Abstract
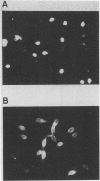
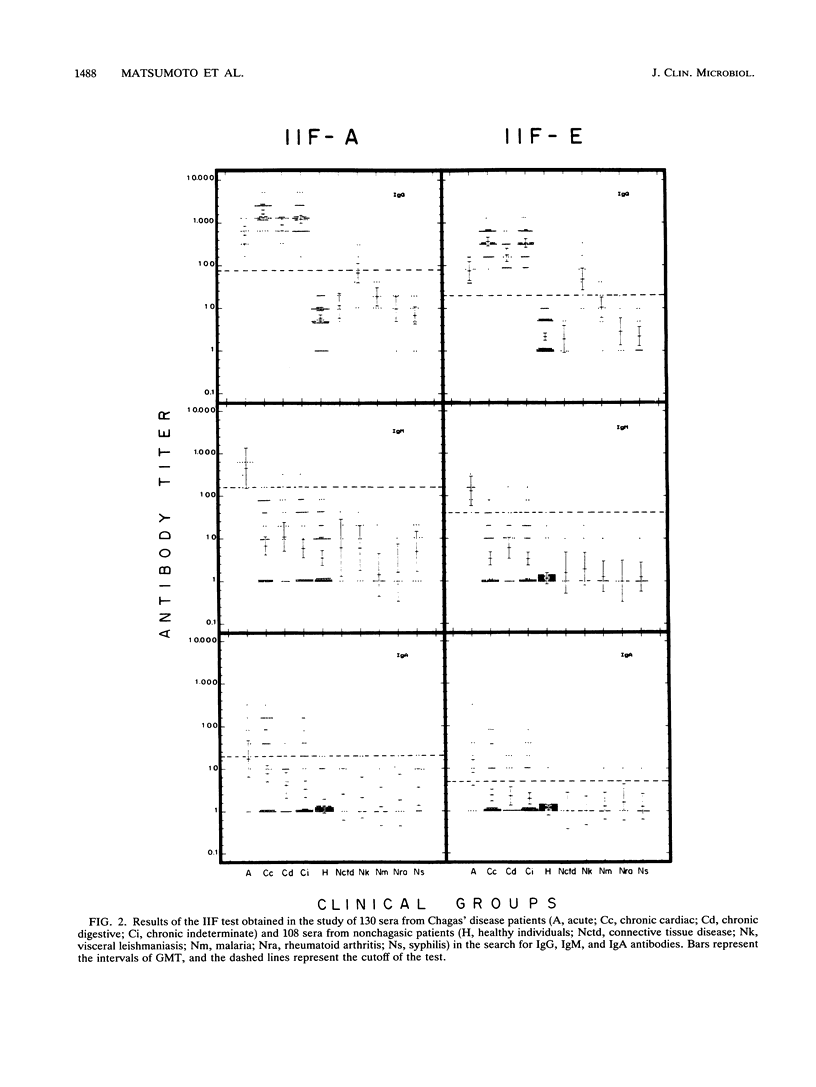
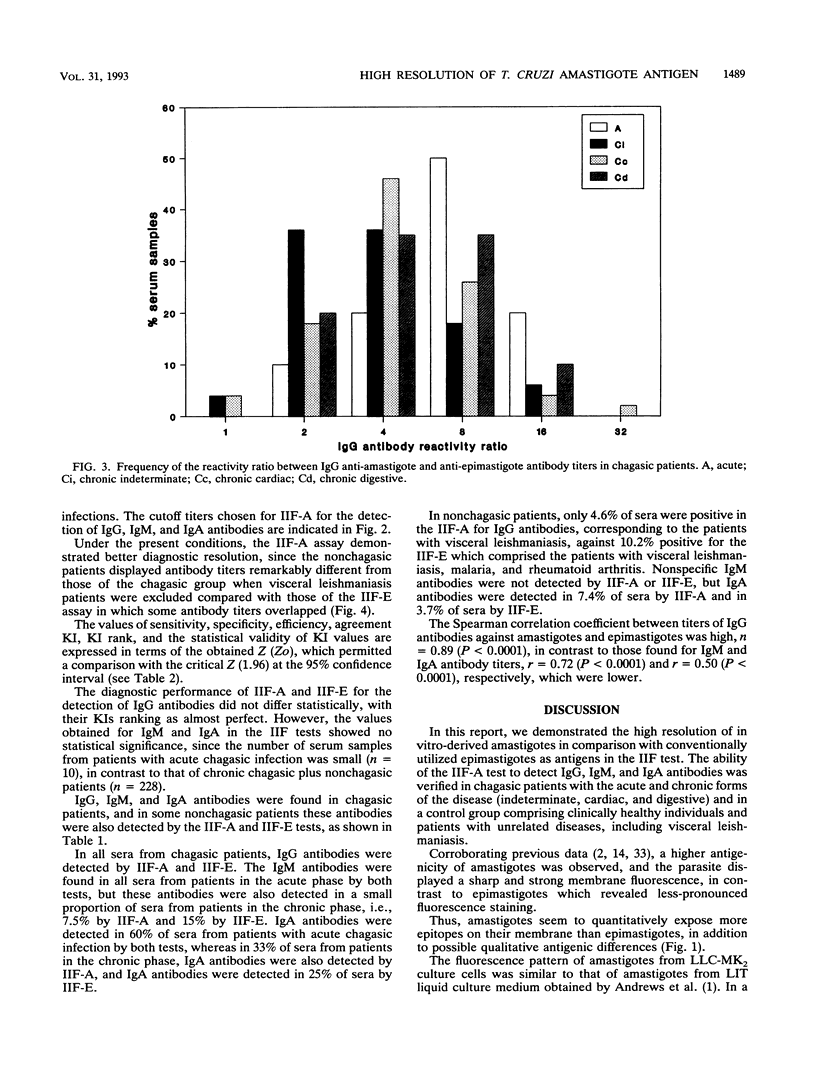
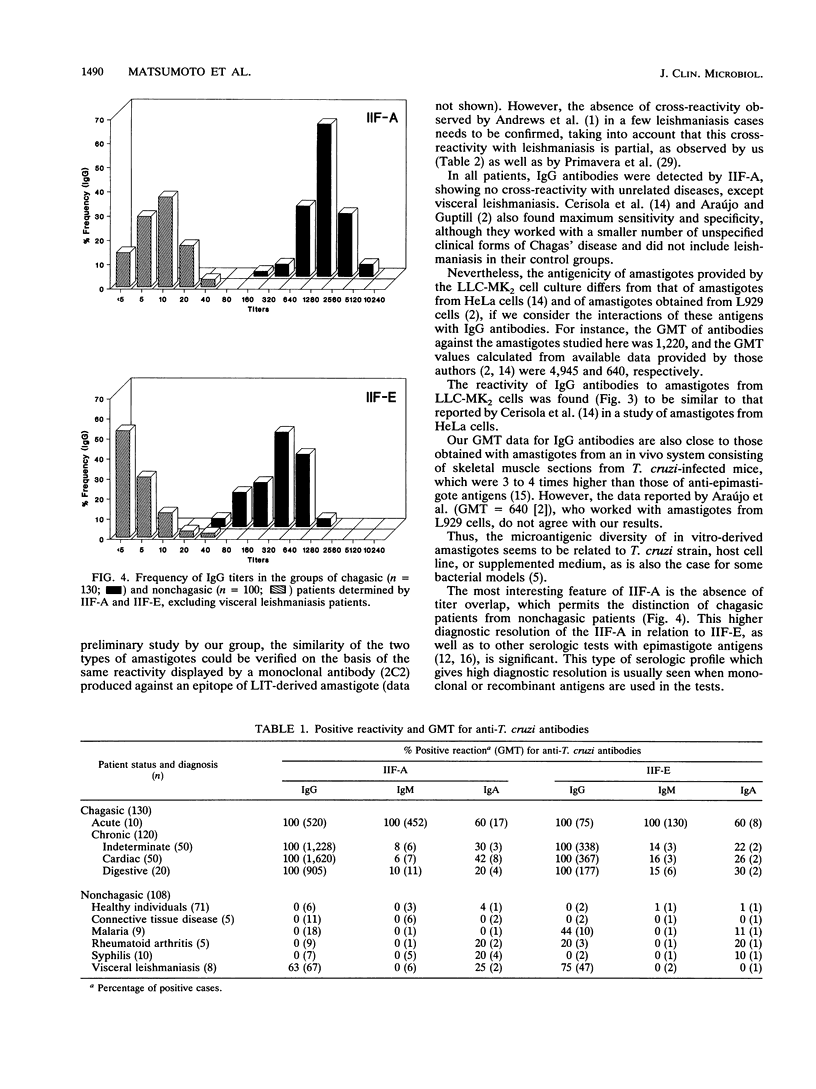
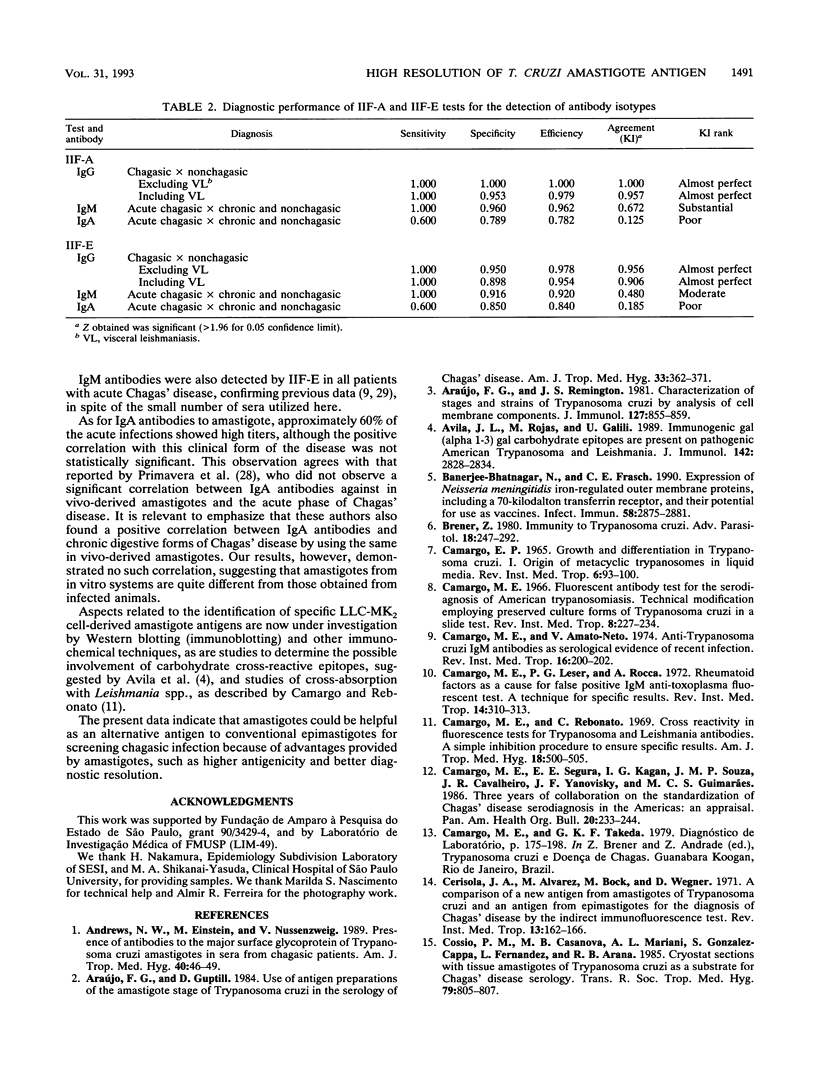
The serodiagnosis of Chagas' disease, a highly prevalent disorder in South American countries, is usually made by the detection of antibodies to Trypanosoma cruzi epimastigote antigen. In this study, we assess the diagnostic performance of the immunofluorescence test with T. cruzi (Y strain) amastigote antigen from an LLC-MK2-infected cell supernatant in comparison with a test with the conventional epimastigote antigen. A total of 238 serum samples from patients in the acute and chronic phases of the disease, with the chronic indeterminate, cardiac, and digestive forms, and from nonchagasic individuals were tested for the presence of immunoglobulin G (IgG), IgM, and IgA antibodies. The reactivity of the amastigote antigen in terms of geometric mean titers was 2 to 4 times higher than that of the epimastigote antigen. Clear-cut results were obtained with the amastigote antigen, with no overlapping of true and false positives. IgG antibodies to amastigotes were found in all patients with Chagas' disease, whereas all sera from nonchagasic patients were negative, except for those from patients with visceral leishmaniasis, in which 63% cross-reactivity was observed. IgM antibodies to amastigotes were detected in 100% of sera from patients with acute Chagas' disease and in 7.5% of sera from patients with chronic Chagas' disease, whereas IgA antibodies were found in 60% of sera from patients in the acute phase