Abstract
We examined the effects of urease and hydrogenase assembly gene deletions on NikR activation in H. pylori strains 26695 and G27. The loss of any component of urease assembly increased NikR activity under Ni2+-limiting conditions, as measured by reduced transcript levels and 63Ni accumulation. Additionally, SlyD functioned in urease assembly in strain 26695.
A diverse complement of proteins is dedicated to the acquisition, trafficking, and regulation of intracellular transition metal ions. The mechanisms by which these activities are integrated to allocate the appropriate proportion of metal to different metal-binding proteins are not yet understood. Additionally, studies of the equilibrium metal-binding properties of transcriptional regulatory proteins important for metal homeostasis have revealed that they avidly bind their cognate metal ions (10−21 M ≤ Kd ≤ 10−9 M [8, 9, 22, 33, 42]). These observations suggest that competition may exist between metalloenzyme assembly and metalloregulation. Detailed investigations of this hypothesis are encumbered by the presence of numerous essential metalloenzymes for metals such as zinc and iron. Microbial nickel physiology provides an ideal system for studying intracellular metal competition due to the small number of enzymes that require nickel ions (30) and their nonessentiality under laboratory growth conditions.
We have studied the effect of disrupting Ni2+-dependent enzyme assembly pathways on nickel-dependent gene regulation in the gram-negative gastric pathogen Helicobacter pylori (3). The two Ni2+-dependent enzymes of H. pylori, urease and hydrogenase, are required for efficient colonization of animal models of infection (15, 16, 31). Both enzymes require conserved, GTP-dependent pathways for metal cofactor assembly that include an absolute requirement for nickel insertion chaperones under metal-limiting conditions (30). Hausinger and coworkers identified UreE as the Ni2+-binding protein required for urease assembly in Klebsiella aerogenes (10, 38). Similarly, Bock and coworkers identified two chaperones, HypA and HypB, required for the nickel insertion step of E. coli hydrogenases (21, 26). Recently, the E. coli SlyD protein was shown to associate with HypB and participate in hydrogenase assembly (25, 46). Interestingly, in H. pylori, the hydrogenase chaperones HypA and HypB function in both hydrogenase and urease assembly (32).
NikR regulates the expression of several H. pylori genes in response to increased nickel ion levels. Genetic and biochemical studies have shown direct NikR-dependent regulation of genes required for nickel import (nixA [5, 17, 44], fecA3 [18], frpB4 [14, 18], and exbBD tonB [18]), Ni2+-dependent enzyme activity (ureAB [1, 5, 17]), and nickel storage (hpn [11]). Because NikR represses all currently known nickel import genes (nixA, fecA3, frpB4, and exbBD tonB) (14, 17, 18, 44), Ni2+-dependent enzyme biosynthesis pathways must acquire Ni2+ before NikR, and the subsequent repression of nickel uptake genes. Such competition, if present, would be manifested as a change in NikR activity independently of a change in total nickel levels. In the absence of competition, NikR activity would correlate with a fixed total nickel concentration, independent of Ni2+-dependent enzyme expression or biosynthesis. Competition between metalloenzymes and metalloregulatory proteins has not been tested. Demonstration of the nature of such competition would facilitate subsequent studies to understand the molecular basis of metal ion partitioning within cells.
We examined the effects of Ni2+-dependent enzyme assembly pathway gene deletions on NikR activity using several assays. In each case, cells were grown under identical conditions and manipulated in the same way for the same length of time. Cells were grown for 20 h (26695) or 24 h (G27) to an optical density at 600 nm of 1.0 in brucella broth (BD Difco) with 5% fetal bovine serum (Sigma) and then exposed to either 100 μM dimethylglyoxime (DMG), a Ni2+-selective chelator, or 100 μM NiCl2 for 40 min. Cells were then assayed for transcript levels (20) or urease activity (6, 37) as previously described. 63Ni content was measured as previously described (23) after 40 min accumulation in the absence of DMG treatment. Genes necessary for urease or hydrogenase assembly were individually deleted in strains 26695 or G27 using standard approaches (7, 13). Stop codons were introduced in all three frames to avoid polar effects due to gene disruption (see Tables S1 and S2 in the supplemental material for strains and oligonucleotide sequences).
