Abstract
Recent neuroimaging and postmortem studies have demonstrated abnormalities in glutamatergic transmission in major depression. Glutamate NMDA (N-methyl-D-aspartate) receptors are one of the major mediators of excitatory neurotransmission in the central nervous system. At synaptic sites, NMDA receptors are linked with postsynaptic density protein-95 (PSD-95) that plays a key role in mediating trafficking, clustering, and downstream signaling events, following receptor activation. In this study, we examined the expression of NMDA receptor subunits NR1, NR2A, and NR2B as well as PSD-95 in the anterior prefrontal cortex (PFC) using Western blot method. Cortical samples were obtained from age, gender and postmortem interval matched depressed and psychiatrically healthy controls. The results revealed that there was a reduced expression of the NMDA receptor subunits NR2A (−54%) and NR2B (−48%), and PSD-95 protein level (−40%) in the PFC of depressed subjects relative to controls, with no change in the NR1 subunit. The alterations in NMDA receptor subunits, especially the NR2A and NR2B, as well as PSD-95 suggest an abnormality in the NMDA receptor signaling in the PFC in major depression. Our findings in conjunction with recent clinical, cellular, and neuroimaging studies further implicate the involvement of glutamate neurotransmission in the pathophysiology of depression. This study provides additional evidence that NMDA receptor complex is a target for discovery of novel antidepressants.
Keywords: major depressive disorder, glutamate receptors, postmortem, postsynaptic density protein, anterior prefrontal cortex
1. Introduction
A disturbance of glutamatergic transmission in the central nervous system has been suggested to contribute to the pathophysiology of major depressive disorder (MDD). The N-methyl-D-aspartate (NMDA) receptor has received the most attention with respect to the biology of depression and its treatment (for recent reviews see Kugaya and Sanacora, 2005; Pittenger et al., 2007). Furthermore, there is evidence for efficacy of NMDA receptor antagonists in depression. For example, drugs that modulate NMDA receptor functional activity have demonstrated antidepressant-like properties in animal models. Functional antagonists of the NMDA receptor including ligands at the glutamate, glycine, polyamine and ionophore recognition sites have demonstrated similar activity to currently marketed antidepressants in a range of preclinical screening procedures (for reviews see Paul and Skolnick, 2003; Kugaya and Sanacora, 2005). Clinically, ketamine, an NMDA receptor channel blocker, produces robust and rapid antidepressant effects in treatment-resistant depression (Berman et al., 2000; Zarate et al., 2006).
Ionotropic glutamate receptors are the principal mediators of fast excitatory neurotransmission in the central nervous system. These receptors include three subfamilies: alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA), kainate, and NMDA receptors. The NMDA receptors are tetrameric complexes composed of obligatory NR1 subunits co-assembled with varying expression of NR2 family of subunits, i.e. NR2(A-D) and less commonly NR3(A-B) subunits. At synaptic sites, NMDA receptors are clustered and anchored to the postsynaptic membrane via a family of specialized proteins which contain PDZ (Postsynaptic density 95, Discs large, and Zonula occludens-1) binding domains (Kornau et al., 1995; Cousins et al., 2008). Postsynaptic density-95 (PSD-95) is a prominent example of a postsynaptic protein (Kornau et al., 1995; Sheng and Kim, 2002), which belongs to a membrane associated guanylate kinase (MAGUK) family of scaffold proteins. It contains three PDZ binding domains, which enable anchoring of NMDA receptors via the C-terminal of NR2 subunits (Ziff, 1997). In addition to their role as scaffolding protein for the NMDA receptor, PSD-95 is required for receptor trafficking, signaling, membrane targeting and internalization of receptor complexes.
Previously, we have demonstrated increased protein expression of specific subunits of the NMDA receptor in the locus coeruleus (LC) and amygdala from depressed subjects (Karolewicz et al., 2005; Karolewicz et al., 2008b). Neuronal activity of the LC and amygdala is controlled by glutamatergic inputs from the prefrontal cortex (PFC). Thus, abnormalities detected previously in these subcortical regions may reflect adaptive responses to altered cortical inputs. The PFC has long been implicated in the pathobiology of depression and suicide. Earlier studies have demonstrated reduced glutamate level and gross cellular abnormalities in the PFC in MDD (Cotter et al., 2002; Drevets, 1999; Hasler et al., 2007; Rajkowska et al., 1999; Rajkowska et al., 2005; Rajkowska et al., 2007). Thus, within the framework of our current understanding of depression and its anatomical matrix, the PFC seems to be an area where pathophysiology is likely to contribute to certain aspects of depressive symptoms. To date the NMDA receptors have not been examined in the anterior PFC in depression. Hence, there is a need for such studies especially in the light of the recent findings that NMDA receptors are potential targets for rapidly acting experimental antidepressant medication, such as ketamine (Zarate et al., 2006). Thus, we hypothesize that the expression of NMDA receptor subunits is altered in the anterior PFC in depression. Therefore, in the present study, levels of relevant subunits of the NMDA receptor (NR1, NR2A, NR2B), and a major anchoring, scaffolding protein associated with the receptor (PSD-95) were investigated in the anterior PFC from subjects diagnosed with major depression and psychiatrically healthy control cases.
