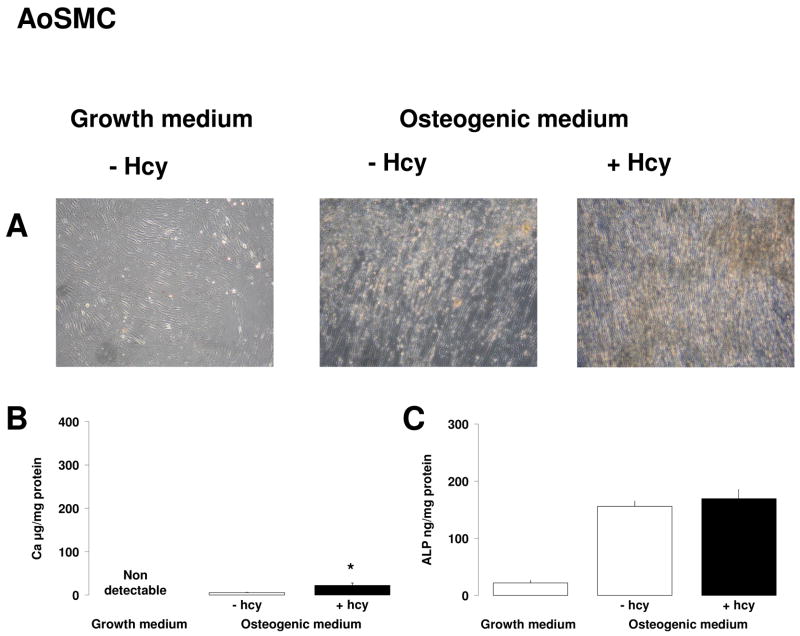
Gene array experiments suggested that Hcy was not able to induce osteogenic differentiation of human AoSMC. No differences in expression levels of osteogenic genes (ALP, matrix Gla protein, cbfa1, osteocalcin, osteonectin, osteopontin, bone morphogenetic proteins) could be observed in AoSMC which have been cultured in growth medium in the presence of 0, 10 and 100μmol/L Hcy for 14 days. Furthermore, 16 days incubation in osteogenic medium in the absence and presence of Hcy also failed to induce osteogenic differentiation measured by Ca deposition and ALP activity. However, when AoSMC were pre-incubated for 12 days in osteogenic medium, culturing an additional 12 days in osteogenic medium with or without Hcy triggered the cells into osteogenic differentiation. This was evidenced by a small but significant Ca deposition and higher ALP activity in these calcifying vascular cells compared to parallel incubation in growth medium (Figure 4A). The presence of Hcy mildly enhanced Ca deposition (23±5 vs 6±1 μg Ca/mg protein in the absence of Hcy, p=0.05) (Figure 4A1) and mineralised deposits (Figure 3B).
Figure 4.
Effect of Hcy on AoSMCs. AoSMC were pre-incubated for 12 days prior to culturing an additional 12 days in growth medium or osteogenic medium with or without 100μmol/L Hcy. The effect of Hcy on calcification was assessed by (A) Von Kossa staining, (B) Ca deposition and (C) ALP activity. Results are expressed as mean±sem of 3 separate experiments with measurements in triplicate. * p ≤ 0.05 in the non-parametric Mann-Whitney comparison with osteogenic culture in the absence of Hcy.