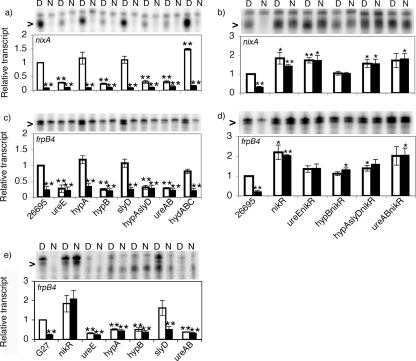
As expected, nixA and frpB4 levels were repressed five- and sixfold (Fig. 1a to d), respectively, in a NikR-dependent manner (14, 17, 18, 44). nixA and frpB4 levels in the nikR mutant strain treated with DMG or NiCl2 were also increased relative to levels in the parent strain (Fig. 1b and d), suggesting that some level of NikR-dependent repression occurs under these conditions. Deletion of the fur gene did not alleviate nickel-dependent repression of nixA and frpB4 under these conditions (data not shown). Deletion of ureE, hypB, or the urease structural genes (ureAB) resulted in significantly decreased nixA and frpB4 levels after DMG treatment compared to the parent strain, whereas deletion of hypA, slyD, or the hydrogenase structural genes (hydABC) had no effect (Fig. 1a and c). The differential effect of the hypA and hypB deletions was unexpected, because both HypA and HypB participate in urease assembly (32). Therefore, we also measured NikR regulation in a hypA slyD double mutant, which behaved similarly to all other urease pathway mutants, suggesting that HypA and SlyD play redundant roles in urease assembly in strain 26695. Together, these data suggest that an intact urease assembly pathway is required to compete with NikR for intracellular nickel.
FIG. 1.
Deletion of genes necessary for urease assembly results in NikR activation under Ni2+-limiting conditions in strains 26695 and G27. Cells were exposed to DMG (D) or NiCl2 (N) as described in the text. RNA was isolated from 26695 (a to d) or G27 (e) cells using Trizol (Invitrogen), and levels of nixA (a and b) or frpB4 (c to e) transcript were measured using 10 μg input RNA. One representative gel (10% denaturing polyacrylamide) for each probe (input, 2 × 105 cpm) is shown, and arrowheads indicate bands corresponding to digested probes for nixA or frpB4. Parent and nikR strains were always assayed in parallel to control for variations in growth. Plotted data in each panel are the averages of three independent cultures normalized to the transcript level of the parent strain. Calculated standard errors are shown. Each experiment was repeated at least twice. The upper bands in all gels represent undigested probe. Lower bands in panel e likely represent cross-hybridization of the frpB4 probe with one or more existing frpB paralogs present in H. pylori (4, 41). *, P < 0.1; **, P < 0.05.
The decrease in transcript level was always NikR dependent (Fig. 1b and d). De novo synthesis of NikR was not required for the mutant phenotypes, because inhibition of translation by chloramphenicol or erythromycin had no effect (data not shown). Single or double mutant strains with deletions of genes involved in Ni2+ transport (nixA [28] and exbBD tonB [36]), Ni2+ storage (hpn and hpn-like [6, 19, 29, 37]), and Ni2+ efflux (cznABC [39]) also showed no changes in NikR-dependent regulation (data not shown). This contrasts with results from E. coli, where the Ni2+ efflux protein RcnA impedes NikR activation under nickel-limiting conditions (23).
As expected, deletion of ureE, ureAB, or hypB decreased urease activity to ≤1% of the parent strain level (Table 1). Deletion of hypA resulted in ∼30% activity, while the hypA slyD double mutant had <1% activity. These data provide the first functional evidence of SlyD participation in H. pylori Ni2+-dependent enzyme assembly and suggest partially overlapping functions of HypA and SlyD in urease assembly in this strain. The loss of urease activity itself was not linked to NikR function, because treatment of 26695 cells with fluorofamide, a competitive inhibitor of holo-urease (2, 34), had no effect on nixA and frpB4 levels in the 26695 parent or ureAB strains but decreased urease activity >100-fold in the parent strain (data not shown).
TABLE 1.