2. Methods
2.1. Human subjects
The tissue samples containing the anterior prefrontal cortex (PFC, Brodmann’s area 10) were collected at autopsy at the Cuyahoga County Coroner’s Office in Cleveland, OH. Informed written consent was obtained from the legal next-of-kin of all subjects. Next-of-kin were interviewed and retrospective psychiatric assessments were conducted in accordance with Institutional Review Board policies at Case Western Reserve University and the University of Mississippi Medical Center. A trained interviewer administered the Schedule for Affective Disorders and Schizophrenia: lifetime version (SADS-L) and/or the Structured Clinical Interview for DSM-IV Psychiatric Disorders (SCID-IV) to knowledgeable next-of-kin of subjects in the study. Fourteen subjects met criteria for major depressive disorder and 14 subjects did not meet criteria for an Axis I disorder (termed psychiatrically healthy controls) except for nicotine and alcohol dependence (as noted in Table 2). Among the fourteen depressed subjects, 10 were suicide victims. Blood and urine samples from all subjects were examined by the coroner’s office for psychotropic medications and substances of abuse, including ethanol (Table 1 and 2).
Table 2.
Summary of demographic characteristics of subjects
| Parameter | Controls (n=14) | Major Depression (n=14) |
|---|---|---|
| Age (years)* | 48 ± 4.3 | 49 ± 4.2 |
| PMI (hours)* | 19 ± 1.7 | 22 ± 1.5 |
| pH* | 6.7 ± 0.08 | 6.6 ± 0.08 |
| Gender (F/M) | 3/11 | 3/11 |
| Medication history a | none | n=4 (sertraline, n=2; fluoxetine, n=1; fluoxetine, paroxetine, amitriptyline, n=1) |
| Comorbid diagnosis | History of alcohol dependence, n=2 | Alcohol abuse/dependence, n=2 |
| History of alcohol abuse, n=1 | ||
| Polysubstance abuse, n=1 | ||
| Smoking | Smokers, n=4 | Smokers, n=4 |
| History of smoking, n=2 | History of smoking, n=2 |
Treatment with antidepressants within 4 wk of time of death;
mean ± S.E.M; PMI, postmortem interval; F, female; M, male. The average ages, PMI, and pH values of depressed and control subjects were not statistically different.
Table 1.
Demographic data of controls and major depressive subjects
| Control (pair no.) | Age | Sex | PMI | Brain pH | Toxicology | Cause of death |
|---|---|---|---|---|---|---|
| 1 | 35 | M | 25 | 6.74 | Lidocaine | Heart disease |
| 2 | 30 | M | 19 | 6.98 | Clean | Heart disease |
| 3 | 33 | M | 23 | 6.86 | Clean | Heart disease |
| 4 | 27 | F | 15 | 7.01 | Clean | Heart disease |
| 5 | 43 | M | 23 | 6.49 | Propoxyphene, norpropoxyphene, oxycodone | Pulmonary thromboemboli |
| 6 | 37 | M | 13 | 5.93 | Clean | Viral myocarditis |
| 7 | 46 | M | 11 | 6.95 | Clean | Heart disease |
| 8 | 54 | M | 17 | 6.87 | Brompheniramine | Heart disease |
| 9 | 67 | F | 28 | 6.39 | Clean | Heart disease |
| 10 | 69 | F | 26 | 6.7 | Clean | Heart disease |
| 11 | 70 | M | 20 | 6.81 | Clean | Heart disease |
| 12 | 74 | M | 21 | 6.62 | Clean | Abdominal aortic aneurysm |
| 13 | 59 | M | 6 | 6.79 | Lidocaine | Heart disease |
| 14 | 34 | M | 24 | 6.61 | Ethanol | Thrombophlebitis |
|
| ||||||
| MDD (pair no.) | ||||||
| 1 | 36 | M | 11 | 6.96 | Diphenhydramine | Undetermined |
| 2 | 41 | M | 19 | 6.24 | Clean | Hypertrophic cardiomyopathy |
| 3 | 30 | M | 18 | 6.91 | Ethanol | Suicide by gun shot to chest |
| 4 | 34 | F | 27 | 6.91 | Ethanol, CO, alprazolam | Suicide by CO poisoning |
| 5 | 45 | M | 29 | 6.27 | Ethanol | Suicide by gun shot to chest |
| 6 | 40 | M | 25 | 6.86 | Morphine, codeine, hydrocodone, diphenhydramine | Heart disease |
| 7 | 46 | M | 17 | 6.26 | Clean | Homicide |
| 8 | 54 | M | 23 | 6.24 | Phenobarbital, phenytoin, CO | Suicide by CO poisoning |
| 9 | 42 | F | 24 | 6.62 | Acetaminophen, propoxyphene | Suicide by overdose of propoxyphene and acetaminophen |
| 10 | 69 | F | 26 | 6.85 | Ethanol | Suicide by gun shot to head |
| 11 | 74 | M | 25 | 6.67 | Ethanol | Suicide by gun shot to head |
| 12 | 81 | M | 33 | 6.78 | Clean | Suicide by drowning |
| 13 | 60 | M | 20 | 6.31 | Ethanol | Suicide by gun shot to chest |
| 14 | 42 | M | 20 | 6.8 | Clean | Suicide by gun shot to chest |
M, male; F, female; PMI, postmortem interval; MDD, major depressive disorder; CO, carbon monoxide.
2.2. Dissection and anatomical positioning of measurements
Tissue was collected from frozen blocks containing the right anterior PFC. The anterior PFC is also known as the frontal pole or rostral frontal cortex, and comprises the most anterior part of the frontal lobe (Ramnani and Owen, 2004) and PFC was identified according to the cytoarchitectural criteria outlined previously (John et al., 2007; Rajkowska et al., 1999; Ramnani and Owen, 2004). Frozen blocks were cut into 50μm-thick sections for collecting tissue for Western blotting and 20μm-thick sections for immunohistochemistry. Dissected tissues (diameter 5 mm) containing the gray matter of anterior PFC were collected and placed in centrifuge tubes and kept at −80° C until further processing.