The effect of Hcy on the interaction between monocytic cells and AoSMC
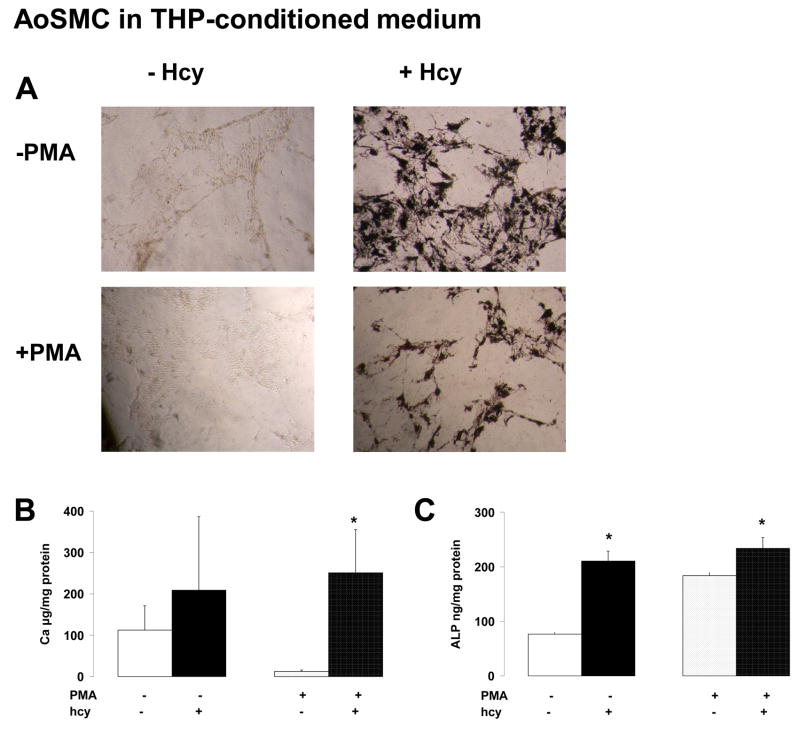
Experiments were carried out to study if Hcy influenced the ability of non-activated and PMA-activated THP-1 cells to alter osteogenic differentiation of AoSMC.
As compared to non-conditioned osteogenic medium (see above), Ca quantification in the AoSMC cell layer was much more pronounced when cultured in THP-conditioned medium (Figure 4B1 versus 4A1). Moreover, the presence of Hcy in the conditioned media of activated THP-1 cells significantly increased the Ca depostion of AoSMC (Figure 4B1). This was confirmed by Von Kossa staining showing darker mineral deposition in the presence of Hcy (Figure 3C). In addition, the presence of Hcy in the conditioned media of non-activated as well as activated THP-1 cells significantly enhanced the ALP activity of AoSMC (Figure 4B2).
4. Discussion
Our results provide evidence for the independent association between plasma tHcy and vascular calcification. Circulating tHcy concentration has been previously associated with abdominal aortic calcification in two small studies involving a total of only 77 patients 33, 34. Hirose and colleagues used CT to assess infrarenal aortic calcification in 28 patients with hyperlipidaemia 33. They assessed the percentage of calcification on two CT slices and provided no reproducibility data. More recently Jamal et al assessed 52 renal failure patients on haemodialysis using abdominal X-ray 34. Unlike these studies we assessed calcification involving the infrarenal abdominal aorta in a larger group of patients with PAD or AAA using an accurate CTA based technique. We demonstrated an association between plasma tHcy and aortic calcification which was independent of other atherosclerotic risk factors and medication (Table 2).