Urease activity in different H. pylori strains
| Genotype | Urease activity (μmol NH3 min−1 mg protein−1)a
|
|||
|---|---|---|---|---|
| 26695
|
G27
|
|||
| DMG | NiCl2 | DMG | NiCl2 | |
| Wild type | 13.55 (0.52) | 20.71 (1.88) | 25.76 (1.98) | 36.65 (2.25) |
| nikR | 17.64 (1.43)* | 26.80 (0.89)* | 29.56 (0.95)* | 30.23 (0.64)** |
| ureE | 0.11 (0.03)** | 0.24 (0.07)** | 0.13 (0.02)** | 0.16 (0.00)** |
| hypA | 4.76 (0.31)** | 10.52 (0.53)* | 0.05 (0.02)** | 0.01 (0.00)** |
| hypB | 0.09 (0.00b)** | 0.23 (0.02)** | 0.12 (0.00)** | 0.17 (0.02)** |
| slyD | 13.96 (0.56) | 25.80 (0.58) | 32.92 (2.53)* | 27.00 (0.69)* |
| hypA slyD | 0.07 (0.01)** | 0.23 (0.04)** | 0.01 (0.00)** | 0.01 (0.00)** |
| ureAB | 0.04 (0.04)** | 0.01 (0.00)** | 0.01 (0.00)** | 0.01 (0.00)** |
| hydABC | 15.21 (5.05) | 25.26 (3.15) | ND | ND |
Values are the averages for three independent cultures (standard error are in parentheses) for each strain and condition. Cells were lysed by sonication and either used directly (low activity) or diluted 20-fold (high activity) in 50 mM HEPES (pH 7.0). Samples were incubated for 10 (high activity) or 30 min (low activity) at 37°C. ND, not determined. * and **, P < 0.1 and P < 0.01 for mutant versus parent strain.
Error is <0.01. The lowest measurable value by this method (A625 = 0.001) corresponds to ∼0.5 to 1 nM NH3.
A consequence of increased NikR activity was decreased Ni2+ accumulation, consistent with reduced frpB4 and nixA transcript levels. 63Ni accumulation was measured as previously described (23) in cells exposed to either low (10 nM 63NiCl2; specific activity, 9.87 mCi mg−1; Perkin-Elmer, Boston, MA) or high (50 nM 63NiCl2 plus 100 μM NiCl2) Ni2+ concentrations. The ureE, hypB, hypA slyD, and ureAB strains accumulated less 63Ni relative to the parent strain under low- and high-Ni2+ conditions, whereas the hypA, slyD, and hydABC strains took up levels of 63Ni similar to those in the parent strain (Table 2). Interestingly, the chaperone mutants showed a greater difference relative to the parent strain than the ureAB strain. This suggests that the Ni2+-binding capacity of the cell does not reside entirely with UreAB. Deletion of nikR in each mutant background restored 63Ni accumulation to levels similar to those of a nikR mutant strain (∼120 to 150% of the level in the parental strain). These data provide further evidence that NikR activity is increased in the urease pathway mutants and indicate that the decrease in nixA and frpB4 levels results in decreased NixA and FrpB4 protein levels during this assay, consistent with a previous report of the rate of NixA turnover under similar conditions (44).
TABLE 2.
Short-term 63Ni accumulation in H. pylori strains
| Genotype | Total 63Ni/cella
|
|||
|---|---|---|---|---|
| 26695
|
G27
|
|||
| Low Nib | High Nic | Low Nib | High Nic | |
| Wild type | 134.3 (1.9) | 8.52 (0.34) | 536.5 (40.3) | 6.45 (0.23) |
| nikR | 156.0 (7.5)* | 10.19 (0.79) | 867.5 (28.0)** | 6.99 (0.23)* |
| ureE | 92.2 (7.1)* | 6.86 (0.19)* | 196.8 (2.1)** | 5.26 (0.11)* |
| hypA | 128.1 (11.7) | 7.82 (0.45) | 191.2 (1.9)** | 8.71 (0.28)** |
| hypB | 124.6 (8.8)** | 5.46 (0.63)** | 213.6 (5.4)** | 5.18 (0.22)* |
| slyD | 134.0 (2.0) | 7.34 (0.36) | 564.0 (38.0) | 6.75 (0.17) |
| hypA slyD | 96.1 (1.8)** | 6.33 (0.41)** | 156.4 (5.5)** | 9.75 (0.08)** |
| ureAB | 120.7 (1.7)** | 7.68 (0.29) | 155.7 (5.1)** | 6.60 (0.35) |
| hydABC | 137.9 (5.1) | 8.09 (0.44) | ND | ND |
Values are the averages for three independent cultures (standard errors are in parentheses) for each strain and condition. Cells were incubated with 63NiCl2 as described in the text, harvested by centrifugation (16,000 × g, 1 min), washed with 900 μl of 50 mM HEPES (pH 7.0)-50 mM EDTA, and resuspended in 200 μl 10 mM acetic acid before being mixed with 1 ml scintilliation fluid. 63Ni levels were determined by scintillation counting using a preprogrammed 10-min acquisition window (0 to 1.31 MeV). Counts per minute were converted to atoms of 63Ni/CFU using a standard curve determined for cells grown under identical conditions. ND, not determined. * and **, P < 0.1 and P < 0.05 for mutant versus parent strain.