2.3. Immunoblotting
Tissue samples were prepared as published previously (Karolewicz et al., 2005, Karolewicz et al., 2008a). Samples were homogenized in ice-cold TE buffer (10 mM Tris-HCl and 1 mM ethylene-diaminetetraacetate, EDTA) containing protease inhibitors (Protease Inhibitor Cocktail Tablets - Complete™, Boehringer Mannheim GmbH, Mannheim, Germany). The homogenized tissue was centrifuged at 900 × g for 10 min. Total protein concentration was determined in the resulting supernatant using the bicinchoninic acid (BCA) method (Pierce Biotechnology, Inc, Rockford, IL, USA). Samples were mixed with sample buffer (125 mM Tris base, 20% glycerol, 4% SDS, 10% mercaptoethanol, 0.05% bromophenol blue, pH 6.8) and heated at 95°C for 8 min. Solubilized protein (20 μg per lane) was subjected to 7.5 or 10 % Criterion Precast Tris-HCl gel electrophoresis (Bio-Rad Laboratories, Hercules, CA, USA) and transferred to nitrocellulose membrane (Hybond ECL; Amersham Biosciences, Piscataway, NJ, USA). Nitrocellulose blots were blocked in 5% non-fat milk/TBS (20 mM Tris base and 0.5 M NaCl, pH 7.5) for 2 h, and then incubated (overnight at 4° C) with the primary antibodies (see Antibodies section below). Membranes were then washed two times for 15 min in TBS buffer and incubated with secondary anti-mouse antibody (diluted 1:2000; Amersham Biosciences, no. NA931). After incubation, blots were washed 3 times for 15 min and developed using enhanced chemiluminescence detection (ECL; Perkin-Elmer Life Sciences Inc., Boston, MA, USA) and immediately exposed to film (Hyperfilm-ECL; Amersham Biosciences).
2.4. Antibodies
All antibodies used in this study were commercially available. Mouse monoclonal antibodies were used to label NR1 (1:1000; BD Biosciences/Pharmingen, San Diego, CA, USA; no. 556308), NR2A (1:500; Millipore, Temecula, CA, USA; no MAB5216), NR2B (1:500; Chemicon International, Temecula, CA, USA; no MAB5220), and PSD-95 (1:1000; Chemicon International, Temecula, CA, USA; no MAB1596). Actin was used as a control for transfer and loading, and was detected on blots using an anti-actin antibody (Chemicon, Temecula, CA, USA; no MAB1596).
2.5. Experimental design
Fourteen depressed subjects and 14 psychiatrically healthy controls were arranged into pairs matched as close as possible for age, gender, postmortem interval (PMI) and brain pH (Table 1). Pairs of subjects were immunoblotted on the same gel (maximum 5 pairs) with duplicates on separate gels. To minimize inter-blot variability and to aid in quantifying blots, each gel was loaded with 3 concentrations of a cortical tissue standard (dissected from a psychiatrically healthy subject) consisting of 10, 20, and 40 μg of total protein. The same cortical tissue standard was used for all experimental gels.
2.6. Data analysis
Immunoreactive bands were analyzed using MCID Elite 7.0 (Imaging Research, St. Catherines, ON, Canada). Linear regression (GraphPad Prism 4.0, GraphPad Software Inc., San Diego, CA, USA) was used to plot a standard curve for each gel, from which relative optical density (ROD) values of samples were converted to cortical standard protein units for each experimental sample for each gel. To control for accuracy of tissue loading and efficiency of transfer, data were normalized to actin detected on the same blots. The final data are expressed in cortical standard protein units and presented as a ratio of [protein of interest]/[actin]. The data were analyzed statistically using a two-tailed paired Student’s t-test (GraphPad Prism 4.0). Linear regression analysis was performed to test for potential associations between age, pH, postmortem interval and each protein investigated. Analysis of covariance (ANCOVA; SPSS 15.0.1, Chicago, IL, USA) was used to correct the test for the effect of diagnosis on the protein expression, whenever we detected a significant correlation between protein immunoreactivities and one of the aforementioned covariates. A p value <0.05 was considered significant. All data are presented as means ± SEM and “n” indicates the number of subjects.
3. Results
3.1. Relationship between optical density and amount of protein
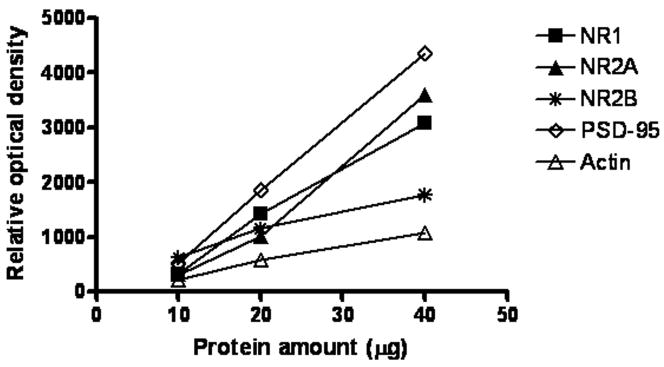
In order to determine the relationship between optical density values and the amounts of protein immunoreactivities, 10, 20, and 40 μg of cortical protein was immunoblotted with all antibodies used in this study. Analyses of blots revealed a linear relationship between optical density values and protein amounts (Fig. 1).
Figure 1.
Relationship between the optical density values of Western-blotted protein immunoreactivities and protein amounts for NR1, NR2A, NR2B, PSD-95 and actin. Wells were loaded with three concentrations of cortical tissue consisting of 10, 20, and 40 μg total protein.
