Abstract
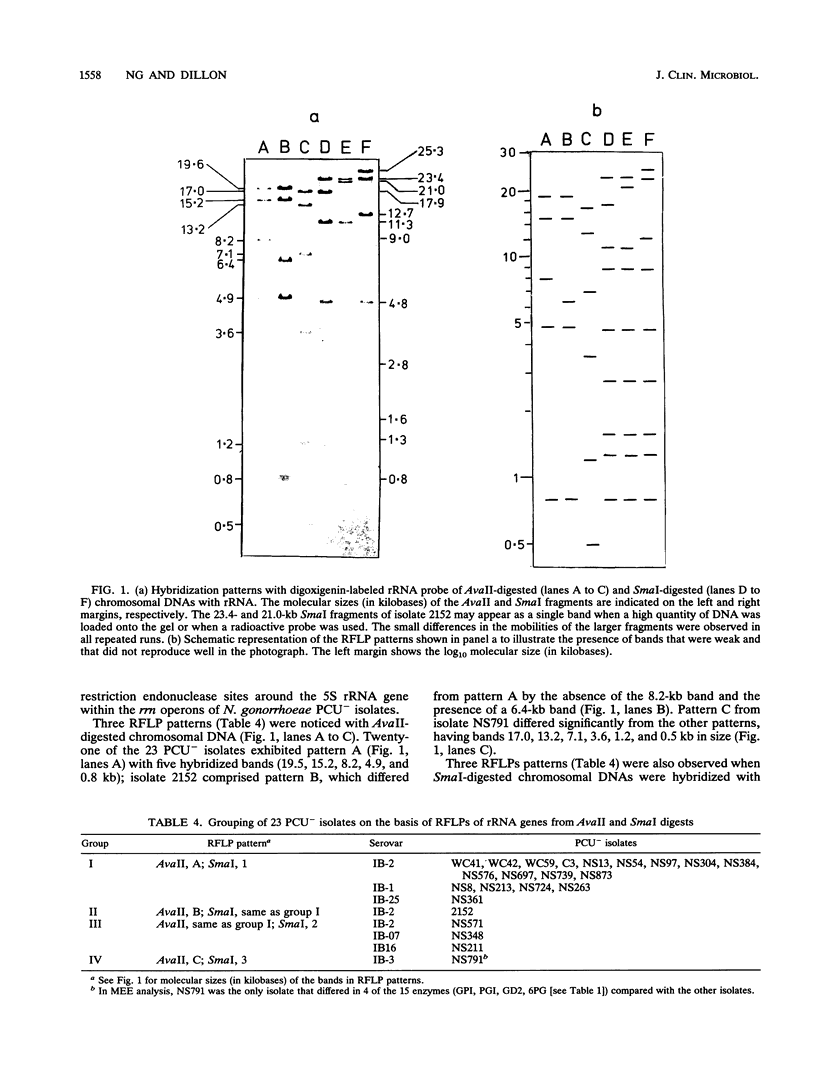
Neisseria gonorrhoeae isolates requiring proline, citrulline, and uracil for growth (PCU-) have homogeneous phenotypes; most are plasmid-free, belong to few serovars, and are significantly associated with intermediate levels of susceptibility to penicillin, tetracycline, erythromycin, and cefoxitin. Because of their lack of variation by these criteria, molecular typing methods, ribotyping (restriction fragment length polymorphism [RFLP] of rRNA genes), and multilocus enzyme electrophoresis were explored as tools for further distinguishing PCU- isolates. By ribotyping, selected PCU- isolates could be separated into four groups on the basis of the hybridization patterns (RFLPs) of SmaI- and AvaII-digested DNA with probes containing rRNA sequences. Most of the isolates (18 of 23 isolates) belonged to a single RFLP (group I). One isolate each was in groups II and IV, and three isolates were in group III. All isolates except one, isolate NS791, had similar multilocus enzyme electrophoresis patterns. Strain NS791 was unusual in that it contained a variant cryptic plasmid with an insert in the 0.46-kb MspI-HinfI fragment of the 4.2-kb plasmid, it was the only isolate belonging to RFLP group IV, and it differed in its multilocus enzyme electrophoresis pattern, having different mobilities for glyceraldehyde phosphate dehydrogenase, phosphoglucose isomerase, 6-phosphogluconate dehydrogenase, and glutamate dehydrogenase. Serovars of PCU- isolates appeared to be more indicative of strain divergence than RFLP or isoenzyme typing. Multilocus enzyme electrophoresis indicated that PCU- isolates are clonal.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brosius J., Ullrich A., Raker M. A., Gray A., Dull T. J., Gutell R. R., Noller H. F. Construction and fine mapping of recombinant plasmids containing the rrnB ribosomal RNA operon of E. coli. Plasmid. 1981 Jul;6(1):112–118. doi: 10.1016/0147-619x(81)90058-5. [DOI] [PubMed] [Google Scholar]
- Brunham R. C., Plummer F., Slaney L., Rand F., DeWitt W. Correlation of auxotype and protein I type with expression of disease due to Neisseria gonorrhoeae. J Infect Dis. 1985 Aug;152(2):339–343. doi: 10.1093/infdis/152.2.339. [DOI] [PubMed] [Google Scholar]
- De la Fuente L., Vázquez J. A. Multilocus enzyme analysis of African type penicillinase-producing Neisseria gonorrhoeae (PPNG) strains isolated in Spain. Sex Transm Dis. 1991 Jul-Sep;18(3):150–152. doi: 10.1097/00007435-199107000-00005. [DOI] [PubMed] [Google Scholar]
- Dillon J. A., Yeung K. H. Beta-lactamase plasmids and chromosomally mediated antibiotic resistance in pathogenic Neisseria species. Clin Microbiol Rev. 1989 Apr;2 (Suppl):S125–S133. doi: 10.1128/cmr.2.suppl.s125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon J. R., Bygdeman S. M., Sandström E. G. Serological ecology of Neisseria gonorrhoeae (PPNG and non-PPNG) strains: Canadian perspective. Genitourin Med. 1987 Jun;63(3):160–168. doi: 10.1136/sti.63.3.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon J. R., Carballo M., King S. D., Brathwaite A. R. Auxotypes, plasmid contents, and serovars of gonococcal strains (PPNG and non-PPNG) from Jamaica. Genitourin Med. 1987 Aug;63(4):233–238. doi: 10.1136/sti.63.4.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon J. R., Pauzé M. Introductory address: resistance to antimicrobial agents. What next for Neisseria gonorrhoeae? Sex Transm Dis. 1984 Oct-Dec;11(4 Suppl):353–359. [PubMed] [Google Scholar]
- Dillon J. R., Pauzé M. Relationship between plasmid content and auxotype in Neisseria gonorrhoeae isolates. Infect Immun. 1981 Aug;33(2):625–628. doi: 10.1128/iai.33.2.625-628.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimont F., Grimont P. A. Ribosomal ribonucleic acid gene restriction patterns as potential taxonomic tools. Ann Inst Pasteur Microbiol. 1986 Sep-Oct;137B(2):165–175. doi: 10.1016/s0769-2609(86)80105-3. [DOI] [PubMed] [Google Scholar]