10 nM 63NiCl2.
50 nM 63NiCl2 plus 100 μM NiCl2. Values are in hundred thousands.
H. pylori is well known for interstrain variability in gene content and physiology (24, 35). Gene deletions were also constructed in H. pylori strain G27, a clinical isolate used in laboratory studies (12), to determine if urease assembly in this genetic background similarly affects NikR activity. Deletion of ureE, hypB, or ureAB had the same effect on G27 frpB4 transcript levels, urease activity, and 63Ni accumulation under Ni2+-limiting conditions as in strain 26695 (Fig. 1e; Tables 1 and 2). However, in G27, deletion of hypA alone decreased frpB4 levels, urease activity, and short-term 63Ni accumulation, while deletion of slyD had no effect in any assay. These data suggest that HypA is essential for urease assembly and nickel competition in strain G27 but not in strain 26695. HypA is also essential for urease assembly in strain 43504 (32), although activity was assayed under different conditions. A comparison of the predicted HypA, HypB, and SlyD sequences from the 26695 and G27 strains (4, 41) indicates that HypA is absolutely conserved, while HypB and SlyD both contain a few key amino acid differences that occur in domains of each protein necessary for a HypB-SlyD interaction that is required for hydrogenase assembly in E. coli (25). It is also possible that differences between strains could result from changes in protein levels of these chaperones or other proteins not examined in this study.
Our results indicate that the intact urease assembly pathway of H. pylori is required to compete with NikR for nickel ions. This competition occurs despite the substantial apparent difference in Ni2+ affinities between the chaperones and NikR (Kd = 10−6 M [6, 27] versus 10−9 [45] to 10−12 M [1, 5]). Hausinger and coworkers have shown that K. aerogenes UreE inserts Ni2+ into apo-urease in the presence of strong Ni2+ chelators (iminodiacetic or nitrilotriacetic acid; Kd = 10−9 and 10−12 M, respectively [38]), indicating that UreE, in conjunction with the urease assembly complex, can shield nickel ions from chelation (38). This observation provides a plausible model for the competition that we have observed, wherein a set of Ni2+ transfer reactions is refractory to competition from Ni2+ scavengers, such as NikR. Additionally, localized urease assembly near the inner membrane (43) could provide spatial separation from NikR, and different local concentrations of nickel ions would be sensed by each pathway within the cell.
The specific roles of the chaperones in urease assembly are not fully established. A recent study with strain 26695 identified SlyD in a complex with other urease chaperones, including HypB (40), further suggesting that SlyD functions in urease nickel insertion. The role of different chaperone functions in preventing NikR activation can now be assessed by genetic approaches using specific point mutations known to abrogate metal-binding and other activities, such as GTP hydrolysis. Additionally, the effects of the amino acid substitutions in the SlyD and HypB proteins of G27 and 26695 on urease assembly and nickel competition can be examined using both genetic and biochemical approaches.
The dynamics of intracellular metal trafficking are poorly understood. We have taken advantage of the prominent nickel physiology of H. pylori to begin to examine mechanisms of metal partitioning. Different transition metals are found either sparingly within cells (copper), used for only a few specific functions (nickel and manganese), or widely required (iron and zinc), making it likely that the nature of the competition between regulators and metalloenzymes will differ. Nonetheless, common mechanistic features are likely to emerge for the trafficking of different metals in cells.