The epidemiology data linking Hcy with atherosclerosis is strong 24, 25 but the mediating cellular and molecular mechanisms for this association remain unclear. Experimentally Hcy stimulates a range of potentially pro-atherosclerotic changes, including inflammation and thrombosis and causes apoptosis of endothelial cells 25, 41, 42. In the present study, we demonstrate for the first time to our knowledge that Hcy is present within advanced atheroma and that the amount of tHcy is elevated in calcified biopsies (Figure 2). This finding suggests that Hcy could modulate cells present in atherosclerotic arteries to promote calcification. Previous reports studying the cellular component of calcification have mainly focused on AoSMC which are present in the intima of advanced atherosclerosis. They showed that AoSMC calcification can be promoted by transforming growth factor β1, lipid oxidation products and reactive oxygen species present in the atherosclerotic arteries 43–45. Hcy has previously been shown to potentiate calcification of cultured rat AoSMC 46, however human AoSMC are much less responsive to calcification induction than rodent cells illustrating the requirement for study of human cells 47. Given the presence of Hcy in the atheroma and its association with calcification, we investigated the influence of Hcy on the osteogenic potential of human AoSMC. Our findings indicate that the direct effect of Hcy on AoSMC calcification was slow and limited. The rather slow and highly variable calcification rates of human AoSMC compared to those of other species has been documented before 47. When we incubated Hcy with monocytic THP-1 cells however, application of the conditioned media on the AoSMC resulted in a much more marked promotion of Ca deposition (Figure 4). In order to minimise experimental variation, the use of the human monocyte THP-1 cell line was preferred over freshly isolated PBMNCs, since the later represents a heterogeneous cell mixture. In addition, our experimental set-up would have required >200 ml of blood to isolate enough PBMCs for each refreshment step. In practise this would have required multiple donors thereby introducing significant experimental variability which would have likely obscured any effects of Hcy. In line with our findings, macrophages are reported to be the predominant cell type associated with different stages of calcification in atherosclerotic plaques 48. They are suggested to enhance vascular cell calcificiation via the production of soluble factors such as tumor necrosis factor-α and reactive oxygen species 49. Our results indicate that the presence of Hcy in the atheroma may further enhance vascular calcification by activating the monocytic cells to secrete factors able to enhance the osteogenic differentiation of AoSMC. This is supported by the observation that pathophysiologic concentrations of Hcy activate human monocytes to secrete inflammatory cytokines including tumor necrosis factor-α 50.
Circulating progenitor cells have also been suggested to contribute to vascular calcification 16–22. Recently a subset of circulating CD14+ monocytes has been identified which has the capacity to exhibit mesenchymal differentiation, including osteogenesis 17, 18. MSC have been detected in circulating blood 20, 22. Eghbali-Fatourechi et al. identified circulating mononuclear cells expressing bone markers and capable of carrying out calcification in vitro and in vivo 19. In this study, we demonstrated that Hcy promoted in vitro Ca deposition by MSC, suggesting another mechanism by which Hcy may favour calcification (Figure 3A). Whether such bone marrow derived cells enter atheromas in vivo is currently controversial 51–53.
Our study has a number of limitations. Firstly, we included patients with both PAD and AAA since these were the subjects on which we routinely carry out CTA. Although the role of calcification in the progression of these diseases remains unclear, a number of studies highlight the importance of calcification in relation to adverse effects as well as impaired treatment options and outcome 6–9. The pathology underlying athero-thrombosis and AAA have a number of differences 54. In carrying out our multiple logistic regression analysis we did include maximum aortic diameter in the model to adjust for any effect this may have caused (Table 2). Similar to other recent studies we failed to demonstrate any association with some conventional risk factors for atherosclerosis and aortic calcification (Table 2) 55. Since a large proportion of our patients were receiving aspirin, statins, beta blockers, angiotensin II inhibitors and oral hypoglycaemics, the later finding may relate to the concurrent treatment of other risk factors.
The independent importance of both arterial calcification and Hcy in cardiovascular disease is controversial 23. One possibility is that Hcy is important in calcification but that calcification is just a marker of atherosclerosis and not associated with its complications. Following on from this Hcy would simply be a marker of atherosclerosis presence rather than pathological in atherosclerosis complications. These associations would explain the inability of therapies which lower plasma Hcy to significantly reduce cardiovascular events. A number of other possibilities still exist such as present failure to identify a suitable therapy to appropriately lower circulating Hcy 23. Further investigations will be required to address this.
Our mechanistic studies were limited to in vitro investigations of human cells. We used this approach as relating findings from animal models of calcification to human can be problematic. Osteoprotegerin deficiency in mice for example is associated with increased atherosclerosis and calcification while serum concentrations of osteoprotegerin in humans are positively correlated with calcification and atherosclerosis 56–58.