- Hendry A. T., Stewart I. O. Auxanographic grouping and typing of Neisseria gonorrhoeae. Can J Microbiol. 1979 Apr;25(4):512–521. doi: 10.1139/m79-075. [DOI] [PubMed] [Google Scholar]
- Holten E. Glucokinase and glucose 6-phosphate dehydrogenase in Neisseria. Acta Pathol Microbiol Scand B Microbiol Immunol. 1974 Apr;82(2):201–206. doi: 10.1111/j.1699-0463.1974.tb02312.x. [DOI] [PubMed] [Google Scholar]
- Holten E. Glutamate dehydrogenases in genus Neisseria. Acta Pathol Microbiol Scand B Microbiol Immunol. 1973 Feb;81(1):49–58. doi: 10.1111/j.1699-0463.1973.tb02186.x. [DOI] [PubMed] [Google Scholar]
- Knapp J. S., Holmes K. K., Bonin P., Hook E. W., 3rd Epidemiology of gonorrhea: distribution and temporal changes in auxotype/serovar classes of Neisseria gonorrhoeae. Sex Transm Dis. 1987 Jan-Mar;14(1):26–32. [PubMed] [Google Scholar]
- Knapp J. S., Tam M. R., Nowinski R. C., Holmes K. K., Sandström E. G. Serological classification of Neisseria gonorrhoeae with use of monoclonal antibodies to gonococcal outer membrane protein I. J Infect Dis. 1984 Jul;150(1):44–48. doi: 10.1093/infdis/150.1.44. [DOI] [PubMed] [Google Scholar]
- Kohl P. K., Knapp J. S., Hofmann H., Gruender K., Petzoldt D., Tams M. R., Holmes K. K. Epidemiological analysis of Neisseria gonorrhoeae in the Federal Republic of Germany by auxotyping and serological classification using monoclonal antibodies. Genitourin Med. 1986 Jun;62(3):145–150. doi: 10.1136/sti.62.3.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng L. K., Dillon J. A. Molecular fingerprinting of isolates of the genus Peptostreptococcus using rRNA genes from Escherichia coli and P. anaerobius. J Gen Microbiol. 1991 Jun;137(6):1323–1331. doi: 10.1099/00221287-137-6-1323. [DOI] [PubMed] [Google Scholar]
- Poh C. L., Khng H. P., Lim C. K., Loh G. K. Molecular typing of Neisseria gonorrhoeae by restriction fragment length polymorphisms. Genitourin Med. 1992 Apr;68(2):106–110. doi: 10.1136/sti.68.2.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poh C. L., Ocampo J. C., Loh G. K. Genetic relationships among Neisseria gonorrhoeae serovars analysed by multilocus enzyme electrophoresis. Epidemiol Infect. 1992 Feb;108(1):31–38. doi: 10.1017/s0950268800049475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy R. N., Bigelow N., Dillon J. A. A novel insertion sequence in the cryptic plasmid of Neisseria gonorrhoeae may alter the B protein at the translational level. Plasmid. 1988 Jan;19(1):39–45. doi: 10.1016/0147-619x(88)90061-3. [DOI] [PubMed] [Google Scholar]
- Sandström E. G., Rudén A. K. Markers of Neisseria gonorrhoeae for epidemiological studies. Scand J Infect Dis Suppl. 1990;69:149–156. [PubMed] [Google Scholar]
- Selander R. K., Caugant D. A., Ochman H., Musser J. M., Gilmour M. N., Whittam T. S. Methods of multilocus enzyme electrophoresis for bacterial population genetics and systematics. Appl Environ Microbiol. 1986 May;51(5):873–884. doi: 10.1128/aem.51.5.873-884.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stewart I. O., Hendry A. T. Association between the auxogroup of Neisseria gonorrhoeae and the minimal inhibitory concentration of penicillin. Sex Transm Dis. 1979 Oct-Dec;6(4):247–252. doi: 10.1097/00007435-197910000-00004. [DOI] [PubMed] [Google Scholar]
- de la Fuente L., Vázquez J. A. Analysis of genetic variability of penicillinase non-producing Neisseria gonorrhoeae strains with different levels of resistance to penicillin. J Med Microbiol. 1992 Aug;37(2):96–99. doi: 10.1099/00222615-37-2-96. [DOI] [PubMed] [Google Scholar]