Supplementary Material
Acknowledgments
We thank Doug Berg, Shumin Tan, and Helene Kling-Bäckhed for sharing their expertise with H. pylori growth and genetics and for generously providing strains and reagents, and Stéphane Benoit and Rob Maier for technical help with the urease assays. We also thank Rainer Haas for the generous gift of pHel2 and pHel3 plasmid DNA, and Scott Merrell and Beth Carpenter for sharing the G27 genomic sequence prior to publication.
This work was supported by National Science Foundation grant MCB0520877.
Footnotes
Published ahead of print on 23 January 2009.
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Abraham, L. O., Y. Li, and D. B. Zamble. 2006. The metal- and DNA-binding activities of Helicobacter pylori NikR. J. Inorg. Biochem. 1001005-1014. [DOI] [PubMed] [Google Scholar]
- 2.Amtul, Z., A. U. Rahman, R. A. Siddiqui, and M. I. Choudhary. 2002. Chemistry and mechanism of urease inhibition. Curr. Med. Chem. 91323-1348. [DOI] [PubMed] [Google Scholar]
- 3.Atherton, J. C. 2006. The pathogenesis of Helicobacter pylori-induced gastro-duodenal diseases. Annu. Rev. Pathol. 163-96. [DOI] [PubMed] [Google Scholar]
- 4.Baltrus, D. A., M. R. Amieva, A. Covacci, T. M. Lowe, D. S. Merrell, K. M. Ottemann, M. Stein, N. R. Salama, and K. Guillemin. 2009. The complete genome sequence of Helicobacter pylori strain G27. J. Bacteriol. 191447-448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benanti, E. L., and P. T. Chivers. 2007. The N-terminal arm of the Helicobacter pylori Ni2+-dependent transcription factor NikR is required for specific DNA binding. J. Biol. Chem. 28220365-20375. [DOI] [PubMed] [Google Scholar]
- 6.Benoit, S., and R. J. Maier. 2003. Dependence of Helicobacter pylori urease activity on the nickel-sequestering ability of the UreE accessory protein. J. Bacteriol. 1854787-4795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chalker, A. F., et al. 2001. Systematic identification of selective essential genes in Helicobacter pylori by genome prioritization and allelic replacement mutagenesis. J. Bacteriol. 1831259-1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Changela, A., K. Chen, Y. Xue, J. Holschen, C. E. Outten, T. V. O'Halloran, and A. Mondragon. 2003. Molecular basis of metal-ion selectivity and zeptomolar sensitivity by CueR. Science 3011383-1387. [DOI] [PubMed] [Google Scholar]
- 9.Chivers, P. T., and R. T. Sauer. 2002. NikR repressor: high-affinity nickel binding to the C-terminal domain regulates binding to operator DNA. Chem. Biol. 91141-1148. [DOI] [PubMed] [Google Scholar]
- 10.Colpas, G. J., and R. P. Hausinger. 2000. In vivo and in vitro kinetics of metal transfer by the Klebsiella aerogenes urease nickel metallochaperone, UreE. J. Biol. Chem. 27510731-10737. [DOI] [PubMed] [Google Scholar]
- 11.Contreras, M., J. M. Thiberge, M. A. Mandrand-Berthelot, and A. Labigne. 2003. Characterization of the roles of NikR, a nickel-responsive pleiotropic autoregulator of Helicobacter pylori. Mol. Microbiol. 49947-963. [DOI] [PubMed] [Google Scholar]
- 12.Covacci, A., et al. 1993. Molecular characterization of the 128-kDa immunodominant antigen of Helicobacter pylori associated with cytotoxicity and duodenal ulcer. Proc. Natl. Acad. Sci. USA 905791-5795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dailidiene, D., G. Dailide, D. Kersulyte, and D. E. Berg. 2006. Contraselectable streptomycin susceptibility determinant for genetic manipulation and analysis of Helicobacter pylori. Appl. Environ. Microbiol. 725908-5914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davis, G. S., E. L. Flannery, and H. L. Mobley. 2006. Helicobacter pylori HP1512 is a nickel-responsive NikR-regulated outer membrane protein. Infect. Immun. 746811-6820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eaton, K. A., C. L. Brooks, D. R. Morgan, and S. Krakowka. 