In conclusion we confirm the independent association of aortic calcification and circulating plasma tHcy and provide a number of cellular mechanisms which may underlie this phenomenon. Our results support a cooperative role for AoSMC and monocytic cells in the calcification process, modulated by the presence of Hcy in the atherosclerotic plaque.
Figure 5.
Effect of Hcy on monocytic cell induced osteogenic differentiation of AoSMC. AoSMC were cutured in osteogenic media for 12 days followed by culturing in condition media from monocytic cells which had been exposed to 100μmol/L Hcy. After a further 12 days cells were assessed for: (A) Von Kossa staining, (B) Ca deposition and (C) ALP activity. Results are expressed as mean±sem of 3 separate experiments with measurements in triplicate. * p ≤ 0.05 in the non-parametric Mann-Whitney comparison with osteogenic culture in the absence of Hcy.
Acknowledgments
This work was supported by Funding from the National Institute of Health, USA (RO1 HL080010-01). JG is a Practitioner’s Fellow of the NHMRC, Australia (431503). CSM is a fellow of The National Heart Foundation, Australia.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Shaw LJ, Raggi P, Schisterman E, Berman DS, Callister TQ. Prognostic value of cardiac risk factors and coronary artery calcium screening for all-cause mortality. Radiology. 2003;228(3):826–33. doi: 10.1148/radiol.2283021006. [DOI] [PubMed] [Google Scholar]
- 2.Budoff MJ, Shaw LJ, Liu ST, Weinstein SR, Mosler TP, Tseng PH, et al. Long-term prognosis associated with coronary calcification: observations from a registry of 25,253 patients. J Am Coll Cardiol. 2007;49(18):1860–70. doi: 10.1016/j.jacc.2006.10.079. [DOI] [PubMed] [Google Scholar]
- 3.Reaven PD, Sacks J. Reduced coronary artery and abdominal aortic calcification in Hispanics with type 2 diabetes. Diabetes Care. 2004;27(5):1115–20. doi: 10.2337/diacare.27.5.1115. [DOI] [PubMed] [Google Scholar]
- 4.Wilson PW, Kauppila LI, O’Donnell CJ, Kiel DP, Hannan M, Polak JM, et al. Abdominal aortic calcific deposits are an important predictor of vascular morbidity and mortality. Circulation. 2001;103(11):1529–34. doi: 10.1161/01.cir.103.11.1529. [DOI] [PubMed] [Google Scholar]
- 5.Jayalath RW, Jackson P, Golledge J. Quantification of abdominal aortic calcification on CT. Arterioscler Thromb Vasc Biol. 2006;26(2):429–30. doi: 10.1161/01.ATV.0000198390.34524.ba. [DOI] [PubMed] [Google Scholar]
- 6.Matsushita M, Nishikimi N, Sakurai T, Nimura Y. Relationship between aortic calcification and atherosclerotic disease in patients with abdominal aortic aneurysm. Int Angiol. 2000;19(3):276–9. [PubMed] [Google Scholar]
- 7.Raggi P, Boulay A, Chasan-Taber S, Amin N, Dillon M, Burke SK, et al. Cardiac calcification in adult hemodialysis patients. A link between end-stage renal disease and cardiovascular disease? J Am Coll Cardiol. 2002;39(4):695–701. doi: 10.1016/s0735-1097(01)01781-8. [DOI] [PubMed] [Google Scholar]
- 8.Wain RA, Marin ML, Ohki T, Sanchez LA, Lyon RT, Rozenblit A, et al. Endoleaks after endovascular graft treatment of aortic aneurysms: classification, risk factors, and outcome. J Vasc Surg. 1998;27(1):69–80. doi: 10.1016/s0741-5214(98)70293-9. [DOI] [PubMed] [Google Scholar]