3.2. Immunoblotting of NR1, NR2A, NR2B, and PSD-95
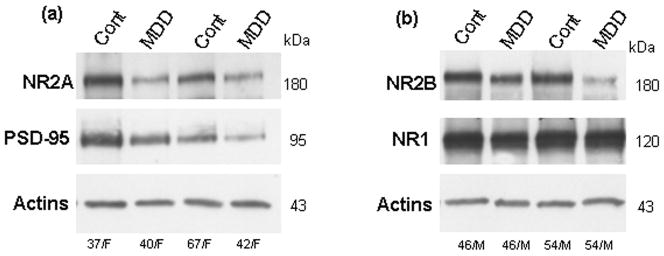
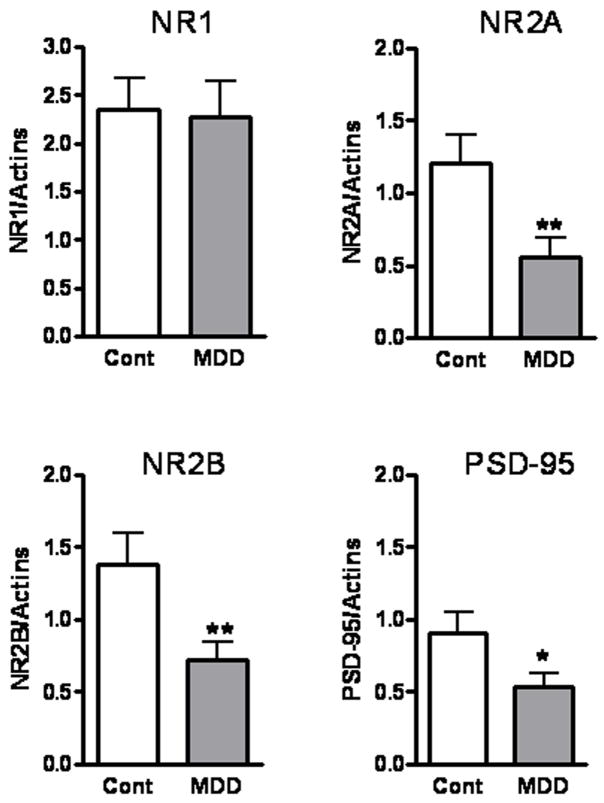
Immunoreactive bands corresponding to molecular masses of 180 and 95 kDa were revealed for the NR2A subunit and PSD-95 (Fig. 2a). Single bands with molecular masses of 180 and 120 kDa were detected for the NR2B and NR1 subunits in tissue homogenates of anterior PFC (Fig. 2b). The amount of NR2A immunoreactivity from depressed subjects (0.56 ± 0.14) was significantly lower (−54 %) than that of control subjects (1.22± 0.19, t=3.2, df=12, p<0.01; Fig. 3). Similarly, there was a robust reduction in the level of NR2B (−48 %) in depressed subjects (0.72 ± 0.13) compared to control subjects (1.38 ± 0.22, t=4.140, df=12, p<0.01; Fig. 3). The amount of PSD-95 immunoreactivity from depressed subjects (0.54 ± 0.1) was also significantly lower (−40 %) than that from control subjects (0.91 ± 0.15, t=2.174, df=13, p<0.05; Fig. 3). However, we did not detect changes in the expression of NR1 subunit between the two groups (t=0.2401, df=12, p=0.80; Fig. 3).
Figure 2.
Immunoblots of NR2A, PSD-95, and actin (a) NR2B, NR1, and actin (b) from eight representative subjects used in the analysis. Each well was loaded with 20 μg of total protein. Cont, control; MDD, major depressive disorder; F, female; M, male.
Figure 3.
Amounts of NR1, NR2A, NR2B, and PSD-95 immunoreactivities in the anterior PFC from control subjects (open bars; n=13–14) and depressed subjects (filled bars; n=13–14). Significant reductions in the NR2A, NR2B, and PSD-95 immunoreactivities were observed in depressed subjects as compared to controls; * p<0.05; **p<0.01.
3.3. Associations between age, pH, PMI and proteins investigated
There was a significant negative correlation between age and the amounts of NR2A immunoreactivity in the control subjects (r2=0.64, p<0.01). However, using ANCOVA with age as a covariate for NR2A, an effect of diagnosis on this subunit was still detected (p<0.01). A significant negative correlation was also found between age and amounts of NR2B immunoreactivity in the control subjects (r2=0.42, p<0.05). Once more, using ANCOVA with age as a covariate for NR2B, an effect of diagnosis on this subunit was detected (p<0.01). Linear regression analysis showed no significant correlations between amounts of NR1 or PSD-95 and pH, age, or PMI.
4. Discussion
The present study is the first to analyze levels of the NMDA receptor subunits NR1, NR2A, NR2B, and their anchoring protein PSD-95 in the anterior PFC from subjects diagnosed with MDD. Significant reductions in the expression of NR2A, NR2B, and PSD-95, were observed in depressed subjects as compared to psychiatrically healthy controls. In contrast, the level of the NR1 subunit was unchanged in depressed subjects.
Abnormalities in the NMDA receptor system have been previously observed in postmortem brain tissue from major depressives and suicide victims. Previously, we have reported elevated protein expression of the NR2A subunit in the amygdala (Karolewicz et al., 2008b) and the NR2C subunit in the locus coeruleus (Karolewicz et al., 2005) in depressed subjects. Now we demonstrate that NR2A and NR2B proteins are reduced in the anterior PFC in depression. In line with our present study is the recent observation by Beneyto and Meador-Woodruff (2008) who reported reduced expression of the NR2A transcript in the dorsolateral PFC in depression. Additionally, the same group found a reduction in expression of both NR2A and NR2B transcripts in the perirhinal cortex in depression (Beneyto et al., 2007). Moreover, specific binding of the NMDA receptor antagonist CGP-39653 was significantly reduced in the anterior PFC from suicide victims (Nowak et al., 1995) suggesting dysfunction of NMDA receptors in frontal cortices from suicide victims. Taken together, these studies highlight the specific regional distribution of NMDA receptor pathology and support the hypothesis that NMDA receptor signaling is altered in depression.