1991. Essential role of urease in pathogenesis of gastritis induced by Helicobacter pylori in gnotobiotic piglets. Infect. Immun. 592470-2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eaton, K. A., and S. Krakowka. 1994. Effect of gastric pH on urease-dependent colonization of gnotobiotic piglets by Helicobacter pylori. Infect. Immun. 623604-3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ernst, F. D., E. J. Kuipers, A. Heijens, R. Sarwari, J. Stoof, C. W. Penn, J. G. Kusters, and A. H. van Vliet. 2005. The nickel-responsive regulator NikR controls activation and repression of gene transcription in Helicobacter pylori. Infect. Immun. 737252-7258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ernst, F. D., J. Stoof, W. M. Horrevoets, E. J. Kuipers, J. G. Kusters, and A. H. van Vliet. 2006. NikR mediates nickel-responsive transcriptional repression of the Helicobacter pylori outer membrane proteins FecA3 (HP1400) and FrpB4 (HP1512). Infect. Immun. 746821-6828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gilbert, J. V., J. Ramakrishna, F. W. Sunderman, Jr., A. Wright, and A. G. Plaut. 1995. Protein Hpn: cloning and characterization of a histidine-rich metal-binding polypeptide in Helicobacter pylori and Helicobacter mustelae. Infect. Immun. 632682-2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Greene, J. M., and K. Struhl. May 2001, posting date. S1 analysis of messenger RNA using single-stranded DNA probes. John Wiley & Sons, Inc., New York, NY. doi: 10.1002/0471142727.mb0406s45. [Online.] [DOI] [PubMed]
- 21.Hube, M., M. Blokesch, and A. Bock. 2002. Network of hydrogenase maturation in Escherichia coli: role of accessory proteins HypA and HybF. J. Bacteriol. 1843879-3885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iwig, J. S., S. Leitch, R. W. Herbst, M. J. Maroney, and P. T. Chivers. 2008. Ni(II) and Co(II) sensing by Escherichia coli RcnR. J. Am. Chem. Soc. 1307592-7606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iwig, J. S., J. L. Rowe, and P. T. Chivers. 2006. Nickel homeostasis in Escherichia coli—the rcnR-rcnA efflux pathway and its linkage to NikR function. Mol. Microbiol. 62252-262. [DOI] [PubMed] [Google Scholar]
- 24.Kang, J., and M. J. Blaser. 2006. Bacterial populations as perfect gases: genomic integrity and diversification tensions in Helicobacter pylori. Nat. Rev. Microbiol. 4826-836. [DOI] [PubMed] [Google Scholar]
- 25.Leach, M. R., J. W. Zhang, and D. B. Zamble. 2007. The role of complex formation between the Escherichia coli hydrogenase accessory factors HypB and SlyD. J. Biol. Chem. 28216177-16186. [DOI] [PubMed] [Google Scholar]
- 26.Lutz, S., A. Jacobi, V. Schlensog, R. Bohm, G. Sawers, and A. Bock. 1991. Molecular characterization of an operon (hyp) necessary for the activity of the three hydrogenase isoenzymes in Escherichia coli. Mol. Microbiol. 5123-135. [DOI] [PubMed] [Google Scholar]
- 27.Mehta, N., J. W. Olson, and R. J. Maier. 2003. Characterization of Helicobacter pylori nickel metabolism accessory proteins needed for maturation of both urease and hydrogenase. J. Bacteriol. 185726-734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mobley, H. L., R. M. Garner, and P. Bauerfeind. 1995. Helicobacter pylori nickel-transport gene nixA: synthesis of catalytically active urease in Escherichia coli independent of growth conditions. Mol. Microbiol. 1697-109. [DOI] [PubMed] [Google Scholar]
- 29.Mobley, H. L., R. M. Garner, G. R. Chippendale, J. V. Gilbert, A. V. Kane, and A. G. Plaut. 1999. Role of Hpn and NixA of Helicobacter pylori in susceptibility and resistance to bismuth and other metal ions. Helicobacter 4162-169. [DOI] [PubMed] [Google Scholar]
- 30.Mulrooney, S. B., and R. P. Hausinger. 2003. Nickel uptake and utilization by microorganisms. FEMS Microbiol. Rev. 27239-261. [DOI] [PubMed] [Google Scholar]