and in 33% of sera from patients in the chronic phase. Despite the cross-reactivity observed with sera from visceral leishmaniasis patients, the IgG immunofluorescence test with the amastigote antigen had the highest sensitivity, specificity, and efficiency. No relationship was observed between the class-specific antibodies or their titers and the clinical forms of patients in the chronic phase. Amastigotes from the cell culture supernatant proved to be useful as an alternative antigen to epimastigotes because of their high resolution in the serodiagnosis of Chagas' disease.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews N. W., Einstein M., Nussenzweig V. Presence of antibodies to the major surface glycoprotein of Trypanosoma cruzi amastigotes in sera from Chagasic patients. Am J Trop Med Hyg. 1989 Jan;40(1):46–49. doi: 10.4269/ajtmh.1989.40.46. [DOI] [PubMed] [Google Scholar]
- Araujo F. G., Guptill D. Use of antigen preparations of the amastigote stage of Trypanosoma cruzi in the serology of Chagas' disease. Am J Trop Med Hyg. 1984 May;33(3):362–371. doi: 10.4269/ajtmh.1984.33.362. [DOI] [PubMed] [Google Scholar]
- Araujo F. G., Remington J. S. Characterization of stages and strains of Trypanosoma cruzi by analysis of cell membrane components. J Immunol. 1981 Sep;127(3):855–859. [PubMed] [Google Scholar]
- Avila J. L., Rojas M., Galili U. Immunogenic Gal alpha 1----3Gal carbohydrate epitopes are present on pathogenic American Trypanosoma and Leishmania. J Immunol. 1989 Apr 15;142(8):2828–2834. [PubMed] [Google Scholar]