The abnormal expression of NR2 subunits can change the function of the receptor. It is known that different combinations of specific NR2 and NR1 subunits result in NMDA receptor/channel complexes with different characteristics. For example, NR2A or NR2B-containing receptors exhibit higher responsiveness to glutamate and higher fractional Ca2+ current than do heteromers containing the NR2C or NR2D subunits (Monyer et al., 1994; Yamakura and Shimoji, 1999). The majority of the NMDA receptors in the PFC are expressed as heteromeric complexes composed of NR1/NR2A, NR1/NR2B, or NR1/NR2A/NR2B subunits. NR1 subunits are expressed in large excess, and are rapidly degraded when unassembled with NR2 partners (Huh and Wenthold, 1999). Hence the total number of functional NMDA receptors appears to be controlled by expression of the NR2 subunits. Since lower levels of NR2A and NR2B protein could be translated into reduced numbers of functional receptors, one would speculate that depression is associated with hypofunction of the NMDA receptor in the anterior PFC.
Despite a robust reduction in both NR2A and NR2B immunoreactivity, the NR1 subunit was unchanged in the PFC in depression. This is congruent with our previous observation in the amygdala, locus coeruleus and cerebellum where no changes in the expression of NR1 subunit were observed in depression (Karolewicz et al., 2005; Karolewicz et al., 2008b). Similarly, Toro and Deakin (2005) reported unchanged NR1 immunoreactivity in the orbitofrontal cortex and in subregions of the hippocampal dentate gyrus in depressed subjects. Since the specific properties of NMDA receptors such as binding affinities for agonists and antagonists and differences in conductance properties are shaped by the combination of NR1 with NR2 subunits (Lopez de Armentia and Sah, 2003; Prybylowski et al., 2002), the unchanged NR1 immunoreactivity found in this study does not rule out abnormal NMDA receptor expression and/or function in depression.
Our present observation that the PSD-95 is markedly reduced in the anterior PFC in depression is not surprising as this protein plays a crucial role in the trafficking, membrane targeting, and internalization of NMDA receptor complexes. Thus, lower levels of PSD-95 may reflect reduced communication/coupling of the NMDA receptors as well as non-NMDA receptors with intracellular signaling cascades. In contrast, previously we have reported a marked elevation in the levels of PSD-95 protein in the amygdala in depression (Karolewicz et al., 2008b). Interestingly, the difference between the present findings in the PFC and our previous studies in the amygdala suggests that depression-associated pathology of NMDA/PSD-95 is regionally specific. Other reports that have measured PSD-95 in different brain regions have not described changes in the expression of PSD-95. Unchanged levels of PSD-95 mRNA were previously reported in the dorsolateral PFC and striatum (Kristiansen and Meador-Woodruf, 2005; Beneyto and Meador-Woodruf, 2008) or in the hippocampus and orbitofrontal cortex in depression (Toro and Deakin, 2005). Differences between experimental techniques and subjects characteristics (e.g. medication exposure) are key factors that may be associated with discrepancies between postmortem studies.
Alterations in NMDA receptor expression can be a consequence of altered glutamate levels in brains of depressed subjects. In fact, several lines of evidence indicate reduced (Auer et al., 2000; Hasler et al., 2007; Michael et al., 2003 a, b; Mirza et al., 2004; Pfleiderer et al., 2003) or elevated (Hashimoto et al., 2007; Sanacora et al., 2004) glutamate or glutamate/glutamine (Glx) levels in various brain regions in depression. Interestingly, reduced Glx levels were recently reported in the anterior PFC in unmedicated depressed patients (Hasler et al., 2007). Previous postmortem studies have revealed a reduction in cellular size and density in the PFC in depression (Cotter et al., 2002; Rajkowska et al., 1999, Rajkowska et al., 2005). Rajkowska and co-workers (1999) reported reduction in cortical thickness, and reductions in neuronal size and density in the upper and lower cortical layers of the rostral orbitofrontal cortex (Brodmann’s area 10–47) in depressed subjects. Neuronal pathology detected in cortical layers III, V and VI of the dorsolateral PFC and anterior cingulate cortex in depression (Cotter et al., 2001; Rajkowska et al., 1999) is associated with the pathology of glutamatergic pyramidal neurons that express NMDA receptors (Akbarian et al., 1996). It has been established that activation of synaptic NMDA receptors promotes neuronal survival, and enhanced expression of brain derived neurotrophic factor (Hardingham et al., 2002). Thus, it is likely that disturbances in the NMDA receptor system in depression may underlie the impairment in cellular plasticity and resilience, and may contribute to cellular pathology consistently detected in the PFC in depression (for review see Rajkowska, 2000; Rajkowska, 2003). However, further studies are required to elucidate whether aberrations in the NMDA receptor complex are the reason for or consequence of the cellular changes detected in depression.