- 31.Olson, J. W., and R. J. Maier. 2002. Molecular hydrogen as an energy source for Helicobacter pylori. Science 2981788-1790. [DOI] [PubMed] [Google Scholar]
- 32.Olson, J. W., N. S. Mehta, and R. J. Maier. 2001. Requirement of nickel metabolism proteins HypA and HypB for full activity of both hydrogenase and urease in Helicobacter pylori. Mol. Microbiol. 39176-182. [DOI] [PubMed] [Google Scholar]
- 33.Outten, C. E., and T. V. O'Halloran. 2001. Femtomolar sensitivity of metalloregulatory proteins controlling zinc homeostasis. Science 2922488-2492. [DOI] [PubMed] [Google Scholar]
- 34.Pope, A. J., C. D. Toseland, B. Rushant, S. Richardson, M. McVey, and J. Hills. 1998. Effect of potent urease inhibitor, fluorofamide, on Helicobacter sp. in vivo and in vitro. Dig. Dis. Sci. 43109-119. [DOI] [PubMed] [Google Scholar]
- 35.Salama, N., K. Guillemin, T. K. McDaniel, G. Sherlock, L. Tompkins, and S. Falkow. 2000. A whole-genome microarray reveals genetic diversity among Helicobacter pylori strains. Proc. Natl. Acad. Sci. USA 9714668-14673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schauer, K., B. Gouget, M. Carriere, A. Labigne, and H. de Reuse. 2007. Novel nickel transport mechanism across the bacterial outer membrane energized by the TonB/ExbB/ExbD machinery. Mol. Microbiol. 631054-1068. [DOI] [PubMed] [Google Scholar]
- 37.Seshadri, S., S. L. Benoit, and R. J. Maier. 2007. Roles of His-rich Hpn and Hpn-like proteins in Helicobacter pylori nickel physiology. J. Bacteriol. 1894120-4126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Soriano, A., G. J. Colpas, and R. P. Hausinger. 2000. UreE stimulation of GTP-dependent urease activation in the UreD-UreF-UreG-urease apoprotein complex. Biochemistry 3912435-12440. [DOI] [PubMed] [Google Scholar]
- 39.Stahler, F. N., S. Odenbreit, R. Haas, J. Wilrich, A. H. Van Vliet, J. G. Kusters, M. Kist, and S. Bereswill. 2006. The novel Helicobacter pylori CznABC metal efflux pump is required for cadmium, zinc, and nickel resistance, urease modulation, and gastric colonization. Infect. Immun. 743845-3852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stingl, K., K. Schauer, C. Ecobichon, A. Labigne, P. Lenormand, J. C. Rousselle, A. Namane, and H. de Reuse. 2008. In vivo interactome of Helicobacter pylori urease revealed by tandem affinity purification. Mol. Cell. Proteomics 72429-2441. [DOI] [PubMed] [Google Scholar]
- 41.Tomb, J. F., et al. 1997. The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature 388539-547. [DOI] [PubMed] [Google Scholar]
- 42.VanZile, M. L., N. J. Cosper, R. A. Scott, and D. P. Giedroc. 2000. The zinc metalloregulatory protein Synechococcus PCC7942 SmtB binds a single zinc ion per monomer with high affinity in a tetrahedral coordination geometry. Biochemistry 3911818-11829. [DOI] [PubMed] [Google Scholar]
- 43.Voland, P., D. L. Weeks, E. A. Marcus, C. Prinz, G. Sachs, and D. Scott. 2003. Interactions among the seven Helicobacter pylori proteins encoded by the urease gene cluster. Am. J. Physiol. Gastrointest. Liver Physiol. 284G96-G106. [DOI] [PubMed] [Google Scholar]
- 44.Wolfram, L., E. Haas, and P. Bauerfeind. 2006. Nickel represses the synthesis of the nickel permease NixA of Helicobacter pylori. J. Bacteriol. 1881245-1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zambelli, B., M. A. Danielli, V. Scarlato, and S. Ciurli. 2007. The Ni2+ binding properties of Helicobacter pylori NikR. Chem. Commun. (Cambridge) 20073649-3651. [DOI] [PubMed] [Google Scholar]
- 46.Zhang, J. W., G. Butland, J. F. Greenblatt, A. Emili, and D. B. Zamble. 2005. A role for SlyD in the Escherichia coli hydrogenase biosynthetic pathway. J. Biol. Chem. 2804360-4366. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.