- Banerjee-Bhatnagar N., Frasch C. E. Expression of Neisseria meningitidis iron-regulated outer membrane proteins, including a 70-kilodalton transferrin receptor, and their potential for use as vaccines. Infect Immun. 1990 Sep;58(9):2875–2881. doi: 10.1128/iai.58.9.2875-2881.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brener Z. Immunity to Trypanosoma cruzi. Adv Parasitol. 1980;18:247–292. doi: 10.1016/s0065-308x(08)60401-7. [DOI] [PubMed] [Google Scholar]
- Camargo M. E., Amato Neto V. Anti-Trypanosoma cruzi IgM antibodies as serological evidence of recent infection. Rev Inst Med Trop Sao Paulo. 1974 Jul-Aug;16(4):200–202. [PubMed] [Google Scholar]
- Camargo M. E. Fluorescent antibody test for the serodiagnosis of American trypanosomiasis. Technical modification employing preserved culture forms of Trypanosoma cruzi in a slide test. Rev Inst Med Trop Sao Paulo. 1966 Sep-Oct;8(5):227–235. [PubMed] [Google Scholar]
- Camargo M. E., Leser P. G., Rocca A. Rheumatoid factors as a cause for false positive IgM anti-toxoplasma fluorescent tests. A technique for specific results. Rev Inst Med Trop Sao Paulo. 1972 Sep-Oct;14(5):310–313. [PubMed] [Google Scholar]
- Camargo M. E., Rebonato C. Cross-reactivity in fluorescence tests for Trypanosoma and Leishmania antibodies. A simple inhibition procedure to ensure specific results. Am J Trop Med Hyg. 1969 Jul;18(4):500–505. doi: 10.4269/ajtmh.1969.18.500. [DOI] [PubMed] [Google Scholar]
- Camargo M. E., Segura E. L., Kagan I. G., Souza J. M., Carvalheiro J. da R., Yanovsky J. F., Guimarães M. C. Three years of collaboration on the standardization of Chagas' disease serodiagnosis in the Americas: an appraisal. Bull Pan Am Health Organ. 1986;20(3):233–244. [PubMed] [Google Scholar]