- 9.White JV, Ryjewski C. Progress in the endovascular treatment of intermittent claudication: rationale for changes in the TASC classification. Semin Vasc Surg. 2007;20(1):54–61. doi: 10.1053/j.semvascsurg.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 10.Cozzolino M, Gallieni M, Brancaccio D. Vascular calcification in uremic conditions: new insights into pathogenesis. Semin Nephrol. 2006;26(1):33–7. doi: 10.1016/j.semnephrol.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 11.Johnson RC, Leopold JA, Loscalzo J. Vascular calcification: pathobiological mechanisms and clinical implications. Circ Res. 2006;99(10):1044–59. doi: 10.1161/01.RES.0000249379.55535.21. [DOI] [PubMed] [Google Scholar]
- 12.Dhore CR, Cleutjens JP, Lutgens E, Cleutjens KB, Geusens PP, Kitslaar PJ, et al. Differential expression of bone matrix regulatory proteins in human atherosclerotic plaques. Arterioscler Thromb Vasc Biol. 2001;21(12):1998–2003. doi: 10.1161/hq1201.100229. [DOI] [PubMed] [Google Scholar]
- 13.Hunt JL, Fairman R, Mitchell ME, Carpenter JP, Golden M, Khalapyan T, et al. Bone formation in carotid plaques: a clinicopathological study. Stroke. 2002;33(5):1214–9. doi: 10.1161/01.str.0000013741.41309.67. [DOI] [PubMed] [Google Scholar]
- 14.Bostrom KI. Cell differentiation in vascular calcification. Z Kardiol. 2000;89(Suppl 2):69–74. doi: 10.1007/s003920070102. [DOI] [PubMed] [Google Scholar]
- 15.Iyemere VP, Proudfoot D, Weissberg PL, Shanahan CM. Vascular smooth muscle cell phenotypic plasticity and the regulation of vascular calcification. J Intern Med. 2006;260(3):192–210. doi: 10.1111/j.1365-2796.2006.01692.x. [DOI] [PubMed] [Google Scholar]
- 16.Chim H, Schantz JT. Human circulating peripheral blood mononuclear cells for calvarial bone tissue engineering. Plast Reconstr Surg. 2006;117(2):468–78. doi: 10.1097/01.prs.0000201489.65811.e7. [DOI] [PubMed] [Google Scholar]
- 17.Kuwana M, Okazaki Y, Kodama H, Izumi K, Yasuoka H, Ogawa Y, et al. Human circulating CD14+ monocytes as a source of progenitors that exhibit mesenchymal cell differentiation. J Leukoc Biol. 2003;74(5):833–45. doi: 10.1189/jlb.0403170. [DOI] [PubMed] [Google Scholar]
- 18.Seta N, Kuwana M. Human circulating monocytes as multipotential progenitors. Keio J Med. 2007;56(2):41–7. doi: 10.2302/kjm.56.41. [DOI] [PubMed] [Google Scholar]
- 19.Eghbali-Fatourechi GZ, Lamsam J, Fraser D, Nagel D, Riggs BL, Khosla S. Circulating osteoblast-lineage cells in humans. N Engl J Med. 2005;352(19):1959–66. doi: 10.1056/NEJMoa044264. [DOI] [PubMed] [Google Scholar]
- 20.Kuznetsov SA, Mankani MH, Gronthos S, Satomura K, Bianco P, Robey PG. Circulating skeletal stem cells. J Cell Biol. 2001;153(5):1133–40. doi: 10.1083/jcb.153.5.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leskela HV, Satta J, Oiva J, Eriksen H, Juha R, Korkiamaki P, et al. Calcification and cellularity in human aortic heart valve tissue determine the differentiation of bone-marrow-derived cells. J Mol Cell Cardiol. 2006;41(4):642–9. doi: 10.1016/j.yjmcc.2006.07.014. [DOI] [PubMed] [Google Scholar]
