Abstract
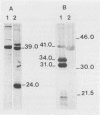
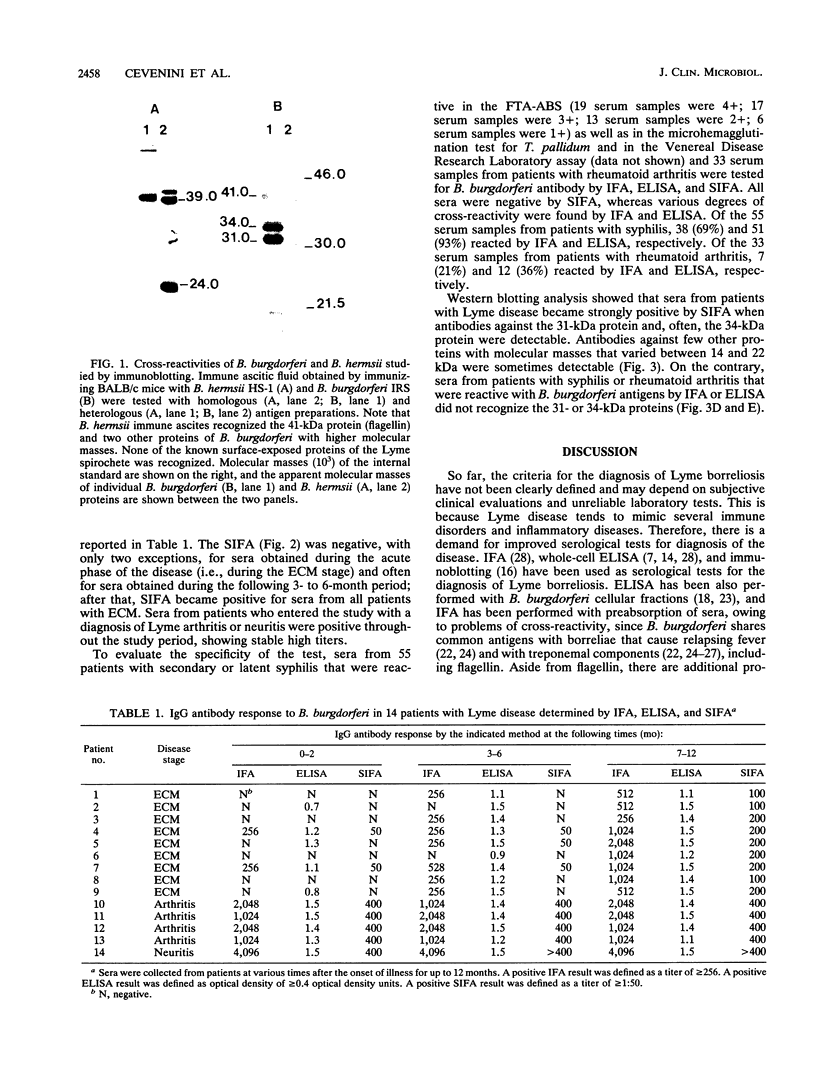
A surface immunofluorescence assay (SIFA) was analyzed and compared with a conventional indirect immunofluorescence assay (IFA) and whole-cell enzyme-linked immunosorbent assay (ELISA) for detecting immunoglobulin G (IgG) antibodies to Borrelia burgdorferi in sera from patients with Lyme disease. Fifty-five patients with syphilis and 33 patients with rheumatoid arthritis were used as disease controls. The sensitivity of the SIFA was low during the acute phase of Lyme disease (sera from seven of nine patients presenting with erythema chronicum migrans were negative during the first 2 months of illness); later, seroconversion was observed in all patients at various times during convalescence. Sera from five patients with complicated Lyme disease were strongly positive. SIFA was found to be highly specific, since sera from all patients with secondary or latent syphilis and patients with rheumatoid arthritis did not react in the test. Strong cross-reactivity occurred when these sera were tested in conventional IFA and ELISA; sera from 38 (69%) patients with syphilis were positive by IFA and sera from 51 (93%) patients were positive by ELISA, whereas 7 (21%) and 12 (36%) of the serum samples from patients with rheumatoid arthritis were positive by IFA and ELISA, respectively. Immunoblot analysis of SIFA-positive sera showed that the 31- and 34-kDa outer surface proteins (proteins A and B, respectively) of B. burgdorferi were the major reactive antigens involved in the test. The results support a role for SIFA in the investigation of complicated Lyme disease as well as in the differentiation of Lyme disease from other diseases associated with B. burgdorferi cross-reactive antibodies.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker-Zander S. A., Lukehart S. A. Antigenic cross-reactivity between Treponema pallidum and other pathogenic members of the family Spirochaetaceae. Infect Immun. 1984 Oct;46(1):116–121. doi: 10.1128/iai.46.1.116-121.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour A. G., Hayes S. F., Heiland R. A., Schrumpf M. E., Tessier S. L. A Borrelia-specific monoclonal antibody binds to a flagellar epitope. Infect Immun. 1986 May;52(2):549–554. doi: 10.1128/iai.52.2.549-554.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour A. G., Heiland R. A., Howe T. R. Heterogeneity of major proteins in Lyme disease borreliae: a molecular analysis of North American and European isolates. J Infect Dis. 1985 Sep;152(3):478–484. doi: 10.1093/infdis/152.3.478. [DOI] [PubMed] [Google Scholar]
- Barbour A. G. Isolation and cultivation of Lyme disease spirochetes. Yale J Biol Med. 1984 Jul-Aug;57(4):521–525. [PMC free article] [PubMed] [Google Scholar]
- Barbour A. G., Tessier S. L., Hayes S. F. Variation in a major surface protein of Lyme disease spirochetes. Infect Immun. 1984 Jul;45(1):94–100. doi: 10.1128/iai.45.1.94-100.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour A. G., Tessier S. L., Todd W. J. Lyme disease spirochetes and ixodid tick spirochetes share a common surface antigenic determinant defined by a monoclonal antibody. Infect Immun. 1983 Aug;41(2):795–804. doi: 10.1128/iai.41.2.795-804.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berardi V. P., Weeks K. E., Steere A. C. Serodiagnosis of early Lyme disease: analysis of IgM and IgG antibody responses by using an antibody-capture enzyme immunoassay. J Infect Dis. 1988 Oct;158(4):754–760. doi: 10.1093/infdis/158.4.754. [DOI] [PubMed] [Google Scholar]
