Abstract
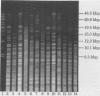
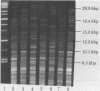
Newer genetic techniques have replaced phenotypic methods of subtyping Pseudomonas aeruginosa strains. Widespread application of newer methodologies, however, may be limited by technologic complexity and the cost of equipment. We conducted restriction endonuclease analysis (REA) of sheared genomic DNAs from 48 clinical P. aeruginosa strains using the enzyme SalI and electrophoresis in horizontal, low-concentration (0.3 to 0.6%) agarose gels. Each REA profile consisted of a smear of lower-molecular-mass bands as well as a countable number of well-resolved bands in the 8.3- to 48.5-kbp range which could easily be compared when isolates were run side-by-side on the same gel. In general, the REA patterns of strains recovered from different patients differed by at least seven bands, and those of serial isolates from individual patients were identical or differed by, at most, two bands over this 8.3- to 48.5-kbp range. REA of strains already subtyped by field inversion gel electrophoresis revealed that the two techniques generally paralleled each other. Overall, some unrelated strains had similar REA profiles, but the relative simplicity and low cost of the approach coupled with the ability to demonstrate differences between most unrelated strains should make this type of REA an attractive first step in the investigation of institutional P. aeruginosa problems.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boukadida J., de Montalembert M., Gaillard J. L., Gobin J., Grimont F., Girault D., Véron M., Berche P. Outbreak of gut colonization by Pseudomonas aeruginosa in immunocompromised children undergoing total digestive decontamination: analysis by pulsed-field electrophoresis. J Clin Microbiol. 1991 Sep;29(9):2068–2071. doi: 10.1128/jcm.29.9.2068-2071.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fegan M., Francis P., Hayward A. C., Fuerst J. A. Heterogeneity, persistence, and distribution of Pseudomonas aeruginosa genotypes in cystic fibrosis patients. J Clin Microbiol. 1991 Oct;29(10):2151–2157. doi: 10.1128/jcm.29.10.2151-2157.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goullet P., Picard B. Pseudomonas aeruginosa isolate typing by esterase electrophoresis. FEMS Microbiol Lett. 1991 Mar 1;62(2-3):195–200. doi: 10.1016/0378-1097(91)90157-6. [DOI] [PubMed] [Google Scholar]
- Grothues D., Koopmann U., von der Hardt H., Tümmler B. Genome fingerprinting of Pseudomonas aeruginosa indicates colonization of cystic fibrosis siblings with closely related strains. J Clin Microbiol. 1988 Oct;26(10):1973–1977. doi: 10.1128/jcm.26.10.1973-1977.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjelm L. N., Branstrom A. A., Warren R. L. Detection of restriction fragment length polymorphisms in clinical isolates and serially passaged Pseudomonas aeruginosa strains. J Clin Microbiol. 1990 Oct;28(10):2178–2182. doi: 10.1128/jcm.28.10.2178-2182.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holloway B. W., Dharmsthiti S., Krishnapillai V., Morgan A. F., Ratnaningsih E., Sinclair M. I., Saffery R. Chromosome organization in Pseudomonas aeruginosa. Antibiot Chemother (1971) 1991;44:23–28. doi: 10.1159/000420293. [DOI] [PubMed] [Google Scholar]
- Kern W., Wolz C., Döring G. Molecular epidemiological study of Pseudomonas aeruginosa isolates from patients with acute leukemia. Eur J Clin Microbiol Infect Dis. 1990 Apr;9(4):257–261. doi: 10.1007/BF01968056. [DOI] [PubMed] [Google Scholar]
- Loutit J. S., Tompkins L. S. Restriction enzyme and Southern hybridization analyses of Pseudomonas aeruginosa strains from patients with cystic fibrosis. J Clin Microbiol. 1991 Dec;29(12):2897–2900. doi: 10.1128/jcm.29.12.2897-2900.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClelland M., Jones R., Patel Y., Nelson M. Restriction endonucleases for pulsed field mapping of bacterial genomes. Nucleic Acids Res. 1987 Aug 11;15(15):5985–6005. doi: 10.1093/nar/15.15.5985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogle J. W., Janda J. M., Woods D. E., Vasil M. L. Characterization and use of a DNA probe as an epidemiological marker for Pseudomonas aeruginosa. J Infect Dis. 1987 Jan;155(1):119–126. doi: 10.1093/infdis/155.1.119. [DOI] [PubMed] [Google Scholar]