- 22.Zvaifler NJ, Marinova-Mutafchieva L, Adams G, Edwards CJ, Moss J, Burger JA, et al. Mesenchymal precursor cells in the blood of normal individuals. Arthritis Res. 2000;2(6):477–88. doi: 10.1186/ar130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Loscalzo J. Homocysteine trials--clear outcomes for complex reasons. N Engl J Med. 2006;354(15):1629–32. doi: 10.1056/NEJMe068060. [DOI] [PubMed] [Google Scholar]
- 24.Wald DS, Wald NJ, Morris JK, Law M. Folic acid, homocysteine, and cardiovascular disease: judging causality in the face of inconclusive trial evidence. Bmj. 2006;333(7578):1114–7. doi: 10.1136/bmj.39000.486701.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jakubowski H. Review: The molecular basis of homocysteine thiolactone-mediated vascular disease. Clin Chem Lab Med. 2007 doi: 10.1515/CCLM.2007.338. [DOI] [PubMed] [Google Scholar]
- 26.Bonaa KH, Njolstad I, Ueland PM, Schirmer H, Tverdal A, Steigen T, et al. Homocysteine lowering and cardiovascular events after acute myocardial infarction. N Engl J Med. 2006;354(15):1578–88. doi: 10.1056/NEJMoa055227. [DOI] [PubMed] [Google Scholar]
- 27.Lonn E, Yusuf S, Arnold MJ, Sheridan P, Pogue J, Micks M, et al. Homocysteine lowering with folic acid and B vitamins in vascular disease. N Engl J Med. 2006;354(15):1567–77. doi: 10.1056/NEJMoa060900. [DOI] [PubMed] [Google Scholar]
- 28.Spence JD, Bang H, Chambless LE, Stampfer MJ. Vitamin Intervention For Stroke Prevention trial: an efficacy analysis. Stroke. 2005;36(11):2404–9. doi: 10.1161/01.STR.0000185929.38534.f3. [DOI] [PubMed] [Google Scholar]
- 29.Kullo IJ, Li G, Bielak LF, Bailey KR, Sheedy PF, 2nd, Peyser PA, et al. Association of plasma homocysteine with coronary artery calcification in different categories of coronary heart disease risk. Mayo Clin Proc. 2006;81(2):177–82. doi: 10.4065/81.2.177. [DOI] [PubMed] [Google Scholar]
- 30.Von Feldt JM, Scalzi LV, Cucchiara AJ, Morthala S, Kealey C, Flagg SD, et al. Homocysteine levels and disease duration independently correlate with coronary artery calcification in patients with systemic lupus erythematosus. Arthritis Rheum. 2006;54(7):2220–7. doi: 10.1002/art.21967. [DOI] [PubMed] [Google Scholar]
- 31.Godsland IF, Elkeles RS, Feher MD, Nugara F, Rubens MB, Richmond W, et al. Coronary calcification, homocysteine, C-reactive protein and the metabolic syndrome in Type 2 diabetes: the Prospective Evaluation of Diabetic Ischaemic Heart Disease by Coronary Tomography (PREDICT) Study. Diabet Med. 2006;23(11):1192–200. doi: 10.1111/j.1464-5491.2006.01950.x. [DOI] [PubMed] [Google Scholar]
- 32.Taylor AJ, Feuerstein I, Wong H, Barko W, Brazaitis M, O’Malley PG. Do conventional risk factors predict subclinical coronary artery disease? Results from the Prospective Army Coronary Calcium Project. Am Heart J. 2001;141(3):463–8. doi: 10.1067/mhj.2001.113069. [DOI] [PubMed] [Google Scholar]
- 33.Hirose N, Arai Y, Ishii T, Tushima M, Li J. Association of mild hyperhomocysteinemia with aortic calcification in hypercholesterolemic patients. J Atheroscler Thromb. 2001;8(3):91–4. doi: 10.5551/jat1994.8.91. [DOI] [PubMed] [Google Scholar]
- 34.Jamal SA, Leiter RE, Bauer DC. Hyperhomocysteinaemia and aortic calcification are associated with fractures in patients on haemodialysis. Qjm. 2005;98(8):575–9. doi: 10.1093/qjmed/hci092. [DOI] [PubMed] [Google Scholar]