- Bergström S., Bundoc V. G., Barbour A. G. Molecular analysis of linear plasmid-encoded major surface proteins, OspA and OspB, of the Lyme disease spirochaete Borrelia burgdorferi. Mol Microbiol. 1989 Apr;3(4):479–486. doi: 10.1111/j.1365-2958.1989.tb00194.x. [DOI] [PubMed] [Google Scholar]
- Burgdorfer W., Barbour A. G., Hayes S. F., Benach J. L., Grunwaldt E., Davis J. P. Lyme disease-a tick-borne spirochetosis? Science. 1982 Jun 18;216(4552):1317–1319. doi: 10.1126/science.7043737. [DOI] [PubMed] [Google Scholar]
- Cevenini R., Moroni A., Sambri V., Perini S., La Placa M. Serological response to chlamydial infection in sheep, studied by enzyme-linked immunosorbent assay and immunoblotting. FEMS Microbiol Immunol. 1989 Dec;1(8-9):459–464. doi: 10.1016/0378-1097(89)90273-5. [DOI] [PubMed] [Google Scholar]
- Cevenini R., Rumpianesi F., Sambri V., La Placa M. Antigenic specificity of serological response in Chlamydia trachomatis urethritis detected by immunoblotting. J Clin Pathol. 1986 Mar;39(3):325–327. doi: 10.1136/jcp.39.3.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cevenini R., Sambri V., Massaria F., La Placa M., Brocchi E., De Simone F. Complement-mediated in vitro bactericidal activity of monoclonal antibodies reactive with outer-surface-protein OspB of Borrelia burgdorferi. FEMS Microbiol Lett. 1992 Jan 1;69(2):147–152. doi: 10.1016/0378-1097(92)90619-y. [DOI] [PubMed] [Google Scholar]
- Cevenini R., Sambri V., Pileri S., Ratti G., La Placa M. Development of transplantable ascites tumours which continuously produce polyclonal antibodies in pristane primed BALB/c mice immunized with bacterial antigens and complete Freund's adjuvant. J Immunol Methods. 1991 Jun 24;140(1):111–118. doi: 10.1016/0022-1759(91)90132-y. [DOI] [PubMed] [Google Scholar]
- Craft J. E., Grodzicki R. L., Steere A. C. Antibody response in Lyme disease: evaluation of diagnostic tests. J Infect Dis. 1984 May;149(5):789–795. doi: 10.1093/infdis/149.5.789. [DOI] [PubMed] [Google Scholar]
- Dorward D. W., Schwan T. G., Garon C. F. Immune capture and detection of Borrelia burgdorferi antigens in urine, blood, or tissues from infected ticks, mice, dogs, and humans. J Clin Microbiol. 1991 Jun;29(6):1162–1170. doi: 10.1128/jcm.29.6.1162-1170.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grodzicki R. L., Steere A. C. Comparison of immunoblotting and indirect enzyme-linked immunosorbent assay using different antigen preparations for diagnosing early Lyme disease. J Infect Dis. 1988 Apr;157(4):790–797. doi: 10.1093/infdis/157.4.790. [DOI] [PubMed] [Google Scholar]
- Hansen K., Bangsborg J. M., Fjordvang H., Pedersen N. S., Hindersson P. Immunochemical characterization of and isolation of the gene for a Borrelia burgdorferi immunodominant 60-kilodalton antigen common to a wide range of bacteria. Infect Immun. 1988 Aug;56(8):2047–2053. doi: 10.1128/iai.56.8.2047-2053.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen K., Hindersson P., Pedersen N. S. Measurement of antibodies to the Borrelia burgdorferi flagellum improves serodiagnosis in Lyme disease. J Clin Microbiol. 1988 Feb;26(2):338–346. doi: 10.1128/jcm.26.2.338-346.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang W., Luft B. J., Munoz P., Dattwyler R. J., Gorevic P. D. Cross-antigenicity between the major surface proteins (ospA and ospB) and other proteins of Borrelia burgdorferi. J Immunol. 1990 Jan 1;144(1):284–289. [PubMed] [Google Scholar]
