Abstract
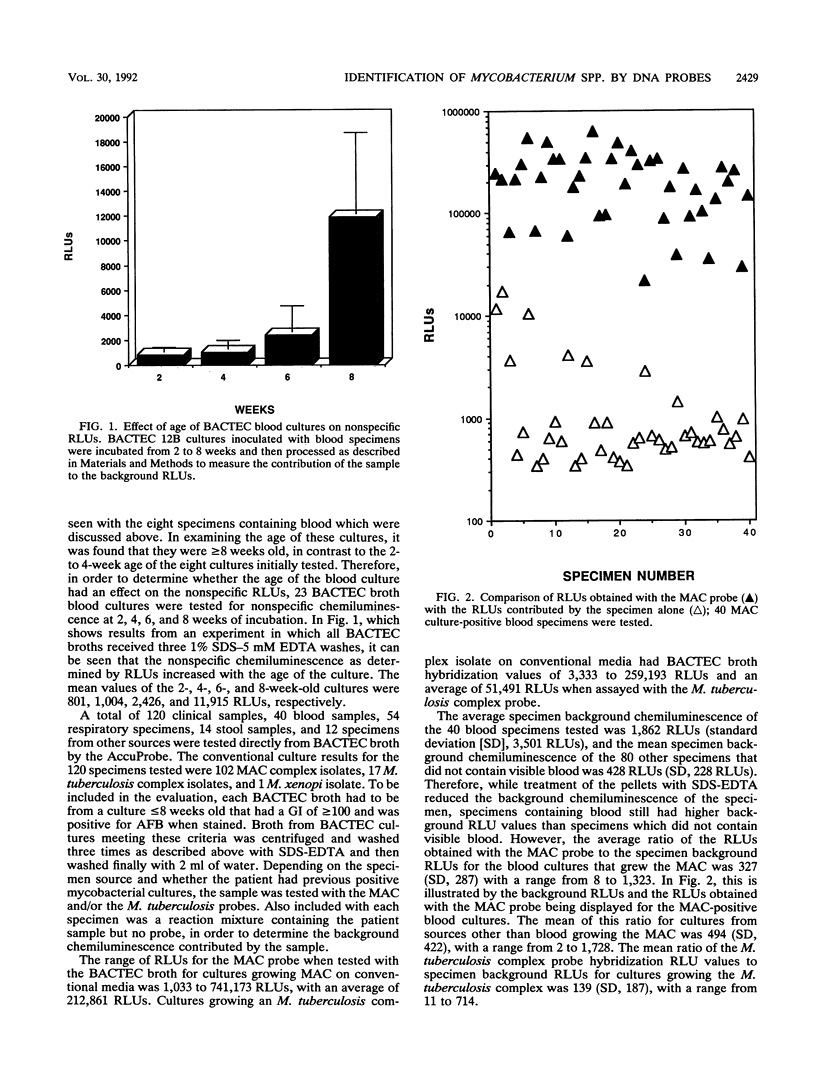
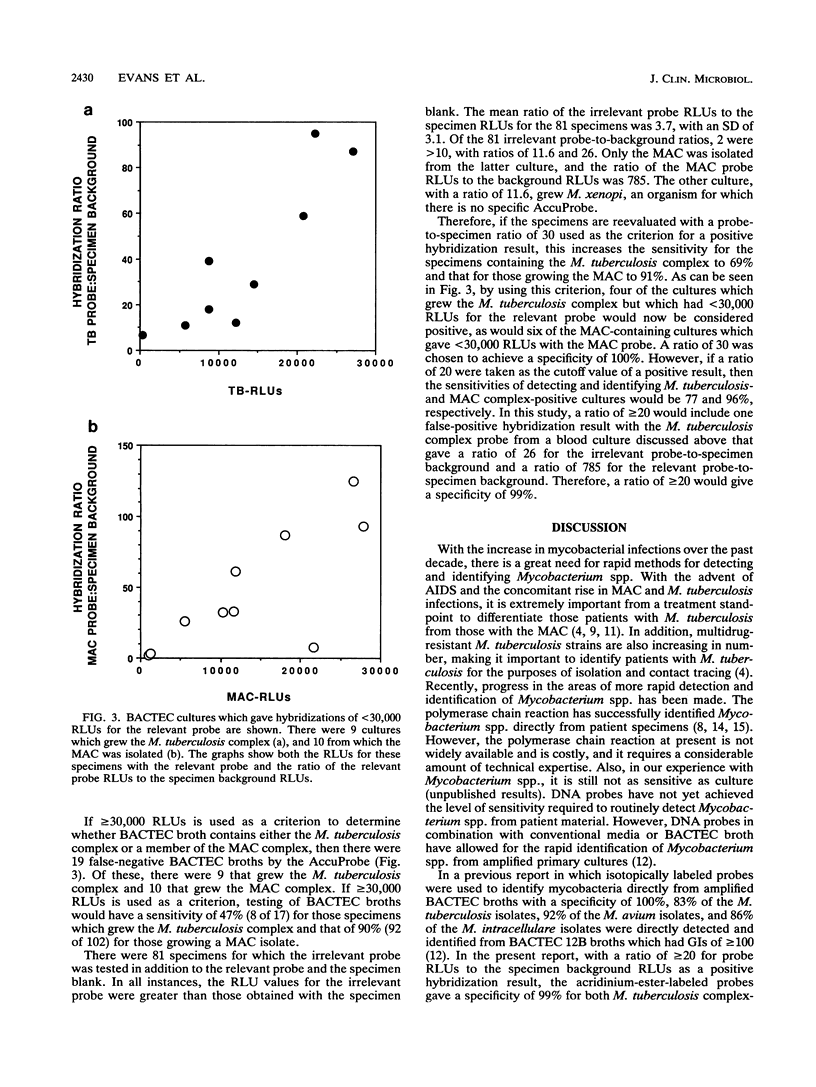
Identification of members of the Mycobacterium tuberculosis complex and the M. avium-M. intracellulare complex (MAC) directly from primary BACTEC cultures was evaluated by using acridinium-ester-labeled DNA probes (AccuProbe; GenProbe, Inc., San Diego, Calif.). In preliminary experiments, blood present in samples was found to interfere with the assay because of nonspecific chemiluminescence, which was measured in relative light units (RLUs). There was a direct relationship between the age of the culture and the number of nonspecific RLUs. A protocol using 1% sodium dodecyl sulfate-5 mM EDTA to treat BACTEC broth cultures which, with specimens containing blood, gave on the average a ninefold reduction in nonspecific chemiluminescence was developed. By using this treatment protocol, 120 specimens were tested directly from BACTEC broth cultures with an AccuProbe for the M. tuberculosis complex and/or the MAC. In order to establish the background of the specimen, the patient sample was assayed without probe. The criteria for the inclusion of BACTEC cultures in the evaluation were a growth index of greater than or equal to 100 and a positive smear for acid-fast bacilli directly from the BACTEC broth. For the 120 cultures tested, if a hybridization result of greater than or equal to 30,000 RLUs was considered positive, the sensitivities for detecting the M. tuberculosis complex and the MAC were 47 and 90%, respectively, with a specificity of 100% for both. However, if a ratio of the RLUs obtained with the MAC or the M. tuberculosis complex probe to those obtained with the specimen background of >/= 20 was considered positive, this gave 77% sensitivity and 100% specificity for BACTEC cultures containing M. tuberculosis complex isolates and 96% sensitivity and 100% specificity for those growing MAC isolates.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Davis T. E., Fuller D. D. Direct identification of bacterial isolates in blood cultures by using a DNA probe. J Clin Microbiol. 1991 Oct;29(10):2193–2196. doi: 10.1128/jcm.29.10.2193-2196.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake T. A., Hindler J. A., Berlin O. G., Bruckner D. A. Rapid identification of Mycobacterium avium complex in culture using DNA probes. J Clin Microbiol. 