Studies have demonstrated that a single dose of the NMDA antagonist ketamine induces a rapid (within hours) antidepressant effect (Berman et al., 2000; Zarate et al., 2006). Recent biochemical and behavioral studies support the contention that the fast antidepressant response to ketamine is mediated by increased AMPA to NMDA throughput (Maeng et al., 2008). Notably, ketamine rapidly increases the release of glutamate (Moghaddam et al., 1997), a process most likely mediated via NMDA receptors expressed on GABAergic interneurons. Our current and previous postmortem studies demonstrate region specific abnormalities in NMDA receptor expression in MDD. Based on these observations it is tempting to hypothesize that ketamine produces its rapid antidepressant responses by correcting these abnormalities in “here and now” manner in critical neuronal circuits. Moreover, based on the current observation, it is plausible to hypothesize that optimal levels of NMDA receptor activation are essential for proper PFC function and could be required for antidepressant activity. However, further studies are needed to elucidate whether aberrations in the NMDA receptor complex coexist with the pathology of AMPA receptors in depressed subjects.
A possible limitation of this study is the confounding effect of medication. In fact, previous animal studies have provided evidence that antidepressant drugs produce region-specific reductions of transcripts for NMDA receptor subunits (Boyer et al., 1998) and produce changes in the radioligand binding properties of the NMDA receptor (for review see Skolnick et al., 1996). The present study includes four depressed subjects with a reported history of antidepressant medication within four weeks before time of death (Table 2). However, antidepressants were not detected in the postmortem toxicology screening. Two of these four subjects had NR2A, NR2B, and PSD-95 levels lower than that of matched controls and comparable to the average level of immunoreactivity of depressive subjects lacking the drug exposure. The other two depressed subjects had NR2A, NR2B and PSD-95 levels nearly unchanged compared to their matched controls. Based on this observation it can be concluded that past antidepressant treatment is unlikely to contribute to the reduction of NR2A, NR2B or PSD-95 levels observed in depression. However, further research is needed to determine the possible influence of antidepressant medication on the expression of NMDA receptor proteins.
Conclusions
In conclusion, the data reported herein, in conjunction with recent clinical, cellular, and neuroimaging studies, further implicate the involvement of the glutamate system in the pathophysiology of depression. Reduced levels of NMDA receptor subunits NR2A and NR2B, as well as PSD-95 suggest an abnormality in glutamate signaling in the anterior PFC in depression. It is tempting to hypothesize that neurotransmission at the NMDA receptor and its downstream signaling system is reduced in the PFC in depressed subjects. Thus, the reductions in the NMDA receptor complex are likely to be associated with cognitive deficits as well as affective symptoms of depression. Consequently, modification of NMDA receptor signaling represents a novel approach for the development of effective antidepressant medication.
Acknowledgments
The authors thank Drs Grazyna Rajkowska, Dorota Maciag, and Jose-Javier Miguel-Hidalgo for assistance in anatomical positioning of measurements and tissue dissection. We thank Dr. John P. Kelly for editing the manuscript. We gratefully acknowledge the work of Drs James C. Overholser, Herbert Y. Meltzer, Bryan L. Roth, George Jurjus, Ginny Dilley, Lisa Konick, Nicole Herbst and Lesa Dieter in the retrospective psychiatric diagnoses. The excellent assistance of the Cuyahoga County Coroner’s Office, Cleveland, OH is greatly appreciated. This publication was supported by RR17701; American Foundation for Suicide Prevention (Young Investigator Award to BK); NARSAD (Young Investigator Award to BK), and MH67996.
Abbreviations
- MDD
major depressive disorder
- AMPA
alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid
- NMDA
N-methyl-D-aspartate
- NR
NMDA receptor subunit
- PFC
prefrontal cortex
- PSD-95
postsynaptic density protein of 95 kilo Dalton
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akbarian S, Sucher NJ, Bradley D, Tafazzoli A, Trinh D, Hetrick WP, et al. Selective alterations in gene expression for NMDA receptor subunits in prefrontal cortex of schizophrenics. J Neurosci. 1996;6:19–30. doi: 10.1523/JNEUROSCI.16-01-00019.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auer DP, Putz B, Kraft E, Lipinski B, Schill J, Holsboer F. Reduced glutamate in the anterior cingulate cortex in depression: an in vivo proton magnetic resonance spectroscopy study. Biol Psychiatry. 2000;47:305–13. doi: 10.1016/s0006-3223(99)00159-6. [DOI] [PubMed] [Google Scholar]
- Beneyto M, Meador-Woodruff JH. Lamina-Specific Abnormalities of NMDA Receptor-Associated Postsynaptic Protein Transcripts in the Prefrontal Cortex in Schizophrenia and Bipolar Disorder. Neuropsychopharmacology. 2008;33:2175–86. doi: 10.1038/sj.npp.1301604. [DOI] [PubMed] [Google Scholar]
