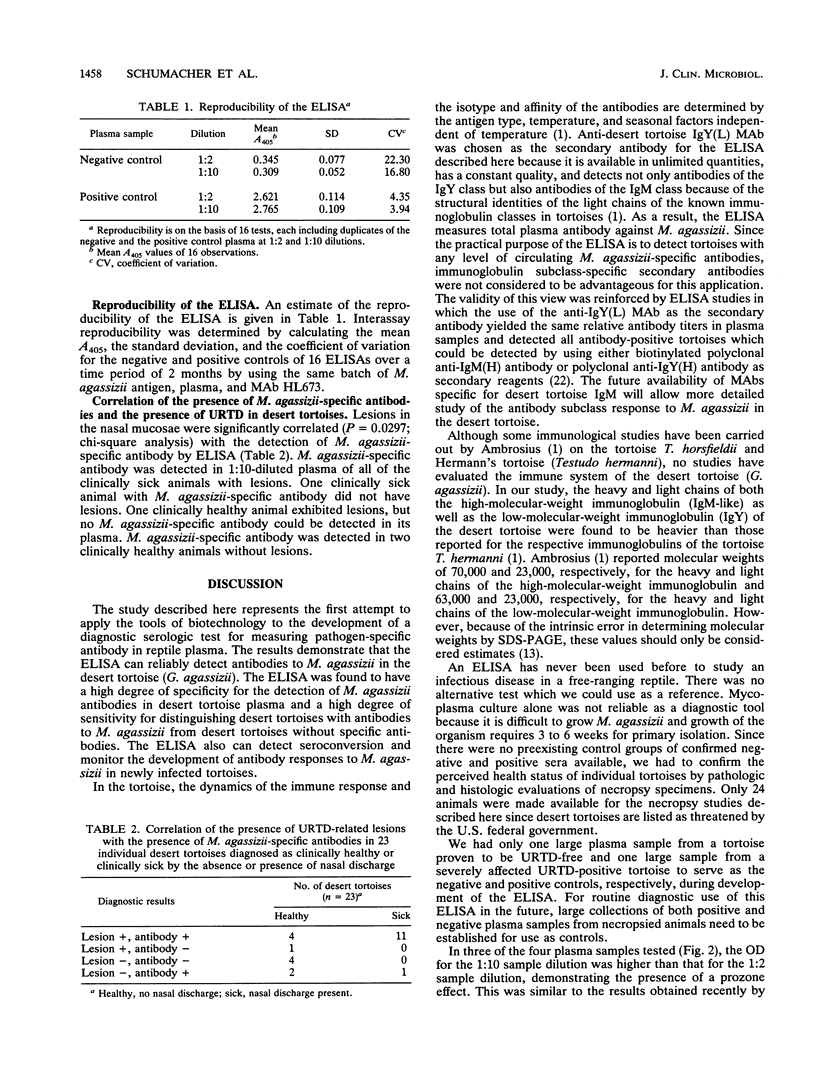
Abstract
Mycoplasma agassizii (proposed species novum) is the etiologic agent of an upper respiratory tract disease in the desert tortoise (Gopherus agassizii), which is threatened in most of its range. An enzyme-linked immunosorbent assay (ELISA) for the detection of M. agassizii-specific antibodies in desert tortoises was developed with a monoclonal antibody with specificity for desert tortoise immunoglobulin light chain. Plasma samples from one group of tortoises were tested immediately before and 1 month after challenge either with nasal exudate containing M. agassizii or with a purified preparation of M. agassizii. Plasma samples from a second group of known healthy and sick tortoises were also tested. In the first group, the ELISA detected seroconversion in individual tortoises following challenge with M. agassizii. In the second group, ELISA results were positively correlated with the health status of the tortoises, as determined by clinical and pathologic findings. In addition, the ELISA revealed that tortoise antimycoplasma antibodies were specific for M. agassizii when samples were assayed against M. agassizii, M. pulmonis, M. testudinis, and M. gallisepticum antigens. The observed direct correlation between the presence of nasal mucosal lesions and M. agassizii-specific antibodies proved that the ELISA reliably diagnosed M. agassizii infection in desert tortoises and advocates its use for monitoring M. agassizii-induced upper respiratory tract disease in free-ranging desert tortoises.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andreas E. M., Ambrosius H. Surface immunoglobulin on lymphocytes of the tortoise Agrionemys horsfieldii. Dev Comp Immunol. 1989 Spring;13(2):167–175. doi: 10.1016/0145-305x(89)90031-1. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Horowitz S. A., Cassell G. H. Detection of antibodies to Mycoplasma pulmonis by an enzyme-linked immunosorbent assay. Infect Immun. 1978 Oct;22(1):161–170. doi: 10.1128/iai.22.1.161-170.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hädge D., Fiebig H., Ambrosius H. Evolution of low molecular weight immunoglobulins. I. Relationship of 7S immunoglobulins of various vertebrates to chicken IgY. Dev Comp Immunol. 1980 Summer;4(3):501–513. doi: 10.1016/s0145-305x(80)80052-8. [DOI] [PubMed] [Google Scholar]
- Jacobson E. R., Gaskin J. M., Brown M. B., Harris R. K., Gardiner C. H., LaPointe J. L., Adams H. P., Reggiardo C. Chronic upper respiratory tract disease of free-ranging desert tortoises (Xerobates agassizii). J Wildl Dis. 1991 Apr;27(2):296–316. doi: 10.7589/0090-3558-27.2.296. [DOI] [PubMed] [Google Scholar]
- Kao K. J., Scornik J. C., Small S. J. Enzyme-linked immunoassay for anti-HLA antibodies--an alternative to panel studies by lymphocytotoxicity. Transplantation. 1993 Jan;55(1):192–196. doi: 10.1097/00007890-199301000-00036. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leslie G. A., Clem L. W. Phylogen of immunoglobulin structure and function. 3. Immunoglobulins of the chicken. J Exp Med. 1969 Dec 1;130(6):1337–1352. doi: 10.1084/jem.130.6.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minion F. C., Brown M. B., Cassell G. H. Identification of cross-reactive antigens between Mycoplasma pulmonis and Mycoplasma arthritidis. Infect Immun. 1984 Jan;43(1):115–121. doi: 10.1128/iai.43.1.115-121.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simrell C. R., Klein P. A. Antibody responses of tumor-bearing mice to their own tumors captured and perpetuated as hybridomas. J Immunol. 1979 Nov;123(5):2386–2394. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tully J. G., Whitcomb R. F., Clark H. F., Williamson D. L. Pathogenic mycoplasmas: cultivation and vertebrate pathogenicity of a new spiroplasma. Science. 1977 Mar 4;195(4281):892–894. doi: 10.1126/science.841314. [DOI] [PubMed] [Google Scholar]