- 35.Golledge J, Jayalath R, Oliver L, Parr A, Schurgers L, Clancy P. Relationship between CT anthropometric measurements, adipokines and abdominal aortic calcification. Atherosclerosis. 2007 doi: 10.1016/j.atherosclerosis.2007.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Golledge J, Leicht A, Crowther RG, Clancy P, Spinks WL, Quigley F. Association of obesity and metabolic syndrome with the severity and outcome of intermittent claudication. J Vasc Surg. 2007;45(1):40–6. doi: 10.1016/j.jvs.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 37.Golledge J, Jones L, Oliver L, Quigley F, Karan M. Folic acid, vitamin B12, MTHFR genotypes, and plasma homocysteine. Clin Chem. 2006;52(6):1205–6. doi: 10.1373/clinchem.2006.069849. [DOI] [PubMed] [Google Scholar]
- 38.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 39.Golledge J, Mangan S, Clancy P. Effects of peroxisome proliferator-activated receptor ligands in modulating tissue factor and tissue factor pathway inhibitor in acutely symptomatic carotid atheromas. Stroke. 2007;38(5):1501–8. doi: 10.1161/STROKEAHA.106.474791. [DOI] [PubMed] [Google Scholar]
- 40.Jaiswal N, Haynesworth SE, Caplan AI, Bruder SP. Osteogenic differentiation of purified, culture-expanded human mesenchymal stem cells in vitro. J Cell Biochem. 1997;64(2):295–312. [PubMed] [Google Scholar]
- 41.Papatheodorou L, Weiss N. Vascular Oxidant Stress and Inflammation in Hyperhomocysteinemia. Antioxid Redox Signal. 2007;9(11):1941–58. doi: 10.1089/ars.2007.1750. [DOI] [PubMed] [Google Scholar]
- 42.Suhara T, Fukuo K, Yasuda O, Tsubakimoto M, Takemura Y, Kawamoto H, et al. Homocysteine enhances endothelial apoptosis via upregulation of Fas-mediated pathways. Hypertension. 2004;43(6):1208–13. doi: 10.1161/01.HYP.0000127914.94292.76. [DOI] [PubMed] [Google Scholar]
- 43.Mody N, Parhami F, Sarafian TA, Demer LL. Oxidative stress modulates osteoblastic differentiation of vascular and bone cells. Free Radic Biol Med. 2001;31(4):509–19. doi: 10.1016/s0891-5849(01)00610-4. [DOI] [PubMed] [Google Scholar]
- 44.Parhami F, Morrow AD, Balucan J, Leitinger N, Watson AD, Tintut Y, et al. Lipid oxidation products have opposite effects on calcifying vascular cell and bone cell differentiation. A possible explanation for the paradox of arterial calcification in osteoporotic patients. Arterioscler Thromb Vasc Biol. 1997;17(4):680–7. doi: 10.1161/01.atv.17.4.680. [DOI] [PubMed] [Google Scholar]
- 45.Watson KE, Bostrom K, Ravindranath R, Lam T, Norton B, Demer LL. TGF-beta 1 and 25-hydroxycholesterol stimulate osteoblast-like vascular cells to calcify. J Clin Invest. 1994;93(5):2106–13. doi: 10.1172/JCI117205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li J, Chai S, Tang C, Du J. Homocysteine potentiates calcification of cultured rat aortic smooth muscle cells. Life Sci. 2003;74(4):451–61. doi: 10.1016/j.lfs.2003.06.028. [DOI] [PubMed] [Google Scholar]
- 47.Olesen P, Nguyen K, Wogensen L, Ledet T, Rasmussen LM. Calcification of human vascular smooth muscle cells: associations with osteoprotegerin expression and acceleration by high-dose insulin. Am J Physiol Heart Circ Physiol. 2007;292(2):H1058–64. doi: 10.1152/ajpheart.00047.2006. [DOI] [PubMed] [Google Scholar]