- Beneyto M, Kristiansen LV, Oni-Orisan A, McCullumsmith RE, Meador-Woodruff JH. Abnormal glutamate receptor expression in the medial temporal lobe in schizophrenia and mood disorders. Neuropsychopharmacology. 2007;32:1888–1902. doi: 10.1038/sj.npp.1301312. [DOI] [PubMed] [Google Scholar]
- Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47:351–54. doi: 10.1016/s0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- Boyer PA, Skolnick P, Fossom LH. Chronic administration of imipramine and citalopram alters the expression of NMDA receptor subunit mRNAs in mouse brain. A quantitative in situ hybridization study. J Mol Neurosci. 1998;10:219–33. doi: 10.1007/BF02761776. [DOI] [PubMed] [Google Scholar]
- Cotter D, Mackay D, Chana G, Beasley C, Landau S, Everall IP. Reduced neuronal size and glial cell density in area 9 of the dorsolateral prefrontal cortex in subjects with major depressive disorder. Cereb Cortex. 2002;12:386–94. doi: 10.1093/cercor/12.4.386. [DOI] [PubMed] [Google Scholar]
- Cotter D, Mackay D, Landau S, Kerwin R, Everall I. Reduced glial cell density and neuronal size in the anterior cingulate cortex in major depressive disorder. Arch Gen Psychiatry. 2001;58:545–53. doi: 10.1001/archpsyc.58.6.545. [DOI] [PubMed] [Google Scholar]
- Cousins SL, Papadakis M, Rutter AR, Stephenson FA. Differential interaction of NMDA receptor subtypes with the post-synaptic density-95 family of membrane associated guanylate kinase proteins. J Neurochem. 2008;104:903–13. doi: 10.1111/j.1471-4159.2007.05067.x. [DOI] [PubMed] [Google Scholar]
- Drevets WC. Prefrontal cortical amygdalar metabolism in major depression. Ann N Y Acad Sci. 1999;877:614–37. doi: 10.1111/j.1749-6632.1999.tb09292.x. [DOI] [PubMed] [Google Scholar]
- Hardingham GE, Fukunaga Y, Bading H. Extrasynaptic NMDARs oppose synaptic NMDARs by triggering CREB shut-off and cell death pathways. Nat Neurosci. 2002;5:405–14. doi: 10.1038/nn835. [DOI] [PubMed] [Google Scholar]
- Hashimoto K, Sawa A, Iyo M. Increased levels of glutamate in brains from patients with mood disorders. Biol Psychiatry. 2007;62:1310–16. doi: 10.1016/j.biopsych.2007.03.017. [DOI] [PubMed] [Google Scholar]
- Hasler G, van der Veen JW, Tumonis T, Meyers N, Shen J, Drevets WC. Reduced prefrontal glutamate/glutamine and gamma-aminobutyric acid levels in major depression determined using proton magnetic resonance spectroscopy. Arch Gen Psychiatry. 2007;64:193–200. doi: 10.1001/archpsyc.64.2.193. [DOI] [PubMed] [Google Scholar]
- Huh KH, Wenthold RJ. Turnover analysis of glutamate receptors identifies a rapidly degraded pool of the N-methyl-D-aspartate receptor subunit, NR1, in cultured cerebellar granule cells. J Biol Chem. 1999;274:151–7. doi: 10.1074/jbc.274.1.151. [DOI] [PubMed] [Google Scholar]
- John JP, Yashavantha BS, Gado M, Veena R, Jain S, Ravishankar S, et al. A proposal for MRI-based parcellation of the frontal pole. Brain Struct Funct. 2007;212:245–53. doi: 10.1007/s00429-007-0157-x. [DOI] [PubMed] [Google Scholar]
- Karolewicz B, Johnson L, Szebeni K, Stockmeier CA, Ordway GA. Glutamate signaling proteins and tyrosine hydroxylase in the locus coeruleus of alcoholics. J Psychiatr Res. 2008a;42:348–55. doi: 10.1016/j.jpsychires.2007.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karolewicz B, Stockmeier CA, Ordway GA. Elevated Levels of the NR2C Subunit of the NMDA receptor in the locus coeruleus in depression. Neuropsychopharmacology. 2005;30:1557–67. doi: 10.1038/sj.npp.1300781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karolewicz B, Szebeni K, Gilmore T, Maciag D, Stockmeier CA, Ordway GA. Elevated levels of NR2A and PSD-95 in the lateral amygdala in depression. Int J Neuropsychopharmacol. 2008b doi: 10.1017/S1461145708008985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornau HC, Schenker LT, Kennedy MB, Seeburg PH. Domain interaction between NMDA receptor subunits and the postsynaptic density protein PSD-95. Science. 1995;269:1737–40. doi: 10.1126/science.7569905. [DOI] [PubMed] [Google Scholar]
- Kristiansen LV, Meador-Woodruff JH. Abnormal striatal expression of transcripts encoding NMDA interacting PSD proteins in schizophrenia, bipolar disorder and major depression. Schizophr Res. 2005;78:87–93. doi: 10.1016/j.schres.2005.06.012. [DOI] [PubMed] [Google Scholar]
- Kugaya A, Sanacora G. Beyond monoamines: glutamatergic function in mood disorders. CNS Spectr. 2005;10:808–19. doi: 10.1017/s1092852900010403. [DOI] [PubMed] [Google Scholar]
- Lopez de Armentia M, Sah P. Development and subunit composition of synaptic NMDA receptors in the amygdala: NR2B synapses in the adult central amygdala. J Neurosci. 2003;23:6876–83. doi: 10.1523/JNEUROSCI.23-17-06876.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeng S, Zarate CA, Jr, Du J, Schloesser RJ, McCammon J, Chen G, Manji HK. Cellular mechanisms underlying the antidepressant effects of ketamine: role of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors. Biol Psychiatry. 2008;63:349–52. doi: 10.1016/j.biopsych.2007.05.028. [DOI] [PubMed] [Google Scholar]
- Michael N, Erfurth A, Ohrmann P, Arolt V, Heindel W, Pfleiderer B, et al. Metabolic changes within the left dorsolateral prefrontal cortex occurring with electroconvulsive therapy in patients with treatment resistant unipolar depression. Psychol Med. 2003a;33:1277–84. doi: 10.1017/s0033291703007931. [DOI] [PubMed] [Google Scholar]