1987 Aug;25(8):1442–1445. doi: 10.1128/jcm.25.8.1442-1445.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto M., Oka S., Okuzumi K., Kimura S., Shimada K. Evaluation of acridinium-ester-labeled DNA probes for identification of Mycobacterium tuberculosis and Mycobacterium avium-Mycobacterium intracellulare complex in culture. J Clin Microbiol. 1991 Nov;29(11):2473–2476. doi: 10.1128/jcm.29.11.2473-2476.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthertz L. S., Damsker B., Bottone E. J., Ford E. G., Midura T. F., Janda J. M. Mycobacterium avium and Mycobacterium intracellulare infections in patients with and without AIDS. J Infect Dis. 1989 Dec;160(6):1037–1041. doi: 10.1093/infdis/160.6.1037. [DOI] [PubMed] [Google Scholar]
- Hermans P. W., Schuitema A. R., Van Soolingen D., Verstynen C. P., Bik E. M., Thole J. E., Kolk A. H., van Embden J. D. Specific detection of Mycobacterium tuberculosis complex strains by polymerase chain reaction. J Clin Microbiol. 1990 Jun;28(6):1204–1213. doi: 10.1128/jcm.28.6.1204-1213.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horsburgh C. R., Jr Mycobacterium avium complex infection in the acquired immunodeficiency syndrome. N Engl J Med. 1991 May 9;324(19):1332–1338. doi: 10.1056/NEJM199105093241906. [DOI] [PubMed] [Google Scholar]
- Lim S. D., Todd J., Lopez J., Ford E., Janda J. M. Genotypic identification of pathogenic Mycobacterium species by using a nonradioactive oligonucleotide probe. J Clin Microbiol. 1991 Jun;29(6):1276–1278. doi: 10.1128/jcm.29.6.1276-1278.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunn P. P., McAdam K. P. Mycobacterial infections and AIDS. Br Med Bull. 1988 Jul;44(3):801–813. doi: 10.1093/oxfordjournals.bmb.a072284. [DOI] [PubMed] [Google Scholar]
- Peterson E. M., Lu R., Floyd C., Nakasone A., Friedly G., de la Maza L. M. Direct identification of Mycobacterium tuberculosis, Mycobacterium avium, and Mycobacterium intracellulare from amplified primary cultures in BACTEC media using DNA probes. J Clin Microbiol. 1989 Jul;27(7):1543–1547. doi: 10.1128/jcm.27.7.1543-1547.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjöbring U., Mecklenburg M., Andersen A. B., Miörner H. Polymerase chain reaction for detection of Mycobacterium tuberculosis. J Clin Microbiol. 1990 Oct;28(10):2200–2204. doi: 10.1128/jcm.28.10.2200-2204.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]