- Ojeniyi B., Høiby N., Rosdahl V. T. Genome fingerprinting as a typing method used on polyagglutinable Pseudomonas aeruginosa isolates from cystic fibrosis patients. APMIS. 1991 Jun;99(6):492–498. [PubMed] [Google Scholar]
- Ojeniyi B., Wolz C., Döring G., Lam J. S., Rosdahl V. T., Høiby N. Typing of polyagglutinable Pseudomonas aeruginosa isolates from cystic fibrosis patients. APMIS. 1990 May;98(5):423–431. [PubMed] [Google Scholar]
- Parrott P. L., Terry P. M., Whitworth E. N., Frawley L. W., Coble R. S., Wachsmuth I. K., McGowan J. E., Jr Pseudomonas aeruginosa peritonitis associated with contaminated poloxamer-iodine solution. Lancet. 1982 Sep 25;2(8300):683–685. doi: 10.1016/s0140-6736(82)90712-7. [DOI] [PubMed] [Google Scholar]
- Pasloske B. L., Joffe A. M., Sun Q., Volpel K., Paranchych W., Eftekhar F., Speert D. P. Serial isolates of Pseudomonas aeruginosa from a cystic fibrosis patient have identical pilin sequences. Infect Immun. 1988 Mar;56(3):665–672. doi: 10.1128/iai.56.3.665-672.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitt T. L. Epidemiological typing of Pseudomonas aeruginosa. Eur J Clin Microbiol Infect Dis. 1988 Apr;7(2):238–247. doi: 10.1007/BF01963095. [DOI] [PubMed] [Google Scholar]
- Plesiat P., Alkhalaf B., Michel-Briand Y. Prevalence and profiles of plasmids in Pseudomonas aeruginosa. Eur J Clin Microbiol Infect Dis. 1988 Apr;7(2):261–264. doi: 10.1007/BF01963098. [DOI] [PubMed] [Google Scholar]
- Römling U., Grothues D., Tümmler B. Whole DNA genome typing. Antibiot Chemother (1971) 1991;44:1–7. [PubMed] [Google Scholar]
- Samadpour M., Moseley S. L., Lory S. Biotinylated DNA probes for exotoxin A and pilin genes in the differentiation of Pseudomonas aeruginosa strains. J Clin Microbiol. 1988 Nov;26(11):2319–2323. doi: 10.1128/jcm.26.11.2319-2323.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schable B., Olson D. R., Smith P. B. Improved, computer-generated system for pyocin typing of Pseudomonas aeruginosa. J Clin Microbiol. 1986 Dec;24(6):1017–1022. doi: 10.1128/jcm.24.6.1017-1022.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speert D. P., Campbell M. E., Farmer S. W., Volpel K., Joffe A. M., Paranchych W. Use of a pilin gene probe to study molecular epidemiology of Pseudomonas aeruginosa. J Clin Microbiol. 1989 Nov;27(11):2589–2593. doi: 10.1128/jcm.27.11.2589-2593.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratton C. W. Pseudomonas aeruginosa. Infect Control. 1983 Jan-Feb;4(1):36–40. doi: 10.1017/s0195941700057647. [DOI] [PubMed] [Google Scholar]
- Thompson J. D., Cuddy K. K., Haines D. S., Gillepsie D. Extraction of cellular DNA from crude cell lysate with glass. Nucleic Acids Res. 1990 Feb 25;18(4):1074–1074. doi: 10.1093/nar/18.4.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tümmler B., Koopmann U., Grothues D., Weissbrodt H., Steinkamp G., von der Hardt H. Nosocomial acquisition of Pseudomonas aeruginosa by cystic fibrosis patients. J Clin Microbiol. 1991 Jun;29(6):1265–1267. doi: 10.1128/jcm.29.6.1265-1267.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vurma-Rapp U., Kayser F. H., Hadorn K., Wiederkehr F. Mechanism of imipenem resistance acquired by three Pseudomonas aeruginosa strains during imipenem therapy. Eur J Clin Microbiol Infect Dis. 1990 Aug;9(8):580–587. doi: 10.1007/BF01967212. [DOI] [PubMed] [Google Scholar]
- Wilson M. A., Morgan M. J., Barger G. E. Comparison of DNA fingerprinting and serotyping for identification of avian Pasteurella multocida isolates. J Clin Microbiol. 1993 Feb;31(2):255–259. doi: 10.1128/jcm.31.2.255-259.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young V. M., Moody M. R. Serotyping of Pseudomonas aeruginosa. J Infect Dis. 1974 Nov;130 (Suppl)(0):S47–S52. doi: 10.1093/infdis/130.supplement.s47. [DOI] [PubMed] [Google Scholar]
- van Ketel R. J., ter Schegget J., Zanen H. C. Molecular epidemiology of Legionella pneumophila serogroup 1. J Clin Microbiol. 1984 Sep;20(3):362–364. doi: 10.1128/jcm.20.3.362-364.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]