- 48.Jeziorska M, McCollum C, Woolley DE. Calcification in atherosclerotic plaque of human carotid arteries: associations with mast cells and macrophages. J Pathol. 1998;185(1):10–7. doi: 10.1002/(SICI)1096-9896(199805)185:1<10::AID-PATH71>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 49.Tintut Y, Patel J, Territo M, Saini T, Parhami F, Demer LL. Monocyte/macrophage regulation of vascular calcification in vitro. Circulation. 2002;105(5):650–5. doi: 10.1161/hc0502.102969. [DOI] [PubMed] [Google Scholar]
- 50.Su SJ, Huang LW, Pai LS, Liu HW, Chang KL. Homocysteine at pathophysiologic concentrations activates human monocyte and induces cytokine expression and inhibits macrophage migration inhibitory factor expression. Nutrition. 2005;21(10):994–1002. doi: 10.1016/j.nut.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 51.Bentzon JF, Weile C, Sondergaard CS, Hindkjaer J, Kassem M, Falk E. Smooth muscle cells in atherosclerosis originate from the local vessel wall and not circulating progenitor cells in ApoE knockout mice. Arterioscler Thromb Vasc Biol. 2006;26(12):2696–702. doi: 10.1161/01.ATV.0000247243.48542.9d. [DOI] [PubMed] [Google Scholar]
- 52.Caplice NM, Bunch TJ, Stalboerger PG, Wang S, Simper D, Miller DV, et al. Smooth muscle cells in human coronary atherosclerosis can originate from cells administered at marrow transplantation. Proc Natl Acad Sci U S A. 2003;100(8):4754–9. doi: 10.1073/pnas.0730743100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sata M, Fukuda D, Tanaka K, Kaneda Y, Yashiro H, Shirakawa I. The role of circulating precursors in vascular repair and lesion formation. J Cell Mol Med. 2005;9(3):557–68. doi: 10.1111/j.1582-4934.2005.tb00488.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Golledge J, Muller J, Daugherty A, Norman P. Abdominal aortic aneurysm: pathogenesis and implications for management. Arterioscler Thromb Vasc Biol. 2006;26(12):2605–13. doi: 10.1161/01.ATV.0000245819.32762.cb. [DOI] [PubMed] [Google Scholar]
- 55.Adler Y, Fisman EZ, Shemesh J, Schwammenthal E, Tanne D, Batavraham IR, et al. Spiral computed tomography evidence of close correlation between coronary and thoracic aorta calcifications. Atherosclerosis. 2004;176(1):133–8. doi: 10.1016/j.atherosclerosis.2004.03.027. [DOI] [PubMed] [Google Scholar]
- 56.Bennett BJ, Scatena M, Kirk EA, Rattazzi M, Varon RM, Averill M, et al. Osteoprotegerin inactivation accelerates advanced atherosclerotic lesion progression and calcification in older ApoE−/− mice. Arterioscler Thromb Vasc Biol. 2006;26(9):2117–24. doi: 10.1161/01.ATV.0000236428.91125.e6. [DOI] [PubMed] [Google Scholar]
- 57.Clancy P, Oliver L, Jayalath R, Buttner P, Golledge J. Assessment of a serum assay for quantification of abdominal aortic calcification. Arterioscler Thromb Vasc Biol. 2006;26(11):2574–6. doi: 10.1161/01.ATV.0000242799.81434.7d. [DOI] [PubMed] [Google Scholar]
- 58.Kiechl S, Schett G, Wenning G, Redlich K, Oberhollenzer M, Mayr A, et al. Osteoprotegerin is a risk factor for progressive atherosclerosis and cardiovascular disease. Circulation. 2004;109(18):2175–80. doi: 10.1161/01.CIR.0000127957.43874.BB. [DOI] [PubMed] [Google Scholar]