- Magnarelli L. A., Anderson J. F., Barbour A. G. Enzyme-linked immunosorbent assays for Lyme disease: reactivity of subunits of Borrelia burgdorferi. J Infect Dis. 1989 Jan;159(1):43–49. doi: 10.1093/infdis/159.1.43. [DOI] [PubMed] [Google Scholar]
- Magnarelli L. A., Anderson J. F. Enzyme-linked immunosorbent assays for the detection of class-specific immunoglobulins to Borrelia burgdorferi. Am J Epidemiol. 1988 Apr;127(4):818–825. doi: 10.1093/oxfordjournals.aje.a114864. [DOI] [PubMed] [Google Scholar]
- Magnarelli L. A., Anderson J. F., Johnson R. C. Cross-reactivity in serological tests for Lyme disease and other spirochetal infections. J Infect Dis. 1987 Jul;156(1):183–188. doi: 10.1093/infdis/156.1.183. [DOI] [PubMed] [Google Scholar]
- Magnarelli L. A., Miller J. N., Anderson J. F., Riviere G. R. Cross-reactivity of nonspecific treponemal antibody in serologic tests for Lyme disease. J Clin Microbiol. 1990 Jun;28(6):1276–1279. doi: 10.1128/jcm.28.6.1276-1279.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raoult D., Hechemy K. E., Baranton G. Cross-reaction with Borrelia burgdorferi antigen of sera from patients with human immunodeficiency virus infection, syphilis, and leptospirosis. J Clin Microbiol. 1989 Oct;27(10):2152–2155. doi: 10.1128/jcm.27.10.2152-2155.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell H., Sampson J. S., Schmid G. P., Wilkinson H. W., Plikaytis B. Enzyme-linked immunosorbent assay and indirect immunofluorescence assay for Lyme disease. J Infect Dis. 1984 Mar;149(3):465–470. doi: 10.1093/infdis/149.3.465. [DOI] [PubMed] [Google Scholar]
- Sambri V., Moroni A., Massaria F., Brocchi E., De Simone F., Cevenini R. Immunological characterization of a low molecular mass polypeptidic antigen of Borrelia burgdorferi. FEMS Microbiol Immunol. 1991 Nov;3(6):345–349. doi: 10.1111/j.1574-6968.1991.tb04260.x. [DOI] [PubMed] [Google Scholar]
- Schaible U. E., Kramer M. D., Eichmann K., Modolell M., Museteanu C., Simon M. M. Monoclonal antibodies specific for the outer surface protein A (OspA) of Borrelia burgdorferi prevent Lyme borreliosis in severe combined immunodeficiency (scid) mice. Proc Natl Acad Sci U S A. 1990 May;87(10):3768–3772. doi: 10.1073/pnas.87.10.3768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steere A. C., Bartenhagen N. H., Craft J. E., Hutchinson G. J., Newman J. H., Rahn D. W., Sigal L. H., Spieler P. N., Stenn K. S., Malawista S. E. The early clinical manifestations of Lyme disease. Ann Intern Med. 1983 Jul;99(1):76–82. doi: 10.7326/0003-4819-99-1-76. [DOI] [PubMed] [Google Scholar]
- Stoenner H. G., Dodd T., Larsen C. Antigenic variation of Borrelia hermsii. J Exp Med. 1982 Nov 1;156(5):1297–1311. doi: 10.1084/jem.156.5.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilske B., Preac-Mursic V., Schierz G., Kühbeck R., Barbour A. G., Kramer M. Antigenic variability of Borrelia burgdorferi. Ann N Y Acad Sci. 1988;539:126–143. doi: 10.1111/j.1749-6632.1988.tb31846.x. [DOI] [PubMed] [Google Scholar]