- Cerisola J. A., Alvarez M., Bock M., Wegner D. A comparison of a new antigen from amastigotes of Trypanosoma cruzi and an antigen from epimastigotes for the diagnosis of Chagas' disease by the indirect immunofluorescence test. Rev Inst Med Trop Sao Paulo. 1971 May-Jun;13(3):162–166. [PubMed] [Google Scholar]
- Cossio P. M., Casanova M. B., Mariani A. L., Gonzalez Cappa S., Fernandez L., Arana R. M. Cryostat sections with tissue amastigotes of Trypanosoma cruzi as a substrate for Chagas' disease serology. Trans R Soc Trop Med Hyg. 1985;79(6):805–807. doi: 10.1016/0035-9203(85)90124-5. [DOI] [PubMed] [Google Scholar]
- Ferreira A. W., Belem Z. R., Moura M. E., Camargo M. E. Aspectos da padronizaço de testes sorológicos para a doença de Chagas: um teste imunoenzimático para a triagem de doadores de sangue. Rev Inst Med Trop Sao Paulo. 1991 Mar-Apr;33(2):123–128. [PubMed] [Google Scholar]
- Gam A. A., Neva F. A. Comparison of cell culture with epimastigote antigens of Trypanosoma cruzi. Am J Trop Med Hyg. 1977 Jan;26(1):47–57. doi: 10.4269/ajtmh.1977.26.47. [DOI] [PubMed] [Google Scholar]
- Hayes R. J., Schofield C. J. Estimación de las tasas de incidencia de infecciones y parasitosis crónicas a partir de la prevalencia: la enfermedad de Chagas en América Latina. Bol Oficina Sanit Panam. 1990 Apr;108(4):308–316. [PubMed] [Google Scholar]
- Maclure M., Willett W. C. Misinterpretation and misuse of the kappa statistic. Am J Epidemiol. 1987 Aug;126(2):161–169. doi: 10.1093/aje/126.2.161. [DOI] [PubMed] [Google Scholar]
