Abstract
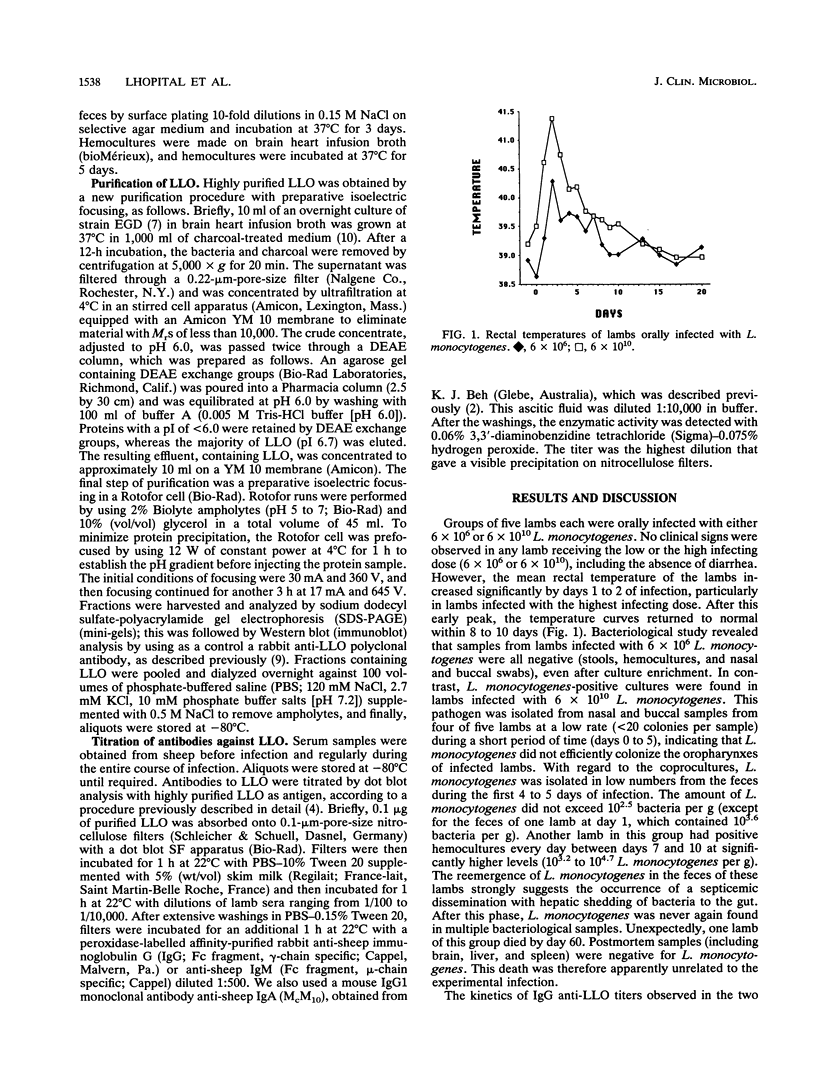
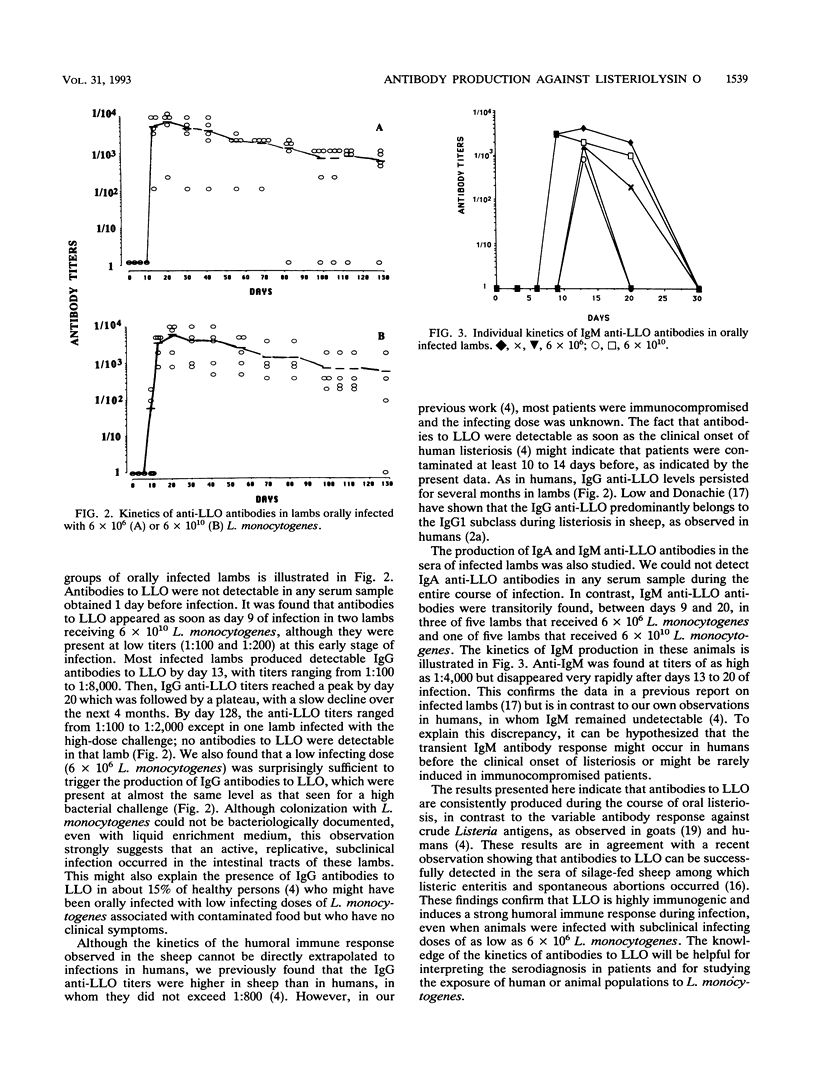
The kinetics of antibody production against listeriolysin O (LLO), a major virulence factor of the intracellular bacterial pathogen Listeria monocytogenes, was studied by dot blot analysis with highly purified LLO during oral infection of sheep. Specific antibodies appeared as soon as day 9 of an oral infection and peaked by day 20 of infection; specific antibody levels then remained almost stable for at least 4 months. A subclinical infecting dose (approximately 10(6) viable bacteria) was capable of eliciting a significant antibody response to LLO, almost at the same level as that observed with a high-dose oral challenge (approximately 10(10)). Antibodies to LLO were mostly constituted by immunoglobulin G (IgG), since an IgA response was not detectable and only a transient and inconstant IgM response was observed between day 9 and day 20 of an oral infection. These results show that antibodies to LLO are constantly produced during oral infection even with a low infecting dose, thus confirming that LLO is highly immunogenic. Detection of antibodies to LLO can therefore be used to detect sheep that have been previously exposed to L. monocytogenes.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beh K. J. Monoclonal antibodies against sheep immunoglobulin light chain, IgM and IgA. Vet Immunol Immunopathol. 1988 Feb;18(1):19–27. doi: 10.1016/0165-2427(88)90033-5. [DOI] [PubMed] [Google Scholar]
- Berche P., Gaillard J. L., Geoffroy C., Alouf J. E. T cell recognition of listeriolysin O is induced during infection with Listeria monocytogenes. J Immunol. 1987 Dec 1;139(11):3813–3821. [PubMed] [Google Scholar]
- Berche P., Reich K. A., Bonnichon M., Beretti J. L., Geoffroy C., Raveneau J., Cossart P., Gaillard J. L., Geslin P., Kreis H. Detection of anti-listeriolysin O for serodiagnosis of human listeriosis. Lancet. 1990 Mar 17;335(8690):624–627. doi: 10.1016/0140-6736(90)90411-w. [DOI] [PubMed] [Google Scholar]