- Michael N, Erfurth A, Ohrmann P, Arolt V, Heindel W, Pfleiderer B. Neurotrophic effects of electroconvulsive therapy: a proton magnetic resonance study of the left amygdalar region in patients with treatment-resistant depression. Neuropsychopharmacology. 2003b;28:720–25. doi: 10.1038/sj.npp.1300085. [DOI] [PubMed] [Google Scholar]
- Mirza Y, Tang J, Russell A, Banerjee SP, Bhandari R, Ivey J, et al. Reduced anterior cingulate cortex glutamatergic concentrations in childhood major depression. J Am Acad Child Adolesc Psychiatry. 2004;43:341–48. doi: 10.1097/00004583-200403000-00017. [DOI] [PubMed] [Google Scholar]
- Moghaddam B, Adams B, Verma A, Daly D. Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neurosci. 1997;17:2921–7. doi: 10.1523/JNEUROSCI.17-08-02921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monyer H, Burnashev N, Laurie DJ, Sakmann B, Seeburg PH. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron. 1994;12:529–40. doi: 10.1016/0896-6273(94)90210-0. [DOI] [PubMed] [Google Scholar]
- Nowak G, Ordway GA, Paul IA. Alterations in the N-methyl-D-aspartate (NMDA) receptor complex in the frontal cortex of suicide victims. Brain Res. 1995;675:157–64. doi: 10.1016/0006-8993(95)00057-w. [DOI] [PubMed] [Google Scholar]
- Paul IA, Skolnick P. Glutamate and depression: clinical and preclinical studies. Ann N Y Acad Sci. 2003;1003:250–72. doi: 10.1196/annals.1300.016. [DOI] [PubMed] [Google Scholar]
- Pfleiderer B, Michael N, Erfurth A, Ohrmann P, Hohmann U, Wolgast M, et al. Effective electroconvulsive therapy reverses glutamate/glutamine deficit in the left anterior cingulum of unipolar depressed patients. Psychiatry Res. 2003;122:185–92. doi: 10.1016/s0925-4927(03)00003-9. [DOI] [PubMed] [Google Scholar]
- Pittenger C, Sanacora G, Krystal JH. The NMDA receptor as a therapeutic target in major depressive disorder CNS. Neurol Disord Drug Targets. 2007;6:101–15. doi: 10.2174/187152707780363267. [DOI] [PubMed] [Google Scholar]
- Prybylowski K, Fu Z, Losi G, Hawkins LM, Luo J, Chang K, et al. Relationship between availability of NMDA receptor subunits and their expression at the synapse. J Neurosci. 2002;22:8902–10. doi: 10.1523/JNEUROSCI.22-20-08902.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkowska G. Histopathology of the prefrontal cortex in major depression: what does it tell us about dysfunctional monoaminergic circuits? Prog Brain Res. 2000;126:397–412. doi: 10.1016/S0079-6123(00)26026-3. [DOI] [PubMed] [Google Scholar]
- Rajkowska G. Depression: what we can learn from postmortem studies. Neuroscientist. 2003;9:273–84. doi: 10.1177/1073858403252773. [DOI] [PubMed] [Google Scholar]
- Rajkowska G, Miguel-Hidalgo JJ, Dubey P, Stockmeier CA, Krishnan KR. Prominent reduction in pyramidal neurons density in the orbitofrontal cortex of elderly depressed patients. Biol Psychiatry. 2005;58:297–306. doi: 10.1016/j.biopsych.2005.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkowska G, Miguel-Hidalgo JJ, Wei J, Dilley G, Pittman SD, Meltzer HY, et al. Morphometric evidence for neuronal and glial prefrontal cell pathology in major depression. Biol Psychiatry. 1999;45:1085–98. doi: 10.1016/s0006-3223(99)00041-4. [DOI] [PubMed] [Google Scholar]
- Rajkowska G, O’Dwyer G, Teleki Z, Stockmeier CA, Miguel-Hidalgo JJ. GABAergic neurons immunoreactive for calcium binding proteins are reduced in the prefrontal cortex in major depression. Neuropsychopharmacology. 2007;32:471–82. doi: 10.1038/sj.npp.1301234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramnani N, Owen AM. Anterior prefrontal cortex: insights into function from anatomy and neuroimaging. Nat Rev Neurosci. 2004;5:184–94. doi: 10.1038/nrn1343. [DOI] [PubMed] [Google Scholar]
- Sanacora G, Gueorguieva R, Epperson CN, Wu YT, Appel M, Rothman DL, et al. Subtype-specific alterations of gamma-aminobutyric acid and glutamate in patients with major depression. Arch Gen Psychiatry. 2004;61:705–13. doi: 10.1001/archpsyc.61.7.705. [DOI] [PubMed] [Google Scholar]
- Sheng M, Kim MJ. Postsynaptic signaling and plasticity mechanisms. Science. 2002;298:776–80. doi: 10.1126/science.1075333. [DOI] [PubMed] [Google Scholar]
- Skolnick P, Layer RT, Popik P, Nowak G, Paul IA, Trullas R. Adaptation of N-methyl-D-aspartate (NMDA) receptors following antidepressant treatment: implications for the pharmacotherapy of depression. Pharmacopsychiatry. 1996;29:23–6. doi: 10.1055/s-2007-979537. [DOI] [PubMed] [Google Scholar]
- Toro C, Deakin JF. NMDA receptor subunit NRI and postsynaptic protein PSD-95 in hippocampus and orbitofrontal cortex in schizophrenia and mood disorder. Schizophr Res. 2005;80:323–30. doi: 10.1016/j.schres.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Yamakura T, Shimoji K. Subunit- and site-specific pharmacology of the NMDA receptor channel. Prog Neurobiol. 1999;59:279–98. doi: 10.1016/s0301-0082(99)00007-6. [DOI] [PubMed] [Google Scholar]
- Zarate CA, Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psych. 2006;63:856–64. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
- Ziff EB. Enlightening the postsynaptic density. Neuron. 1997;19:1163–74. doi: 10.1016/s0896-6273(00)80409-2. [DOI] [PubMed] [Google Scholar]