- Neva F. A., Gam A. A. A complement-fixing antigen from Trypanosoma cruzi grown in cell cultures. Am J Trop Med Hyg. 1977 Jan;26(1):37–46. doi: 10.4269/ajtmh.1977.26.37. [DOI] [PubMed] [Google Scholar]
- Primavera K. S., Hoshino-Shimizu S., Umezawa E. S., Peres B. A., Manigot D. A., Camargo M. E. Immunoglobulin A antibodies to Trypanosoma cruzi antigens in digestive forms of Chagas' disease. J Clin Microbiol. 1988 Oct;26(10):2101–2104. doi: 10.1128/jcm.26.10.2101-2104.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Primavera K. S., Umezawa E. S., Peres B. A., Camargo M. E., Hoshino-Shimizu S. Chagas'disease: IgA, IgM and IgG antibodies to T. cruzi amastigote, trypomastigote and epimastigote antigens in acute and in different chronic forms of the disease. Rev Inst Med Trop Sao Paulo. 1990 May-Jun;32(3):172–180. doi: 10.1590/s0036-46651990000300005. [DOI] [PubMed] [Google Scholar]
- Shikanai-Yasuda M. A., Lopes M. H., Tolezano J. E., Umezawa E., Amato Neto V., Barreto A. C., Higaki Y., Moreira A. A., Funayama G., Barone A. A. Doença de Chagas aguda: vias de transmissão, aspectos clínicos e resposta à terapêutica específica em casos diagnosticados em um centro urbano. Rev Inst Med Trop Sao Paulo. 1990 Jan-Feb;32(1):16–27. doi: 10.1590/s0036-46651990000100004. [DOI] [PubMed] [Google Scholar]
- Silveira F. T., Souza A. A., Lainson R., Shaw J. J., Braga R. R., Ishikawa E. E. Cutaneous leishmaniasis in the Amazon region: natural infection of the sandfly Lutzomyia ubiquitalis (Psychodidae: Phlebotominae) by Leishmania (Viannia) Lainsoni in Pará State, Brazil. Mem Inst Oswaldo Cruz. 1991 Jan-Mar;86(1):127–130. doi: 10.1590/s0074-02761991000100021. [DOI] [PubMed] [Google Scholar]