- Bouwer H. G., Nelson C. S., Gibbins B. L., Portnoy D. A., Hinrichs D. J. Listeriolysin O is a target of the immune response to Listeria monocytogenes. J Exp Med. 1992 Jun 1;175(6):1467–1471. doi: 10.1084/jem.175.6.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farber J. M., Peterkin P. I. Listeria monocytogenes, a food-borne pathogen. Microbiol Rev. 1991 Sep;55(3):476–511. doi: 10.1128/mr.55.3.476-511.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaillard J. L., Berche P., Mounier J., Richard S., Sansonetti P. In vitro model of penetration and intracellular growth of Listeria monocytogenes in the human enterocyte-like cell line Caco-2. Infect Immun. 1987 Nov;55(11):2822–2829. doi: 10.1128/iai.55.11.2822-2829.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaillard J. L., Berche P., Sansonetti P. Transposon mutagenesis as a tool to study the role of hemolysin in the virulence of Listeria monocytogenes. Infect Immun. 1986 Apr;52(1):50–55. doi: 10.1128/iai.52.1.50-55.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaillard J. L., Beretti J. L., Boulot-Tolle M., Wilhelm J. M., Bertrand J. L., Herbelleau T., Berche P. Serological evidence for culture-negative listeriosis of central nervous system. Lancet. 1992 Aug 29;340(8818):560–560. doi: 10.1016/0140-6736(92)91765-z. [DOI] [PubMed] [Google Scholar]
- Geoffroy C., Gaillard J. L., Alouf J. E., Berche P. Production of thiol-dependent haemolysins by Listeria monocytogenes and related species. J Gen Microbiol. 1989 Mar;135(3):481–487. doi: 10.1099/00221287-135-3-481. [DOI] [PubMed] [Google Scholar]
- Geoffroy C., Gaillard J. L., Alouf J. E., Berche P. Purification, characterization, and toxicity of the sulfhydryl-activated hemolysin listeriolysin O from Listeria monocytogenes. Infect Immun. 1987 Jul;55(7):1641–1646. doi: 10.1128/iai.55.7.1641-1646.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray M. L., Killinger A. H. Listeria monocytogenes and listeric infections. Bacteriol Rev. 1966 Jun;30(2):309–382. doi: 10.1128/br.30.2.309-382.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harty J. T., Bevan M. J. CD8+ T cells specific for a single nonamer epitope of Listeria monocytogenes are protective in vivo. J Exp Med. 1992 Jun 1;175(6):1531–1538. doi: 10.1084/jem.175.6.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havell E. A. Synthesis and secretion of interferon by murine fibroblasts in response to intracellular Listeria monocytogenes. Infect Immun. 1986 Dec;54(3):787–792. doi: 10.1128/iai.54.3.787-792.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leimeister-Wächter M., Chakraborty T. Detection of listeriolysin, the thiol-dependent hemolysin in Listeria monocytogenes, Listeria ivanovii, and Listeria seeligeri. Infect Immun. 1989 Aug;57(8):2350–2357. doi: 10.1128/iai.57.8.2350-2357.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low J. C., Davies R. C., Donachie W. Purification of listeriolysin O and development of an immunoassay for diagnosis of listeric infections in sheep. J Clin Microbiol. 1992 Oct;30(10):2705–2708. doi: 10.1128/jcm.30.10.2705-2708.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low J. C., Donachie W. Clinical and serum antibody responses to lambs to infection by Listeria monocytogenes. Res Vet Sci. 1991 Sep;51(2):185–192. doi: 10.1016/0034-5288(91)90012-d. [DOI] [PubMed] [Google Scholar]
- MACKANESS G. B. Cellular resistance to infection. J Exp Med. 1962 Sep 1;116:381–406. doi: 10.1084/jem.116.3.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miettinen A., Husu J., Tuomi J. Serum antibody response to Listeria monocytogenes, listerial excretion, and clinical characteristics in experimentally infected goats. J Clin Microbiol. 1990 Feb;28(2):340–343. doi: 10.1128/jcm.28.2.340-343.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pamer E. G., Harty J. T., Bevan M. J. Precise prediction of a dominant class I MHC-restricted epitope of Listeria monocytogenes. Nature. 1991 Oct 31;353(6347):852–855. doi: 10.1038/353852a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portnoy D. A., Jacks P. S., Hinrichs D. J. Role of hemolysin for the intracellular growth of Listeria monocytogenes. J Exp Med. 1988 Apr 1;167(4):1459–1471. doi: 10.1084/jem.167.4.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yewdell J. W., Bennink J. R. The binary logic of antigen processing and presentation to T cells. Cell. 1990 Jul 27;62(2):203–206. doi: 10.1016/0092-8674(90)90356-j. [DOI] [PubMed] [Google Scholar]


